Abstract
Aim
The potential bond between pentraxin-3 levels and neonatal sepsis has been the center of research in many primary studies. The aim of the current meta-analysis is to examine whether there are differences among pentraxin-3 levels in septic and in healthy neonates.
Materials and Methods
Our search strategy included the systematic search of the following databases: MEDLINE, Clinicaltrials.gov, Cochrane Central Register of Controlled Trials (CENTRAL), Google Scholar, using a structured algorithm. Statistical analysis of the overall outcome was done using Revman 5.4 software while leave-one-out and meta-regression analysis were done using the R software. Quality assessment of the included studies was done using the Newcastle-Ottawa scale.
Results
Pentraxin-3 levels were found to be higher in newborns affected by sepsis than in healthy neonates with an MD = 7.66 [95% CI 0.89, 14.42 (p = .03, I2 = 99%)]. Subgroup analysis, based on the country of origin of the included study, led to I2 = 0 with an MD = 1.25 with 95% CI [0.82, 1.69], p < 10−5. Publication bias was assessed using the trim and fill method together with visual inspection of the funnel plots, showcasing no missing studies.
Conclusion
The results of our study show that pentraxin-3 is elevated in neonates with sepsis making it a potential biomarker that needs to be assessed for its diagnostic accuracy in future cohort studies.
Introduction
Rationale
Neonatal sepsis (NS) is defined as the clinical syndrome with signs-symptoms of systemic infection which may be accompanied by positive blood cultures [Citation1]. This clinical syndrome has two subtypes: a) Early-onset neonatal sepsis (EOS) b) Late-onset neonatal sepsis (LOS), which happen <72 h and >72 h, respectively [Citation2]. Epidemiological data show that NS is the third leading cause of mortality in the neonatal period [Citation3].
To this day, the diagnosis of neonatal sepsis remains challenging as signs and symptoms may vary depending on maternal and newborn risk factors [Citation4]. Additionally, a large number of biomarkers have been studied for their diagnostic accuracy as far as NS is concerned. However, blood cultures remain as the gold standard method for the diagnosis of NS [Citation5]. Furthermore, some multiparametric calculators which combine some patient characteristics have shown promising results in diagnosing NS without causing additional mortality [Citation6]. Nevertheless, these calculators do not use biomarkers such as C-Reactive Protein (CRP), presepsin, procalcitonin and proadrenomedullin which have all shown very promising results alone in diagnosing NS [Citation7–9].
Pentraxins are a family of proteins consisted of CRP, serum amyloid A and pentraxin-3 that regulate the complement system helping the human organism in states of infections [Citation10]. Pentraxin-3 is produced by immune and vascular cells, providing help with microorganism recognition and antibody production. Therefore, its role in human infection is clear and it has been elevated in adults with sepsis [Citation11].
Objectives
Pentraxin-3 could serve as a biomarker for the diagnosis of NS, given it has already been studied in an adult population. In the current meta-analysis we aim to elucidate the value of pentraxin-3 in newborns with sepsis.
Methods
Study design (eligibility criteria)
Our systematic review and meta-analysis was written according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [Citation12]. The PRISMA checklist is presented in Supplementary Figure 1. Every single study comparing pentraxin-3 levels among neonates with sepsis and healthy controls was deemed eligible for inclusion. The current meta-analysis aims to evaluate whether pentraxin-3 levels are increased in neonates affected by sepsis in contrast with otherwise healthy neonates in order to elucidate whether pentraxin-3 can accomplish accurate diagnosis of neonatal sepsis. The process of study selection was done in three steps. First of all, the titles and abstracts of all search results were examined and evaluated for relevance. To continue with, studies that were potentially eligible for inclusion after the initial assessment were accessed in full. Last but not least, studies fulfilling our inclusion criteria were included in the systematic review and studies reporting their results in an appropriate (i.e. quantitative) manner were included in the meta-analysis. Review articles, animal studies, letters to the editor and non-comparative studies (case reports, case series) were excluded. Any disagreement between the authors on the methodological approach, the retrieval of articles and the statistical analysis was resolved through the consensus of all authors.
Literature search and data collection (information sources, search strategy, selection process, data collection process, data items)
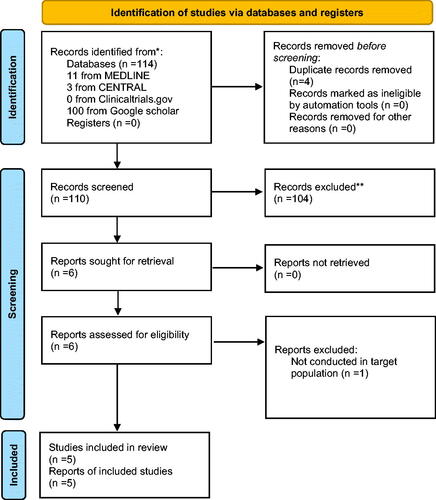
All studies reporting pentraxin-3 levels among septic newborns and healthy controls were accepted for inclusion. Two reviewers (G.P.M., V.I.) independently utilized the following structured algorithm: ("pentraxin-3") AND ("neonatal sepsis" OR "NS") which was applied in the databases MEDLINE (1966–2020), Clinicaltrials.gov (2008–2020), Cochrane Central Register of Controlled Trials (CENTRAL) (1999–2020) and Google Scholar (2004–2020). Depending on the database that was searched if needed the algorithm was altered using critical search terms. In order to achieve a wider appraisal of the literature, citations found within the included articles were manually screened (snow-ball method). No language and date restrictions were applied. The last search was conducted on the 7th of September of 2021. The flowchart of the literature search is depicted in . The extracted data from each study included the following: name of the first author, date of publication, study design, inclusion criteria, early onset or late onset neonatal sepsis, pentraxin-3 levels(μg/L), exclusion criteria, assay used, (NOS) Newcastle-Ottawa scale evaluation and certain patient characteristics: sample size, male gender, weight, vaginal birth and gestational age.
Figure 1. PRISMA flow diagram. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
Study risk of bias assessment
Studies that were approved for inclusion on our meta-analysis were independently evaluated by two researchers with the use of the Newcastle-Ottawa scale (NOS) [Citation13]. This scale applies to nonrandomized studies and assigns stars (maximum score: 9) according to the selection of the study groups, comparability of the groups and the ascertainment of exposure (case- control) or outcome of interest (cohort studies).
Statistical analysis (effect measures, synthesis methods, reporting bias assessment)
The statistical meta-analysis was performed using Revman 5.4 software [Review Manager (RevMan) Version 5.4, The Cochrane Collaboration, 2020] and the Confidence Intervals (CI) were set at 95% [Citation14]. Heterogeneity among the included studies was significant and therefore the Der Simonian-Laird random effect model [Citation15] was applied to calculate mean differences (MD) and 95% confidence intervals (CI). The influence of each individual study on the overall outcome was explored through leave-one-out meta-analysis; each study was sequentially omitted in order to examine how each study affects the overall estimate. Subgroup analysis based on the country of origin of the included studies and on the population included (early onset or late onset neonatal sepsis) was planned post-hoc. Meta-regression analysis was performed using R software [Citation16]. Meta-regression was conducted in order to evaluate the effect of potential confounding factors such as study design, country of origin, year of publication, NOS score, sample size, male gender on the overall effect. However, according to the Cochrane Handbook, at least 10 studies are required for this analysis to be robust [Citation17]. For the evaluation of publication bias funnel plots were created and examined with the trim and fill method [Citation18] through R software using the “meta” package [Citation16].
Certainty assessment
Credibility of outcomes was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework (ranging from very low to high). Examination of the quality of evidence was done through the following domains: study limitations, directness, consistency, precision and publication bias [Citation19].
Results
Study selection, study characteristics
A total of 114 neonates were evaluated for inclusion in the current meta-analysis. In the quantitative analysis a total of 4 studies were included [Citation20–23] (). In addition, in the qualitative synthesis one extra study was included [Citation24]. Study characteristics (country of origin, study design, inclusion criteria, exclusion criteria, assay used, NOS scale, early onset or late onset neonatal sepsis and pentraxin-3 levels in neonates with sepsis) are presented in and patient characteristics are presented in .
Table 1. Study characteristics of the included studies.
Table 2. Patient characteristics of the included studies.
The study of Battal et al. [Citation23] include 58 neonates overall and compared pentraxin-3 among neonates with sepsis-healthy controls with other biomarkers such as CRP, procalcitonin. Additionally, this study had a case-control design, was conducted in Turkey and the study population included only term neonates. However, even though the results of this study were promising regarding pentraxin-3 the authors state that they failed to showcase superiority of this marker to already used biomarkers such as CRP, procalcitonin.
Fahmey et al. [Citation20] conducted a prospective case control study in Egypt among 60 neonates affected by late onset neonatal sepsis. Moreover, this study found higher levels of pentraxin-3 levels among neonates with sepsis and among non-survivor neonates showcasing the significance of this marker in the prognosis of neonatal sepsis.
The third study included in our quantitative analysis is that of Sabry et al. [Citation22] which contained both term and preterm neonates affected by early onset neonatal sepsis in Egypt and measured also CRP values in this population. While the authors of this study found that pentraxin levels were increased in neonates with sepsis they didn’t report a specific cutoff nor sensitivity-specificity values of this marker.
Last but not least, the study of Tunc et al. [Citation21] is the only cohort study in our meta-analysis and was conducted in Turkey. This study was conducted in Turkey and included neonates with early onset neonatal sepsis in which other than pentraxin-3 also CRP and procalcitonin were measured. The authors concluded that there isn’t a statistically significant difference among pentraxin-3 levels between healthy and diseased neonates, which could be possibly attributed to the fact that pentraxin was measured after antibiotic administration.
One study was excluded after full-text assessment [Citation25]. This study was not conducted in neonates and therefore didn’t meet the target population we are examining in the current meta-analysis.
Risk of bias in studies
The quality assessment of each study is shown in under the NOS evaluation header. Two studies (40%) scored 4 stars, two studies (40%) scored 5 stars and one study (20%) scored 6 stars in the NOS scale evaluation.
Results of individual studies
Quantitative synthesis
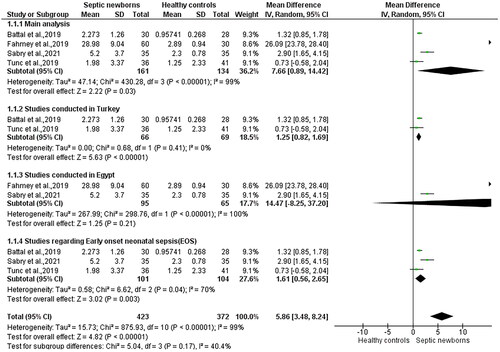
The MD of pentraxin-3 between neonates with sepsis and healthy neonates is depicted in . In a total of 161 neonates with sepsis and 134 healthy neonates the MD between the two mentioned groups is 7.66 μg/L [95% CI 0.89, 14.42 (p = .03, I2 = 99%)].
Qualitative synthesis
One more study [Citation24] was included in the qualitative synthesis with a total of 76 neonates. The study presented a median value of pentraxin-3 levels equal with 8.4558 μg/L [6.3392–10.5725] in neonates with sepsis and a median value of pentraxin-3 levels equal with 4.9627 μg/L [4.3567–5.5688] in healthy neonates (p < .01).
Results of syntheses
Subgroup analysis
Subgroup analysis of studies conducted in Turkey contained 2 studies [Citation21,Citation23]. For this subgroup the MD = 1.25 μg/L with 95% CI [0.82, 1.69] and I2=0. However, in this subgroup one of the two included studies failed to show a statistically significant difference among the two groups [Citation21].The subgroup containing the rest of the studies included in the main analysis contains 2 studies conducted in Egypt [Citation20,Citation22]. In this subgroup the MD = 14.47 μg/L with 95% CI [−8.25, 37.20] and I2=100 with p = 0.21. In addition, subgroup analysis of studies containing solely neonates with early onset neonatal sepsis [Citation21–23] presented an MD = 1.61 μg/L with 95% CI [0.56, 2.65] and I2=70% in a statistically significant manner (p = .003).
Leave-one-out meta-analysis
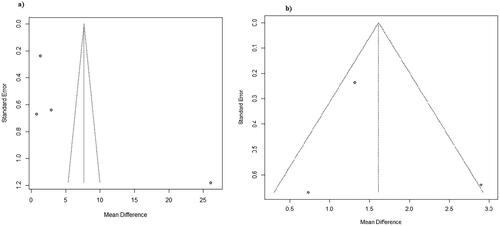
In the leave-one-out analysis () the consecutive omission of each study provided estimates that ranged from 1.6062 μg/L (95% CI (0.5647; 2.6477) I2=69.8%) to 10.0206 μg/L (95% CI (0.1036; 19.9375) I2=99.5%).
Table 3. Leave-one-out meta-analysis.
Meta-regression
Potential confounding factors were evaluated through a meta-regression analysis as shown in Supplementary Table 4. NOS score and sample size significantly affect the MD (estimate= 10.7116, SE= 3.6226 − p = .0031, estimate= 0.5235 SE = 0.2173 − p = .0160, respectively). However, none of the above confounding factors attributed significant heterogeneity from the overall heterogeneity.
Reporting biases (publication bias)
Publication bias was examined using a funnel plot with the aid of the trim and fill method (). From the visual inspection of the funnel plot created there seems to be low suspicion of publication bias regarding the overall outcome as there are no missing studies. The trim and fill method couldn’t be applied to the subgroups regarding country of origin as at least 3 studies are needed. In addition, as far as the early onset neonatal sepsis subgroup is concerned no added studies were calculated using the trim and fill method and therefore there is low suspicion of publication bias ().
Certainty of evidence
Certainty of evidence range from very low to moderate in our meta-analysis as shown in Supplementary Table 5, mainly due to incalculable prediction intervals for some of our subgroups as the number of studies included is small. Publication bias wasn’t suspected in the overall outcome and all subgroups were direct.
Discussion
The current meta-analysis included 295 neonates of which 161 were affected by sepsis. Our results show that pentraxin-3 is a promising biomarker that could aid the neonatologist with the diagnosis of neonatal sepsis. Furthermore, it will help minimize the unnecessary use of antibiotics in neonates as sepsis will be diagnosed more accurately. In addition, our results show that neonates with sepsis had a higher pentraxin-3 than healthy neonates with an MD= 7.66 μg/L [95% CI: 0.89, 14.42 (p = .03, I2=99%). Moreover, subgroup analysis revealed really interesting results as the subgroup containing studies conducted solely in Turkey had I2=0 with an MD= 1.25 μg/L [95% CI: 0.82, 1.69], p < 10−5 and the subgroup analysis regarding early onset neonatal sepsis presented statistically significant results with an MD = 1.61 μg/L with 95% CI [0.56, 2.65] and I2=70% In the leave-one-out meta-analysis I2 ranges from 69.8 to 99.5% showcasing that one study [Citation20] might be introducing significant heterogeneity in our meta-analysis. Meta-regression analysis showcased that potential confounding factors such as study NOS score and sample size could be responsible for slightly different MD’s than the overall outcome, however significant heterogeneity failed to be attributed to these two factors. Publication bias was evaluated using the trim and fill method together with the visual inspection of funnel plots, no suspicion of which was raised.
Pathophysiologically, pentraxin-3 acts as a pattern recognizing receptor such as CRP with the difference however that pentraxin-3 production is not liver related [Citation26]. Furthermore, studies in adults have shown that pentraxin is more sensitive in the acute phases of infection than CRP as it increases in an earlier period [Citation25]. Regarding the head to head comparison of pentraxin-3 diagnostic accuracy against CRP more studies need to be conducted regarding the pediatric population. Moreover, the origin of pentraxin-3 is different from that of procalcitonin therefore making pentraxin-3 an earlier marker of sepsis in adults [Citation27]. These results from the adult population are in concordance with the results of our meta-analysis, more specifically in the early onset neonatal sepsis subgroup where pentraxin-3 was higher than controls in a statistically significant manner. Finally, pentraxin-3 has the advantage of a faster rise than biomarkers already used due to its production being locally at the site of infection and it is not dependent on other molecules to trigger body organs and therefore stimulate them in order to be produced such as other markers(i.e. CRP, procalcitonin) [Citation21].
Strengths and limitations
The current meta-analysis researched the value of pentraxin-3 among neonates with sepsis. We conducted a vast literature search using strict inclusion criteria with no date or language restrictions applied. Furthermore, the majority of our studies had a moderate NOS score rating which together with the moderate quality of evidence arising from the GRADE score makes our results fair. Last but not least, no missing studies were found from the publication bias evaluation and no confounding factors were responsible for the inter-study heterogeneity.
Unfortunately, the current meta-analysis is based on small case-control studies in the majority. These primary studies may introduce selection bias which wasn’t explored in our methodology. Additionally, the overall outcome of our study is based on heterogeneous studies (I2=99%) with small samples and we weren’t able to calculate a specific cutoff due to the small number of primary studies that provided one. Moreover, some of the included studies lacked specific patient characteristics and therefore the meta-regression analysis we performed was limited. Even though we conducted extensive subgroup, meta-regression and leave-one-out analyses we couldn’t attribute the high heterogeneity to a specific factor. In addition, in the subgroup where I2=0 one of the two studies included failed to show a significant difference. In contrast, subgroup analysis regarding early onset neonatal sepsis showcased a statistically significant higher MD than controls, with I2=70%. Furthermore, the results of the current meta-analysis should be read with caution as other noninfectious causes have been found to increase pentraxin-3 levels in the perinatal period and measurement time of pentraxin-3 should also be taken under consideration [Citation28]. Due to scarce data of sepsis definition within the included studies our results concern both clinical and culture proven neonatal sepsis. Finally, the small sample of studies we included didn’t allow us to calculate specific prediction intervals for some subgroups which in addition with the inability to perform the trim and fill method for the examination of publication bias in these groups, lowers the credibility of those outcomes, some of which aren’t even statistically significant.
Implications for practice and future research
We strongly encourage future researchers to conduct prospective cohort studies in order to evaluate pentraxin-3 levels among newborns with sepsis, therefore providing results that arise from higher quality primary studies and are more accurate. In addition, future studies need to provide multiple cutoffs of pentraxin-3 levels above which the diagnosis of neonatal sepsis is made and/or the prognosis of neonatal sepsis is altered. In current practice we urge physicians to use our marker with caution, especially in early onset neonatal sepsis as our results were statistically significant in that subgroup, together with other already studied markers such as pro-ADM, CRP, presepsin and procalcitonin, in a case there is a diagnostic dilemma.
Conclusion
Our findings suggest that pentraxin-3 levels among septic neonates are increased when in contrast with healthy neonates, more specifically regarding neonates with suspicion of early onset neonatal sepsis as pathophysiologically it is expected to rise faster than already used biomarkers such as CRP and procalcitonin. In addition, our results showcase that pentraxin-3 is a very promising biomarker in regards to CRP and procalcitonin. However, larger and more prospective cohort studies need to be done in order to interpret its value more accurately and with a specific cutoff. Furthermore, future studies should be conducted in a more lucid way creating specific subgroups that we were not possible to address in our meta-analysis (such as LOS, term-preterm, time of measurement) in order to help physicians elucidate the true value of this biomarker.
Author contributions
Gerasimos Panagiotis Milas: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing – original draft, writing – review & editing. Vasileios Issaris: data curation, formal analysis, investigation, methodology, software, validation, visualization, writing – original draft, writing - review & editing. Georgios Niotis: supervision.
Supplementary Figure S1
Download PDF (69 KB)Supplementary Tables
Download PDF (179.4 KB)Acknowledgement
No financial support was received for this review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sankar MJ, Agarwal R, Deorari AK, et al. Sepsis in the newborn. Indian J Pediatr. 2008;75:261–266.
- Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390:1770–1780.
- Wattal C, Kler N, Oberoi JK, et al. Neonatal sepsis: mortality and morbidity in neonatal sepsis due to multidrug-resistant (MDR) organisms: part 1. Indian J Pediatr. 2020;87:117–121.
- Procianoy RS, Silveira RC. The challenges of neonatal sepsis management. J Pediatr 2020;96(1):80–86.
- Tzialla C, Manzoni P, Achille C, et al. New diagnostic possibilities for neonatal sepsis. Am J Perinatol. 2018;35:575–577.
- Deshmukh M, Mehta S, Patole S. Sepsis calculator for neonatal early onset sepsis – a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2021;34:1832–1840.
- Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018;22(1):316.
- Bellos I, Fitrou G, Pergialiotis V, et al. The diagnostic accuracy of presepsin in neonatal sepsis: a meta-analysis. Eur J Pediatr. 2018;177(2021):625–632.
- Milas GP, Issaris V. Proadrenomedullin and neonatal sepsis: a systematic review and meta-analysis of diagnostic accuracy. Eur J Pediatr. 2021;181(1):59–71.
- Haapasalo K, Meri S. Regulation of the complement system by pentraxins. Front Immunol. 2019;10:1750.
- Lee YT, Gong M, Chau A, et al. Pentraxin-3 as a marker of sepsis severity and predictor of mortality outcomes: a systematic review and meta-analysis. J Infect. 2018;76(1):1–10.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:2021.
- Wells GA, Shea B, O’Connell D, et al. Ottawa Hospital Research Institute [Internet]. [cited 2021 Sep 20]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- The Nordic Cochrane Centre. The cochrane collaboration. Review manager 5.3. Copenhagen; 2020.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- Schwarzer G. Package “meta” title general package for meta-analysis; 2021 [cited 2021 Jun 9]. Available from: https://www.springer.com/gp/book/9783319214153.
- Cochrane Collaboration. 9.6.4 Meta-regression [Internet]. [cited 2021 Jun 13]. Available from: https://handbook-5-1.cochrane.org/chapter_9/9_6_4_meta_regression.htm.
- Schwarzer G, Carpenter J, Rücker G. Empirical evaluation suggests copas selection model preferable to trim-and-fill method for selection bias in meta-analysis. J Clin Epidemiol. 2010;63:282–288.
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406.
- Fahmey SS, Mostafa N. Pentraxin 3 as a novel diagnostic marker in neonatal sepsis. J Neonatal Perinatal Med. 2019;12:437–442.
- Tunç T, Polat A, Özdemir R, et al. Assessment of novel biomarkers: sTREM-1, pentraxin-3 and pro-adrenomedullin in the early diagnosis of neonatal early onset sepsis. J Neonatal Perinatal Med.. 2020;13:39–45.
- Sabry A, Ibrahim M, Khashana A. Assessment of pentraxin 3 in a systemic inflammatory response occurring with neonatal bacterial infection. J Neonatal Perinatal Med. 2021;14(4):563–568.
- Battal F, Bulut ÖE, Yıldırım Ş, et al. Serum pentraxin 3 concentration in neonatal sepsis. J Pediatr Infect Dis. 2019;14:219–222.
- Baumert M, Surmiak P, Szymkowiak M, et al. The assessment of pentraxin 3: a novel biomarker in early detection of infection in newborns. Biomed Res Int. 2021;2021:6638622.
- Hu C, Zhou Y, Liu C, et al. Pentraxin-3, procalcitonin and lactate as prognostic markers in patients with sepsis and septic shock. Oncotarget. 2017;9:5125–5136.
- Ketter P, Yu JJ, Cap AP, et al. Pentraxin 3: an immune modulator of infection and useful marker for disease severity assessment in sepsis. 2016;12:501–507.
- Hamed S, Behnes M, Pauly D, et al. Diagnostic value of pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions. BMC Infect Dis. 2017;17(1):554.
- Lannergård A, Rosenström F, Normann E, et al. Serum pentraxin 3 concentrations in neonates. Ups J Med Sci. 2014;119(1):62–64.