Abstract
Objective
The purpose of this meta-analysis is to investigate the effect of prophylactic caffeine use in the treatment of apnea and other clinical outcomes in very low birth weight infants.
Methods
We searched PubMed, Embase, Web of Science, Scopus, EBSCO, CNKI, and Cochrane databases for all relevant studies up to May 20, 2022. The meta-analysis was carried out using Stata16.0 and RevMan5.4 software.
Results
Eleven randomized controlled trials were evaluated, including a total of 4375 very low birth weight infants. The results demonstrated that prophylactic caffeine use was linked with a significantly lower probability of AOP (OR 0.31, 95% CI: 0.19–0.49, p < .001), duration of mechanical ventilation and oxygen therapy when compared to the control group. It also reduced the incidence of BPD (OR 0.62, 95% CI: 0.54–0.71, p < .001), PDA (OR 0.49, 95% CI: 0.30–0.80, p = .005) and ROP (OR 0.76, 95% CI: 0.65–0.90, p = .001), without raising the risk of NEC, IVH and death before hospital discharge (p > .05).
Conclusion
This meta-analysis confirmed the beneficial effects of prophylactic caffeine in preventing apnea of prematurity and improving clinical outcomes.
Introduction
Apnea of prematurity (AOP) relies on evidence of prolonged apnea lasting 15–20 s or more, or shorter durations if associated with bradycardia or desaturation [Citation1]. AOP becomes more common with decreased birth weight, affecting 85% of neonates born weighing less than 1500 g [Citation2]. For decades, methylxanthine has been utilized to treat apnea of prematurity effectively. Due to its broad therapeutic window and prolonged serum half-life, the preferred methylxanthine for this indication is caffeine [Citation3]. This finding has been validated in several independent experiments, leading to the widespread use of caffeine as the first-line AOP treatment [Citation4].
Even though caffeine has been widely utilized in neonatal practice, there are no recognized and standardized caffeine administration protocols [Citation5]. Concerns have been expressed about potential safety issues and side effects, some of which may be related to the use of caffeine prophylactically. Several studies have found that taking caffeine prophylactically to preterm infants at risk of apnea reduces the time they need for positive pressure ventilation [Citation6]. Caffeine prophylaxis, in addition to its role in reducing apnea of prematurity, has other benefits on infants’ multiple organ systems, including the brain, lungs, and cardiovascular systems, such as lowering the incidence of bronchopulmonary dysplasia and surgical ligation of patent ductus arteriosus [Citation3]. In contrast, studies have shown that taking caffeine prophylactically may increase the risk of overtreatment, including complications like intracranial bleeding [Citation7], plus research has found that it may increase the mortality rate in premature infants during vulnerable periods [Citation8].
Therefore, there is still controversy concerning whether the use of prophylactic caffeine improves clinical outcomes compared to the strict use of caffeine as a therapeutic pharmacological agent. Definitive studies are needed to prove the comparative effects of prophylactic versus therapeutic caffeine. This meta-analysis aims to assess the effects of the prophylactic initiation of caffeine for apnea and related complications in very low birth weight infants.
Materials and methods
Systematic search and strategy
The meta-analysis was carried out according to Meta-analysis (PRISMA) guidelines [Citation9]. All relevant studies from PubMed, Embase, Web of Science, Scopus, EBSCO, CNKI, and Cochrane databases prior to May 20, 2022 were searched in our research. The search strategy addressed two of five elements of the PICOS model (Population, Intervention/Exposure). Using Boolean operators, the search technique merged two areas as MeSH terms, keywords, and text words: (“Caffeine Citrate” OR “Caffeine” OR “Citrates”) AND (“Apnea” OR “Apnoea”) AND (“Preterm Infants” OR “Infant, Premature” OR “Very Low Birth Weight Infants” OR “Infant, Low Birth Weight” OR “Neonatal Prematurity”)
Inclusion and exclusion criteria
Studies included in this meta-analysis must meet the following inclusion criteria:
All infants weighing <1500 g at birth and gestational age <32 weeks; (2) Randomized controlled studies; (3) A group exposed to prophylactic caffeine therapy (0–3 d of life), a control group of infants not receiving caffeine until they develop AOP, and a comparison between the two groups; (4) At least one of the parameters was included in the outcome measures: The primary outcomes were apnea of prematurity (AOP), duration of mechanical ventilator(days) and duration of oxygen therapy(h); Secondary outcomes included: bronchopulmonary dysplasia(BPD), patent ductus arteriosus (PDA), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH) and death before hospital discharge. All diseases diagnosis relied on the recording of patient charts or surgical data.
Any study that met the exclusion criteria was excluded from the meta-analysis:
Case reports, reviews, or animal trials; (2) Studies with unclear or inadequate exposure/outcome definitions; (3) Studies with insufficient information for extraction data.
Data extraction and quality assessment
Two authors independently performed the study selection and data collection process. The specific format is as follows: (1) General characteristics of study: study, year, number of participants, gestation age. (2) Intervention: mode of administration, timing, and dosage. (3) Outcomes. If there are inconsistent units of outcome indicators in the study, they should be converted to uniform units prior to subsequent processing. We assessed the methodological quality of RCTs using the Cochrane Collaboration’s tool [Citation10]. Furthermore, the bias risk assessment chart could be obtained after entering the evaluation data into the RevMan 5.4 software. Disagreement was resolved by discussion with the third author until an agreement was reached.
Data synthesis and analysis
In the present article, analyses were conducted using Stata software version 16.0 (Stata Corp, College Station, TX). The weighted mean difference (WMD) and confidence interval (CI) of 95% were calculated for continuous outcomes, while the pooled odds ratios (ORs) and confidence interval (CI) of 95% were estimated for dichotomous outcomes. We used I2 statistics to examine the heterogeneity of the studies. I2 < 50% is considered an indicator of homogeneity, and the fixed-effect model was used to analyze. I2 ≥ 50% indicates considerable heterogeneity, and the random-effect model was utilized to analyze. Sensitivity analysis was carried out by deleting each study to assess the results’ stability. Funnel plots, Begg’s and Egger’s tests were used to evaluate and test publication bias, respectively. p < .05 indicates statistical significance.
Results
Search results
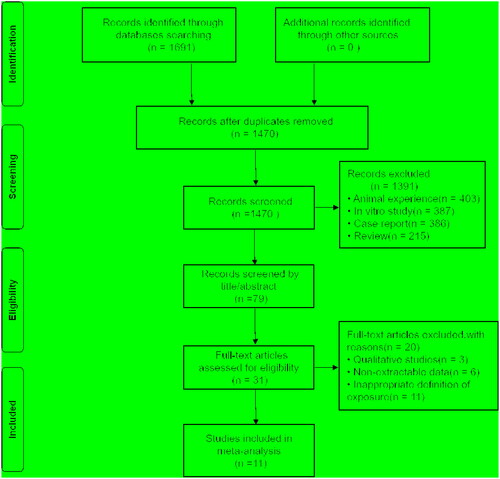
The initial search found 1691 studies, of which 221 duplicate studies were removed. 79 articles remained after analyzing the abstracts and titles of 1470 studies. This meta-analysis includes 11 papers after 68 articles were removed through the full-text review. The search strategy is detailed in Supplementary Table S1. The flowchart of this selection process is shown in , and the PRISMA checklist is presented in the Supplementary Table S2. shows the characteristics of the included research.
Table 1. Characteristics of included studies.
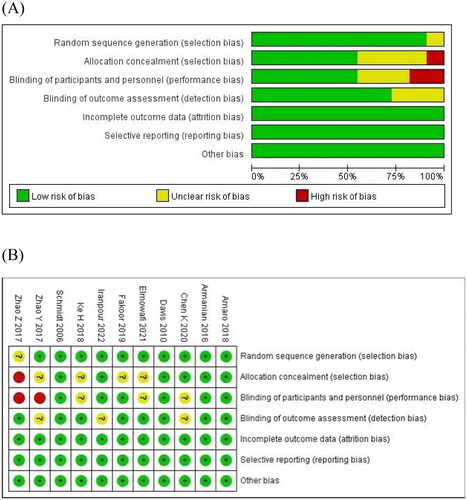
The risk-of-bias assessment is given in . Only four studies showed a low risk of bias [Citation11–14], and seven studies showed an unclear risk of bias [Citation15–21]. Randomization was handled correctly in ten studies [Citation11–20], and allocation concealment was performed adequately in six studies [Citation11–14,Citation17,Citation19]. Information on participants and personnel blinding was provided in six studies [Citation11–14,Citation16,Citation17], and eight studies reported outcome assessment blinding [Citation11–16,Citation18,Citation21]. The cause and number of withdrawals and dropouts were provided for each article.
Meta results
Apnea of prematurity
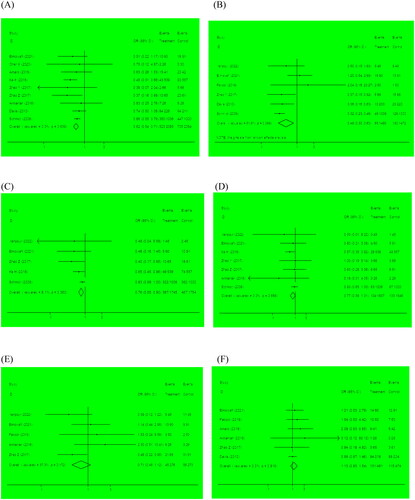
Apnea of prematurity was included as an outcome between the two groups in 5 studies that involved 581 infants. In the meta-analysis, we observed that the prophylactic caffeine group had a lower incidence of apnea than the control group (OR 0.31, 95% CI: 0.19–0.49, p < .001). There was no significant heterogeneity between these studies (I2 = 9.2%, p = .354, ). Therefore, the fixed-effect model was used in the meta-analysis, and sensitivity analysis confirmed that the result was credible.
Figure 3. Forest plots for the primary outcomes. The length of the line segment indicates the 95% confidence interval in each study. The red vertical lines represent the effect size. The black vertical lines represent invalid line. (A) AOP; (B) duration of mechanical ventilation; (C) duration of oxygen therapy.

Duration of mechanical ventilation and oxygen therapy
Five studies reported the duration of mechanical ventilation, and four reported the duration of oxygen therapy. We observed that the duration of mechanical ventilation (OR −0.72, 95% CI: −0.92 to −0.51, p < .001) and oxygen therapy (OR −6.05, 95% CI: −6.56 to −5.54, p < .001) were considerably lower in the prophylactic caffeine group than in the control group. We performed a random-effect model to evaluate the result of mechanical ventilation duration (I2 = 52.2%, p = .079, ), and a fixed-effect model to evaluate the result of oxygen therapy duration(I2 = 23.5%, p = .270, ).
Bronchopulmonary dysplasia
Bronchopulmonary dysplasia was included as an outcome between the two groups in 9 studies that involved 4169 infants. We indicated that the occurrence of BPD was lower in the prophylactic caffeine group than in the control group (OR 0.62, 95% CI: 0.54–0.71, p < .001). There was no significant heterogeneity between these studies (I2 = 0.0%, p = .639, ).
Patent ductus arteriosus
Six studies involving 2962 premature infants included patent ductus arteriosus as an outcome. According to our analysis, premature infants in the prophylactic caffeine group had a decreased chance of developing patent ductus arteriosus (OR 0.49, 95% CI: 0.30–0.80, p = .005). Significant heterogeneity existed among these studies (I2 = 51.6%, p = .066, ), so the random-effect model was adopted.
Retinopathy of prematurity
Retinopathy of prematurity was included as an outcome between the two groups in 5 studies that involved 3499 infants. Compared to the control group, neonates in the prophylactic caffeine group had a lower probability of retinopathy of prematurity (OR 0.76, 95% CI: 0.65–0.90, p = .001). Furthermore, the heterogeneity between studies was low (I2 = 8.1%, p = .360, ).
Necrotizing enterocolitis, intraventricular hemorrhage and death before hospital discharge
We also compared the likelihood of other neonatal complications between the two groups. In terms of necrotizing enterocolitis (OR 0.77, 95% CI: 0.59–1.01, p = .057, ), intraventricular hemorrhage (OR 0.71, 95% CI: 0.45–1.12, p = .145, ) and death before hospital discharge (OR 1.13, 95% CI: 0.83–1.54, p = .44, ), there were no significant statistical differences between the two groups.
Sensitivity analysis and publication bias
The sensitivity analysis indicated that the meta-analysis results were relatively stable (Supplementary Figure S1). We calculated publication bias using tools like funnel plots for all outcomes, and there was no obvious asymmetry (Supplementary Figure S2). Moreover, Begg’s and Egger’s tests revealed no significant publication bias for all outcomes (p > .05).
Discussion
Caffeine is widely used in neonatology and is regarded as the gold standard in preventing and treating apnea of prematurity. The 2019 European consensus guidelines on managing neonatal respiratory distress syndrome emphasized that prophylactic caffeine administration was associated with improving newborn prognosis [Citation22]. However, there were also reviews against the support of the use of prophylactic caffeine for preterm infants at risk of apnea [Citation23]. Therefore, this meta-analysis aims to explore the effect of prophylactic caffeine on AOP and related complications in very low birth weight infants. Compared to the control group, prophylactic caffeine use was found to be substantially linked with a lower incidence of apnea, duration of mechanical ventilation and oxygen therapy, BPD, PDA, and ROP. The incidence of NEC, IVH, and death before hospital discharge was similar in the two groups. In this review, strong evidence from multiple randomized trials supports prophylactic caffeine for the prevention of AOP and related complications.
Firstly, we found that the occurrence of apnea in the prophylactic use of the caffeine group was significantly lower than in the control group, which was consistent with several previous studies [Citation24,Citation25]. The primary effects of caffeine on preventing apnea are stimulating the respiratory center, increasing ventilation per minute, improving carbon dioxide sensitivity, and reducing periodic breathing [Citation26]. It has also been proved to be a neuroprotective anti-inflammatory medication that reduces lung inflammation in premature infants and prevents AOP by activating the pro-inflammatory cascade reaction in newborns [Citation27]. Furthermore, Armanian [Citation12] discovered that caffeine prophylaxis had a greater benefit for more immature newborns with more severe apnea. However, our study only focuses on the impact of very low birth weight infants, and further research is needed to conduct a categorical comparison of newborns of different gestational ages and weights.
Our results indicated that prophylactic initiation of caffeine significantly reduces the duration of mechanical ventilation and oxygen therapy. Such findings were consistent with Park’s previous review [Citation28]. The rationale for prophylactic caffeine administration is to maintain spontaneous respiration by increasing the respiratory drive of newborns [Citation29]. Caffeine may also improve respiratory function by dilating bronchi, improving diaphragmatic contractility, and inducing the transcription of cyclic adenosine surfactant protein B [Citation30]. This may increase the efficiency of noninvasive ventilation while reducing the need for and duration of invasive ventilation. At the same time, it is a manifestation of positive long-term impacts on newborns, since mechanical ventilation and protracted oxygen therapy are both risk factors for bronchopulmonary dysplasia and neurodevelopmental abnormality [Citation31]. Notably, we did not describe the effects of prophylactic caffeine use on the duration of nasal continuous positive airway pressure and CPAP due to the lack of original data, and additional research is required to examine the relationships.
According to this meta-analysis, prophylactic caffeine use was associated with a significant reduction in the incidence of BPD. Our findings were consistent with those of Davis et al. [Citation13], who discovered a 52% reduction in the incidence of BPD in infants with the prophylactic caffeine group, compared with a 23% reduction in infants with the therapeutic caffeine group. Patel et al. [Citation32] also proposed that prophylactic caffeine use could reduce the risk of bronchopulmonary dysplasia, particularly in high-risk preterm infants weighing less than 750 g. It was possible that the number of neutrophils in bronchoalveolar lavage fluid increases fastest shortly after birth and within 4 days after birth [Citation33], while prophylactic caffeine use can reduce the infiltration of neutrophils in lung tissue and decrease the levels of CINC-1, MIP-2, McP-1, TNF-0, and IL-6, thus preventing the development of BPD [Citation34]. Another hypothesis is that caffeine reduces hyper oxygen lung damage by lowering the production of reactive oxygen species [Citation35]. Furthermore, Chen [Citation36] discovered that prophylactic use of high-maintenance doses of caffeine appears to be more effective in promoting lung maturity of preterm infants. However, our study lacked relevant raw data and was not discussed, and more research is needed to assess the safety of caffeine at different maintenance doses.
We have shown that prophylactic use of caffeine significantly reduced the incidence of PDA compared to the control group. Caffeine’s effect on PDA may benefit from its ability to stabilize hemodynamic changes in infants, such as improving cardiac output and blood pressure [Citation37]. Another speculation is that the diuretic effect and prostaglandin antagonistic properties of caffeine may promote ductus arteriosus closure and decrease PDA intervention rates [Citation38]. Although it has been observed in several trials [Citation29], the mechanism of the decline of PDA incidence remains difficult to explain. Additional research on the prophylactic mechanism of caffeine on PDA is required. Furthermore, our study showed that prophylactic use of caffeine was beneficial in reducing the incidence of ROP. Pharmacologic agents with anti-VGEF properties are commonly used for the prevention and treatment of ROP [Citation39]. Caffeine has been shown in preliminary animal studies to prevent ROP by upregulating the sonic hedgehog signaling pathway through vascular endothelial growth factor and insulin-like growth factor, thereby protecting retinal development and angiogenesis [Citation40]. A previous systematic review [Citation8] also found that infants given prophylactic caffeine had a significantly lower risk of PDA and ROP.
Compared with the control group, caffeine prophylaxis was not associated with differences in the incidence of NEC, IVH, or death before hospital discharge. The conclusions of our systematic review were in accordance with previous studies on the same topic [Citation41,Citation42]. NEC is an injury to the intestinal mucosa caused by blood flow redistribution under hypoxia stress. While prophylactic use of caffeine can reduce hypoxia stress in newborns, it can also increase gastric juice secretion, leading to increased intestinal reflux and reduced peristalsis [Citation43]. And caffeine not only has a direct neuroprotective effect on the brain, but also has side effects [Citation44]. Therefore, the benefits and drawbacks for premature infants may thus be balanced. Nevertheless, Kua [Citation8] found that prophylactic use of caffeine increased the mortality rate of infants, contrary to our findings. It was possible that the level of newborn care varies across countries. Another possible explanation is the existence of survival bias, which occurs when the overall survival rate of very low birth weight infants is low. It emphasizes that a more rigorous research design is needed to explore the effects of prophylactic caffeine before strong conclusions can be made.
Limitations
It is worth noting that our meta-analysis has some limitations. Firstly, our study lacked original data on long-term clinical outcomes such as infant respiratory tract morbidity in the early post-natal period, and post-discharge oxygen therapy needs, all of which should be further studied in properly designed RCTs. Furthermore, the standard and dosage scheme of prophylactic caffeine use in our included studies are not completely consistent, which may affect data interpretation.
Conclusion
According to our findings, prophylactic use of caffeine is beneficial compared with caffeine therapy in reducing the incidence of AOP, duration of the mechanical ventilator and oxygen therapy, BPD, PDA, and ROP. Notably, prophylactic caffeine use does not increase the risk of NEC, IVH, and death before hospital discharge. It is believed that our findings will assist clinicians in continuously optimizing their current practice. Furthermore, future studies are needed to investigate the optimal caffeine prophylaxis dosing and plan for long-term follow-up neonatal outcomes.
Supplemental Material
Download Zip (177.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Erickson G, Dobson NR, Hunt CE. Immature control of breathing and apnea of prematurity: the known and unknown. J Perinatol. 2021;41(9):2111–2123.
- Montenegro BL, Amberson M, Veit L, et al. Economics of home monitoring for apnea in late preterm infants. Respir Care. 2017;62(1):42–48.
- Schoen K, Yu T, Stockmann C, et al. Use of methylxanthine therapies for the treatment and prevention of apnea of prematurity. Paediatr Drugs. 2014;16(2):169–177.
- Alhersh E, Abushanab D, Al-Shaibi S, et al. Caffeine for the treatment of apnea in the neonatal intensive care unit: a systematic overview of meta-analyses. Paediatr Drugs. 2020;22(4):399–408.
- Pergolizzi J, Kraus A, Magnusson P, et al. Treating apnea of prematurity. Cureus. 2022;14(1):e21783.
- Henderson-Smart DJ, De Paoli AG. Prophylactic methylxanthine for prevention of apnoea in preterm infants. Cochrane Database Syst Rev. 2010;2010(12):Cd000432.
- Nylander Vujovic S, Nava C, Johansson M, et al. Confounding biases in studies on early-versus late-caffeine in preterm infants: a systematic review. Pediatr Res. 2020;88(3):357–364.
- Kua KP, Lee SW. Systematic review and meta-analysis of clinical outcomes of early caffeine therapy in preterm neonates. Br J Clin Pharmacol. 2017;83(1):180–191.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Amaro CM, Bello JA, Jain D, et al. Early caffeine and weaning from mechanical ventilation in preterm infants: a randomized, placebo-controlled trial. J Pediatr. 2018;196:52–57.
- Armanian AM, Iranpour R, Faghihian E, et al. Caffeine administration to prevent apnea in very premature infants. Pediatr Neonatol. 2016;57(5):408–412.
- Davis PG, Schmidt B, Roberts RS, et al. Caffeine for apnea of prematurity trial: benefits may vary in subgroups. J Pediatr. 2010;156(3):382–387.
- Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–2121.
- Elmowafi M, Mohsen N, Nour I, et al. Prophylactic versus therapeutic caffeine for apnea of prematurity: a randomized controlled trial. J Matern Fetal Neonatal Med. 2022;35(25):6053–6061.
- Fakoor Z, Makooie AA, Joudi Z, et al. The effect of venous caffeine on the prevention of apnea of prematurity in the very preterm infants in the neonatal intensive care unit of Shahid Motahhari Hospital, Urmia, during a year. J Adv Pharm Technol Res. 2019;10(1):16–19.
- Iranpour R, Armanian AM, Miladi N, et al. Effect of prophylactic caffeine on noninvasive respiratory support in preterm neonates weighing 1250–2000 g: a randomized controlled trial. Arch Iran Med. 2022;25(2):98–104.
- Kobaly K, Mandel SJ. Hyperthyroidism and pregnancy. Endocrinol Metab Clin North Am. 2019;48(3):533–545.
- Kou C, Han D, Li Z, et al. Influence of prevention of caffeine citrate on cytokine profile and bronchopulmonary dysplasia in preterm infants with apnea. Minerva Pediatr. 2020;72(2):95–100.
- Zhao Y, Zhao J. PV1: gatekeeper of endothelial permeability. Am J Respir Cell Mol Biol. 2020;63(4):413–414.
- Zulqarnain A, Hussain M, Suleri KM, et al. Comparison of caffeine versus theophylline for apnea of prematurity. Pak J Med Sci. 2019;35(1):113–116.
- Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome – 2019 update. Neonatology. 2019;115(4):432–450.
- Levitt GA, Mushin A, Bellman S, et al. Outcome of preterm infants who suffered neonatal apnoeic attacks. Early Hum Dev. 1988;16(2–3):235–243.
- Dobson NR, Patel RM, Smith PB, et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr. 2014;164(5):992–998.e993.
- Lodha A, Seshia M, McMillan DD, et al. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 2015;169(1):33–38.
- Eichenwald EC, Watterberg KL, Aucott S, et al. Apnea of prematurity. Pediatrics. 2016;137(1):e20153757.
- Kilicdag H, Daglioglu YK, Erdogan S, et al. Effects of caffeine on neuronal apoptosis in neonatal hypoxic-ischemic brain injury. J Matern Fetal Neonatal Med. 2014;27(14):1470–1475.
- Park HW, Lim G, Chung SH, et al. Early caffeine use in very low birth weight infants and neonatal outcomes: a systematic review and meta-analysis. J Korean Med Sci. 2015;30(12):1828–1835.
- Borszewska-Kornacka MK, Hożejowski R, Rutkowska M, et al. Shifting the boundaries for early caffeine initiation in neonatal practice: results of a prospective, multicenter study on very preterm infants with respiratory distress syndrome. PLoS One. 2017;12(12):e0189152.
- Abu-Shaweesh JM, Martin RJ. Caffeine use in the neonatal intensive care unit. Semin Fetal Neonatal Med. 2017;22(5):342–347.
- Geetha O, Rajadurai VS, Anand AJ, et al. New BPD-prevalence and risk factors for bronchopulmonary dysplasia/mortality in extremely low gestational age infants ≤28 weeks. J Perinatol. 2021;41(8):1943–1950.
- Patel RM, Leong T, Carlton DP, et al. Early caffeine therapy and clinical outcomes in extremely preterm infants. J Perinatol. 2013;33(2):134–140.
- Ogden BE, Murphy SA, Saunders GC, et al. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis. 1984;130(5):817–821.
- Weichelt U, Cay R, Schmitz T, et al. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur Respir J. 2013;41(4):966–973.
- Tiwari KK, Chu C, Couroucli X, et al. Differential concentration-specific effects of caffeine on cell viability, oxidative stress, and cell cycle in pulmonary oxygen toxicity in vitro. Biochem Biophys Res Commun. 2014;450(4):1345–1350.
- Chen J, Jin L, Chen X. Efficacy and safety of different maintenance doses of caffeine citrate for treatment of apnea in premature infants: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:9061234.
- Soloveychik V, Bin-Nun A, Ionchev A, et al. Acute hemodynamic effects of caffeine administration in premature infants. J Perinatol. 2009;29(3):205–208.
- Manku MS, Horrobin DF. Chloroquine, quinine, procaine, quinidine, tricyclic antidepressants, and methylxanthines as prostaglandin agonists and antagonists. Lancet. 1976;2(7995):1115–1117.
- Bhatt-Mehta V, Schumacher RE. The effect of ibuprofen and caffeine prophylaxis on retinopathy of prematurity. JAAPOS. 2021;25(5):272.e271–272.e273.
- Aranda JV, Beharry K, Valencia GB, et al. Caffeine impact on neonatal morbidities. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):20–23.
- Park HW, Lim G, Chung SH, et al. Early caffeine use in very low birth weight infants and neonatal outcomes: a systematic review and meta-analysis. J Korean Med Sci. 2015;30(12):1828–1835.
- Schmidt B, Roberts RS, Davis P, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–1902.
- Gounaris A, Kokori P, Varchalama L, et al. Theophylline and gastric emptying in very low birthweight neonates: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):F297–299.
- Atik A, Harding R, De Matteo R, et al. Caffeine for apnea of prematurity: effects on the developing brain. Neurotoxicology. 2017;58:94–102.