Abstract
Objective
This meta-analysis aimed to investigate the relationship between hyperuricemia and maternal and neonatal complications in pregnant women.
Methods
We searched PubMed, Embase, Web of Science, and the Cochrane Library from the databases’ inception to August 12, 2022. We included studies that reported results on the association between hyperuricemia and maternal and fetal outcomes among pregnant women. Using the random-effects model, the pooled odds ratio (OR) with 95% confidence intervals (CIs) was calculated for each outcome analysis.
Results
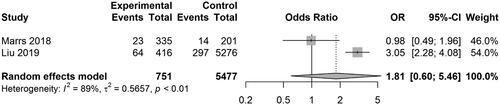
A total of 7 studies, including 8104 participants, were included. The pooled OR for pregnancy-induced hypertension (PIH) was 2.61 [0.26, 26.56] (z = 0.81, p = .4165; I2 = 96.3%). The pooled OR for preterm birth was 2.52 [1.92, 3.30] (z = 6.64, p < .0001; I2 = 0%). The pooled OR for low birth weight (LBW) was 3.44 [2.52, 4.70] (z = 7.77, p < .0001; I2 = 0%). The pooled OR for small gestational age (SGA) was 1.81 [0.60, 5.46] (z = 1.06, p = .2912; I2 = 88.6%).
Conclusion
Results of this meta-analysis indicate a positive relationship between hyperuricemia and PIH, preterm birth, LBW, and SGA in pregnant women.
Background
Uric acid (UA) is the final metabolite of purine metabolism; the liver forms it from endogenous and exogenous precursor proteins, and the kidneys and intestines mainly excrete it [Citation1]. Studies have revealed that physiological levels of UA have a protective function in vivo due to their antioxidant effect [Citation1]. Nevertheless, excessive serum UA levels are associated with kidney disease, cardiovascular disease (CVD), and metabolic syndrome [Citation2,Citation3].
During the uncomplicated pregnancy, the serum UA concentration in early pregnancy decreased by 25–35%, but then increased during late pregnancy, mainly due to the change in renal handling [Citation4]. The kidney’s handling of UA is complex, involving four sequential steps, namely, (1) glomerular filtration, (2) about 98–100% reabsorption in the proximal convoluted tubules, (3) secretion into the lumen of the distal part of the proximal convoluted tubules, and (4) further reabsorption in the distal tubules. The net urine excretion of UA is 6–12% of the filtered amount [Citation5]. Elevated plasma UA is an independent risk factor for premature delivery, low birth weight (LBW), and low Apgar scores of 1 and 5 min [Citation6–8]. UA levels have been used to predict the occurrence of pregnancy complications and adverse and to lower adverse pregnancy outcomes [Citation9–12]. High UA can transfer into fetal circulation via the placenta in mothers with hyper-uric acid (HUA) [Citation13]. Moreover, maternal oxidative stress, the excitation of placental vascular endothelium, and the upregulation of inflammatory response can further induce the formation of dysfunctional placentas and ultimately prevent fetal development [Citation14]. Several studies have examined the correlation between elevated maternal serum UA and adverse maternal and fetal outcomes [Citation15–17]. However, the studies have yielded relevant or irrelevant findings, possibly caused by limitations associated with an individual study.
We endeavored to perform a meta-analysis by synthesizing the most recent results to investigate the relationship between hyperuricemia with maternal and neonatal complications in pregnant women. The results of this study can help early detection, control, treatment, and prevention of maternal and neonatal complications.
Methods
The included studies in this meta-analysis had been published and declared ethical approvals; thus, no ethical approval was needed for this study. This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [Citation18]. The protocol for this meta-analysis was registered with the prospective international registry for systematic reviews, PROSPERO (ID: CRD42022381897).
Literature search and study selection
We systematically searched the databases PubMed, Embase, Web of Science, and the Cochrane Library from the inception of the databases to August 12, 2022. Only the citations in English were considered. A detailed search strategy is attached in Supplementary Table 1. We also searched the reference lists of the included articles to retrieve additional studies not identified through the initial literature search. Furthermore, we contacted ClinicalTrials.gov (US NIH) to retrieve studies that had already been completed but not yet published.
The inclusion criteria of studies eligible for this meta-analysis were as follows: (1) controlled studies reported the association between hyperuricemia and maternal and fetal outcomes among pregnant women. (2) If studies recruited participants over the same period or in the same study institute, only the study with the largest sample size or providing the complete outcomes was included to avoid duplications. Exclusion criteria included case reports, review articles, comments, conference abstracts, and studies with unextractable outcomes. Two reviewers (Jie Tan and Huali Fei) independently screened the titles and abstracts of articles and confirmed the studies for eligibility. Any disagreement was resolved through discussion.
Data extraction and quality assessments
Two researchers (Jie Tan and Huali Fei) independently extracted data from the enrolled studies. The following information was retrieved: name of the first author, year of publication, country, number of participants, the mean age of participants, number of participants with maternal and fetal outcomes (i.e., pregnancy-induced hypertension (PIH), gestational diabetes, preterm birth, low birth weight (LBW), small for gestational age (SGA) in the case and control groups. A third investigator (Xuhong Zhu) resolved disagreements until we achieved a consensus. The risk of bias in included studies was assessed by the modified Newcastle-Ottawa scale (NOS), which consists of three factors: patient selection, comparability of the study groups, and assessment of outcome.
Statistical analysis
We used the R Studio (Version 4.1.2, Comprehensive R Archive Network) for statistical analyses. The pooled odds ratio (OR) with 95% confidence intervals (CIs) was calculated for each analysis of the outcomes (incident rates of PIH, gestational diabetes, preterm birth, LBW, SGA) using the random-effects model [Citation19,Citation20]. A Cochran Q test was used for heterogeneity evaluation between studies, and an I2 statistic was used to evaluate the level of heterogeneity. The I2 classified the magnitude of heterogeneity with I2 > 25%, I2 > 50%, and I2 > 75%, representing moderate, substantial, and considerable heterogeneity, respectively [Citation21,Citation22]. We conducted a sensitivity analysis to assess the robustness of the results. We used Egger’s regression test and funnel plots to assess potential publication bias numerically and visually [Citation23,Citation24]. A p-value <.05 was considered to be of statistical significance.
Results
Study selection and characteristics
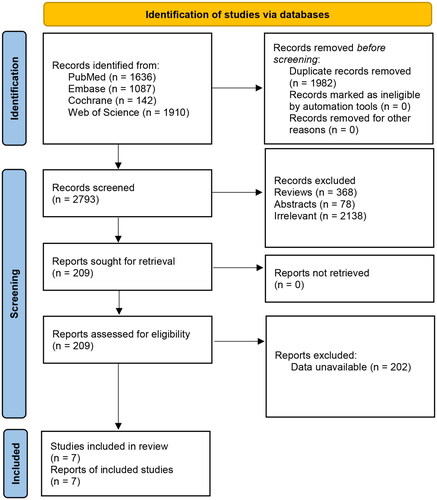
The systematic literature search yielded an identification of 4775 publications, from which 1982 duplicates were removed, and 2584 articles were excluded as they were conference abstracts (78), reviews (368), and topics not relevant to the research question (2138). After a full-text screen of the remaining 209 articles, 7 studies, including 8104 participants, were finally included in this meta-analysis () [Citation4,Citation13,Citation25–30]. Studies were conducted in China, Nigeria, the USA, Brazil, and Malaysia. Two included studies were cross-sectional studies, 2 were case-control studies, 3 were cohort studies. The mean age (years) of participants ranged from 19.8 to 33.1. The quality of included studies was rated as moderate to high according to the NOS scale. shows detailed characteristics of enrolled studies.
Table 1. Baseline features and quality assessment of included studies.
Maternal and fetal outcomes
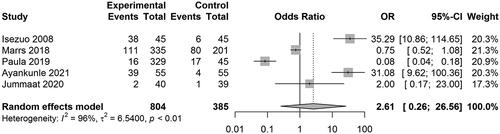
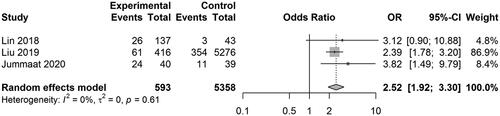
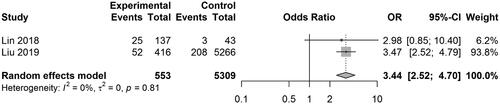
Five studies reported the results on PIH. The pooled OR was 2.61 [0.26, 26.56] (z = 0.81, p = .4165; I2 = 96.3%) (). Three studies reported the results on preterm birth. The pooled OR was 2.52 [1.92, 3.30] (z = 6.64, p < .0001; I2 = 0%) (). Two studies reported the results on LBW. The pooled OR was 3.44 [2.52, 4.70] (z = 7.77, p < .0001; I2 = 0%) (). Two studies reported the results on SGA. The pooled OR was 1.81 [0.60, 5.46] (z = 1.06, p = .2912; I2 = 88.6%) (). In the study of Lin et al. disseminated intravascular coagulation (DIC) was detected in 3 of 173 patients with HUA, and no DIC was detected in normal UA patients.
Sensitivity analysis and publication bias
The sensitivity analysis did not influence the results excessively by omitting any single study. Egger’s tests of funnel plot asymmetry resulted in p-values of .4606 and .2936 for the analysis of PIH and preterm birth; the publication bias was not assessed due to the limited number of included studies (Figures S1 and S2).
Discussion
Asymptomatic hyperuricemia is a common problem, affecting up to 20% of the general population [Citation31]. In pregnancy, hyperuricemia is still a common problem despite the increased glomerular filtration rate (GFR) [Citation30]. Accumulated studies have investigated the relationship between maternal hyperuricemia and pregnant fetal outcomes. However, due to limited sample sizes and heterogeneous results among these single studies, we performed a meta-analysis to explore the association between hyperuricemia and maternal/fetal outcomes. The pooled OR for PIH, preterm birth, LBW, and SGA in the study were 2.61 [0.26, 26.56], 2.52 [1.92, 3.30], 3.44 [2.52, 4.70], 1.81 [0.60, 5.46], respectively.
The association between serum UA and CVD has long been recognized. Nevertheless, it is not yet clear whether serum UA is only a risk marker or pathogen of CVD or whether treatment targeting UA levels will affect outcomes of CVD [Citation32]. Serum UA is closely related to traditional cardiovascular risks factors such as hypertension, dyslipidemia, obesity, impaired glucose metabolism, and metabolic syndrome, which contribute to the pathophysiology of cardiovascular diseases [Citation33,Citation34]. UA may be pathogenic and participate in the pathophysiology of CVD by mediating or enhancing the harmful effects of cardiovascular risk factors on vascular tissue and myocardium as a bridging mechanism [Citation35]. Previous meta-analyses confirmed a significant relationship between UA and CVD. Braga and colleagues identified an overall relative risk (RR) of 1.206 (p = .003) for coronary heart disease (CHD) incidence and a RR of 1.209 (p = .047) for CHD mortality [Citation36]. Another meta-analysis, including 29 studies, revealed that hyperuricemia was associated with an increased risk of CHD incidence (adjusted RR = 1.13) and mortality (adjusted RR = 1.27) [Citation37].
Pregnancy-induced hypertension, including pre-eclampsia, is a common complication in 7 - 10% of all pregnancies, which is responsible for more than 50,000 maternal deaths yearly worldwide, 25% of fetal growth restriction, and 15% of preterm birth [Citation38]. Gestational hypertension, especially preterm pre-eclampsia, is associated with a significant risk of CVD and cerebrovascular disease [Citation39,Citation40]. The American College of Obstetrics and Gynecology (ACOG) defines pre-eclampsia as hypertension and proteinuria occurring in a previously normotensive patient after 20 weeks of gestation [Citation41]. The clinical syndromes of pre-eclampsia begin with abnormal placental formation, followed by the release of antiangiogenic markers, mainly mediated by soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) [Citation42]. High levels of sFlt-1 and sEng are related to endothelial dysfunction, vasoconstriction, and immune dysfunction, negatively affecting each maternal organ and fetus [Citation42]. It has been reported that increased serum UA is a common clinical finding in women with clinically evident pre-eclampsia [Citation38]. In addition, UA clearance usually decreases before a significant decrease in glomerular filtration rate or increases in serum creatinine among women with hypertension [Citation43]. It has been indicated that UA might have a predictive role in pre-eclampsia and play a significant role in maternal and fetal pathogenesis and presentations [Citation44]. However, this study revealed no statistically significant relationship between maternal hyperuricemia and PIH, with an OR value of 2.61. One of the underlying reasons may be the high level of heterogeneity between included studies. Either subgroup analysis or meta-regression was not carried out because insufficient covariates regarding other clinical characteristics of study participants were extracted. Furthermore, Hawkins et al. found that increased UA in pregnant women could lead to a higher risk of adverse fetal outcomes such as small-for-gestational-age infants and premature birth through a retrospective cohort study [Citation6]. Likewise, the results of this meta-analysis demonstrated a positive relationship between maternal hyperuricemia and adverse fetal outcomes such as preterm birth (OR = 2.52) and SGA (OR = 1.81). Moreover, increased serum UA was associated with mild renal impairment and LBW infants, and multivariate regression analysis showed that maternal serum UA level was an independent risk factor for LBW infants [Citation29]. The current study’s findings were consistent with this conclusion, with a larger sample size and insignificant heterogeneity between component studies. The reason for these maternal and fetal abnormalities is that continued high levels of UA may trigger a series of ischemic injuries through the decrease of nitric oxide concentration, obstruction of placental vascular remodeling, and reduction of intramuscular perfusion [Citation29].
In this meta-analysis, two investigators independently did a systematical database search in English to retrieve all relevant studies. The potential factors contributing to heterogeneity may be differences in the sample sizes of included studies, the ethnicity of the study participants, and the design of included studies. However, some limitations in our meta-analysis should be mentioned. Firstly, our results were based on unadjusted estimates; more accurate outcomes would result from adjustments for other confounders such as gender, age, body mass index, and lifestyle. Secondly, data in the meta-analysis need to be more comprehensive, especially when performing subgroup analysis. Thus, potential publication bias or small study effect is very likely despite no evidence from our statistical tests. Thirdly, the language of studies was limited to English, which may result in potential language bias. Fourthly, significant heterogeneity was detected in the pooled outcomes analyses on PIH and SGA. Finally, We only synthesized the outcomes provided in the seven included studies, i.e., PIH, preterm birth, LBW, and SGA, yet these outcomes were not comprehensive. And therefore, the findings should be interpreted with caution, and more relevant studies are needed in the future.
Compared to previous individual observational studies investigating the association between maternal hyperuricemia and neonatal complications, this meta-analysis suggested a positive relationship between hyperuricemia and PIH, preterm birth, LBW, and SGA in pregnant women. The findings of this study may provide a shred of more powerful evidence on managing maternal and neonatal complications in scientific and clinical settings.
Ethical approval
Not Applicable
Consent form
Not Applicable
Author contributions
Jie Tan conceived and designed this study. Xuhong Zhu administrative support. Huali Fei performed the collection, extraction, and analysis of data. Jie Tan was responsible for writing the manuscript. Lumeng Chen modified the English language. All authors reviewed the paper and approved the final manuscript.
Supplemental Material
Download Zip (186.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
The datasets generated and/or analyzed during the current study are available in the article.
Additional information
Funding
References
- Hu J, Xu W, Yang H, et al. Uric acid participating in female reproductive disorders: a review. Reprod Biol Endocrinol. 2021;19(1):65.
- Battelli MG, Bortolotti M, Polito L, et al. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis. 2018;1864(8):2557–2565.
- Richette P, Perez-Ruiz F, Doherty M, et al. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. 2014;10(11):654–661.
- Amini E, Sheikh M, Hantoushzadeh S, et al. Maternal hyperuricemia in normotensive singleton pregnancy, a prenatal finding with continuous perinatal and postnatal effects, a prospective cohort study. BMC Pregnancy Childbirth. 2014;14:104.
- Nair A, Savitha C. Estimation of serum uric acid as an indicator of severity of pre-eclampsia and perinatal outcome. J Obstet Gynaecol India. 2017;67(2):109–118.
- Hawkins TL, Roberts JM, Mangos GJ, et al. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484–492.
- Akahori Y, Masuyama H, Hiramatsu Y. The correlation of maternal uric acid concentration with small-for-gestational-age fetuses in normotensive pregnant women. Gynecol Obstet Invest. 2012;73(2):162–167.
- Chang FM, Chow SN, Huang HC, et al. The placental transfer and concentration difference in maternal and neonatal serum uric acid at parturition: comparison of normal pregnancies and gestosis. Biolo Res Pregnancy Perinatol. 1987;8(1 1ST Half):35–39.
- Bellos I, Pergialiotis V, Loutradis D, et al. The prognostic role of serum uric acid levels in pre-eclampsia: a meta-analysis. J Clin Hypertens. 2020;22(5):826–834.
- Weissgerber TL, Milic NM, Turner ST, et al. Uric acid: a missing link between hypertensive pregnancy disorders and future cardiovascular disease? Mayo Clin Proc. 2015;90(9):1207–1216.
- Laughon SK, Catov J, Provins T, et al. Elevated first-trimester uric acid concentrations are associated with the development of gestational diabetes. Am J Obstet Gynecol. 2009;201(4):402.e1–402.e5.
- Luque-Ramírez M, Alvarez-Blasco F, Uriol Rivera MG, et al. Serum uric acid concentration as non-classic cardiovascular risk factor in women with polycystic ovary syndrome: effect of treatment with ethinyl-estradiol plus cyproterone acetate versus metformin. Hum Reprod. 2008;23(7):1594–1601.
- Liu L, Yu C, Yang F, et al. Maternal hyperuricemia superimposed on maternal hypertension aggravates the risk of small-for-gestational-age fetus. Life Sci. 2019;228:215–220.
- Bainbridge SA, von Versen-Höynck F, Roberts JM. Uric acid inhibits placental system a amino acid uptake. Placenta. 2009;30(2):195–200.
- Redman CW, Beilin LJ, Bonnar J, et al. Plasma-urate measurements in predicting fetal death in hypertensive pregnancy. Lancet. 1976;1(7974):1370–1373.
- Parrish M, Griffin M, Morris R, et al. Hyperuricemia facilitates the prediction of maternal and perinatal adverse outcome in patients with severe/superimposed pre-eclampsia. J Matern Fetal Neonatal Med. 2010;23(12):1451–1455.
- Roberts JM, Bodnar LM, Lain KY, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46(6):1263–1269.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–145.
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10: ed000142.
- Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Mavridis D, Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid Based Ment Health. 2014;17(1):30.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Jummaat F, Adnan AS, Ab Hamid SA, et al. Foetal and maternal outcomes in hyperuricaemia pre-eclampsia patients in Hospital Universiti Sains Malaysia. J Obstet Gynaecol. 2021;41(1):38–43.
- Ayankunle OM, Adeniyi AA, Adewara OE, et al. Maternal serum uric acid: a reliable prognostic indicator of foetal outcome among pre-eclamptic patients in a low resource setting. J Matern Fetal Neonatal Med. 2022;35(25):7695–7700.
- Paula LG, Pinheiro da Costa BE, Hentschke MR, et al. Increased proteinuria and uric acid levels are associated with eclamptic crisis. Pregnancy Hypertens. 2019;15:93–97.
- Marrs CC, Rahman M, Dixon L, et al. The association of hyperuricemia and immediate postpartum hypertension in women without a diagnosis of chronic hypertension. Hypertens Pregnancy. 2018;37(3):126–130.
- Lin J, Hong XY, Tu RZ. The value of serum uric acid in predicting adverse pregnancy outcomes of women with hypertensive disorders of pregnancy. Ginekol Pol. 2018;89(7):375–380.
- Isezuo SA, Ekele BA. Comparison of metabolic syndrome variables among pregnant women with and without eclampsia. J Natl Med Assoc. 2008;100(9):1059–1062.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the national health and nutrition examination survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141.
- Wu AH, Gladden JD, Ahmed M, et al. Relation of serum uric acid to cardiovascular disease. Int J Cardiol. 2016;213:4–7.
- Brand FN, McGee DL, Kannel WB, et al. Hyperuricemia as a risk factor of coronary heart disease: the Framingham study. Am J Epidemiol. 1985;121(1):11–18.
- Rathmann W, Funkhouser E, Dyer AR, et al. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary artery risk development in young adults. Ann Epidemiol. 1998;8(4):250–261.
- Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta. 2018;484:150–163.
- Braga F, Pasqualetti S, Ferraro S, et al. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med. 2016;54(1):7–15
- Li M, Hu X, Fan Y, et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep. 2016;6:19520.
- Powers RW, Bodnar LM, Ness RB, et al. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am J Obstet Gynecol. 2006;194(1):160.
- Tooher J, Thornton C, Makris A, et al. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension. 2017;70(4):798–803.
- Coutinho T, Lamai O, Nerenberg K. Hypertensive disorders of pregnancy and cardiovascular diseases: current knowledge and future directions. Curr Treat Options Cardiovasc Med. 2018;20(7):56.
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstetrics and Gynecology. 2013;122(5):1122–1131.
- Ives CW, Sinkey R, Rajapreyar I, et al. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(14):1690–1702.
- Gallery ED, Györy AZ. Glomerular and proximal renal tubular function in pregnancy-associated hypertension: a prospective study. Eur J Obstet Gynecol Reprod Biol. 1979;9(1):3–12.
- Livingston JR, Payne B, Brown M, et al. Uric acid as a predictor of adverse maternal and perinatal outcomes in women hospitalized with pre-eclampsia. J Obstet Gynaecol Can. 2014;36(10):870–877.