Abstract
Background
Therapeutic regimens for the treatment of toxoplasmosis are not standardized. Treatment strategy mainly at the end of the second and the beginning of the third trimester, especially in cases of negative prenatal diagnosis, is the least uniform. In some situations, the choice of treatment may be ambiguous, and adverse drug reactions of the therapy should be taken into consideration.
Methods
Adverse drug reactions of anti-toxoplasma therapy with spiramycin (n = 77) versus pyrimethamine/sulfadiazine (n = 35) were compared in 112 pregnant women.
Results
Up to 36.6% of women reported adverse reactions to the treatment overall (n = 41). Out of those 38.9% (n = 30) were treated with spiramycin and 31.4% (n = 11) with pyrimethamine/sulfadiazine. Toxic allergic reactions were the only indication for discontinuation of treatment in 8.9% of patients (n = 10), where 9.1% (n = 7) were reported in spiramycin and 8.6% (n = 3) in pyrimethamine/sulfadiazine cohort. Neurotoxic complications (acral paraesthesia) were significantly more frequent during the therapy with spiramycine in 19.5% (n = 15) compared to no cases in pyrimethamine/sulfadiazine group (p = .003). Other adverse drug reactions, such as gastrointestinal discomfort, nephrotoxicity, vaginal discomfort were reported, but the differences between the cohorts were not significant.
Conclusions
The superiority of one of the therapeutic regimens was not statistically demonstrated, since the differences in overall toxicity or incidence of toxic allergic reactions between the cohorts were not confirmed (p = .53 and p = 1.00, respectively). However, although the isolated neurotoxicity of spiramycin was the only significant adverse reaction demonstrated in this study, pyrimethamine/sulfadiazine therapy should be preferred, because it is known to be more effective and with limited adverse reactions.
Introduction
The obligate intracellular protozoan Toxoplasma gondii is the causative agent of toxoplasmosis. Immunocompromised patients, persons on immunosuppressive therapy, pregnant women, newborns, and patients with toxoplasma chorioretinitis are at risk of severe toxoplasmosis. In these patients, prompt diagnosis and timely initiation of proper treatment are very important [Citation1–3].
In the Czech Republic (CR), the seroprevalence levels in men and women are 26.3% and 34.1%, respectively. The prevalence in pregnant women is reported to range from 20.1% to 44.8%, where 4% represent primoinfections in pregnancy. T. gondii is a geopolitical parasite. The seroprevalence varies according to geographical location (increasing toward equatorial countries), environmental conditions, social practices, and animal contact conditions [Citation4].
Therapy in pregnant women is indicated on the basis of serological positivity, morphological pathology (fetus sonography), or direct detection of the parasite (PCR). In the Czech Republic, this therapy is guided by the national recommended guidelines for the diagnosis and treatment of toxoplasmosis. In the prenatal period up to weeks 15–18 of gestation, prophylactic therapy with oral spiramycin at a dose of 3 MIU every 8 h is indicated. After weeks 16–18 of gestation, if infection of the fetus is suspected (according to the results of amniocentesis, ultrasonography, and serology), this treatment is changed to a dual combination therapy of pyrimethamine (oral dose of 50–100 mg/d) and sulfadiazine (oral dose of 1 g every 8 h) with folinic acid (oral dose of 15 mg/d). If infection of the fetus is confirmed, this therapy is maintained until delivery. If infection of the fetus is not confirmed, spiramycin is used until the end of pregnancy. If seroconversion has occurred only in the late second or in the third trimester, dual-combination therapy is indicated immediately [Citation3]. Treatment protocols are not standardized worldwide and there is considerable variation in practice in treatment regimens not only within continents but also within the individual national centers. Spiramycin is indicated in the early stages of pregnancy until amniocentesis with T. gondii DNA testing is performed, and when this diagnostic result is positive, treatment is changed to administration of pyrimethamine/sulfadiazin. A positive correlative pathological finding on sonography of the fetus is of course an integral part of the diagnosis. Some centers indicate amniocentesis from as early as the completed 14 weeks of gestation, others from as late as 18 weeks. In these cases, an interval of several weeks arises, with some pregnant women being treated with spiramycin and others with pyrimethamine/sulfadiazine. When a prenatal diagnosis is negative, spiramycin is usually indicated, but in some centers, dual-combination therapy is given despite this negative diagnosis, especially when serology is unfavorable. The situation when acute seropositivity is detected at the end of the second trimester and in the third trimester is again handled differently. Some centers administer spiramycin pending amniotic fluid testing, while others immediately initiate the dual-combination therapy and do not wait for the amniotic fluid PCR result. Subsequent treatment strategy again varies after a subsequent negative prenatal diagnosis. Sometimes the double combination is retained, while elsewhere spiramycin is given [Citation4–8].
Spiramycin is a macrolidic antibiotic with relatively low antiprotozoal efficacy. The indication is prophylactic, in pregnancies at risk of congenital toxoplasmosis, especially in cases of primoinfection in the first 16-18 weeks of pregnancy. Transplacental passage is limited and, therefore, it does not treat possible infection of the fetus. It is concentrated at several times higher levels in the placenta than in the serum. It resolves present placentitis and probably reduces the transfer of tachyzoites across the placenta to the fetus. Among the adverse reactions described are GIT disorders (nausea, vomiting, or diarrhea), acral paresthesias, and allergy. It can be used at any stage of pregnancy. It does not affect the results of any amniocentesis performed later.
Pyrimethamine is an antimalarial and antiprotozoal drug. It passes through the placenta and is indicated in proven or highly probable infection of the fetus, and in the treatment of congenital toxoplasmosis in newborns, ocular forms, and immunocompromised patients. It is used in combination with sulfadiazine and folinic acid. It is relatively contraindicated until week 16–18 of gestation because of possible teratogenicity. However, it is given earlier in some European centers, around week 14–16. Among adverse reactions, myelotoxicity is the main concern, while hepatotoxicity and allergic reactions have also been described. Treatment with pyrimethamine should be supplemented by the administration of folinic acid to inhibit the blockage of dihydrofolate reductase to prevent the development of megaloblastic anemia.
Sulfadiazine is an antibacterial chemotherapy drug, which passes through the placenta. The indication is similar to that of pyrimethamine with which it is used in combination with folinic acid. It is relatively contraindicated in the first trimester of pregnancy and in the last 4-6 weeks before delivery. Adverse reactions include myelosuppression, allergy, photosensitivity, gastrointestinal intolerance, and acute glomerulonephritis with crystalluria.
During the period of administration of the pyrimethamine/sulfadiazin therapy, periodic monitoring of certain laboratory values is necessary, including blood count, liver function tests and creatinine levels. This therapy is contra-indicated in persons with G6PD deficiency.
Thus, there is a lack of consensus worldwide on the approach to therapy of toxoplasmosis in pregnant women. Therapy with the combination of pyrimethamine/sulfadiazin is highly effective with clear benefits for the patient. However, in many centers there is a hesitation to administer this therapy, especially in situations, where infection of the fetus is not clear, spiramycin is used instead fearing potential adverse effects. This study aims to contribute to fill this gap and supply data regarding the potential side effect of these two types of treatment in clinical settings. Thus, the goal of the study was to compare the adverse reactions of treatment with spiramycin versus the combination pyrimethamine/sulfadiazine in pregnant women including a specification of their most common adverse reactions. Based on the results, the aim was to recommend the preferred treatment regimen in situations where the choice of treatment is ambiguous, does not conform to clear standards, or is managed differently by different centers.
Materials and methods
Adverse reactions to anti-toxoplasma therapy in 112 pregnant women were evaluated retrospectively (for 15 years from 2006 to 2021). The inclusion criteria were the administration of therapy with either spiramycin or the dual combination of pyrimethamine/sulfadiazine for positivity of at least one type of the acute antibodies to toxoplasmosis (IgM, IgA, and/or IgE). IgG avidity values were not considered due to the known limits of this test.
The duration of treatment was at least three weeks. The women did not suffer from any chronic disease, were not taking any other concomitant treatment, and had no history of allergy that might influence the assessment of adverse reactions to the treatment administered. Pregnant women who were taking pharmacy-distributed probiotics, other antibiotics or chemotherapeutics, or magistral preparations during the treatment period were excluded from the study population. All women in the cohorts had regular eating habits.
In addition, women who had other complications related to pregnancy or even outside of pregnancy were excluded. Clinical and laboratory parameters (blood count and differential leukocyte count, glycemia, electrolytes, total bilirubin, ALT, AST, ALP, GMT, urea, creatinine, and urinary sediment) were monitored. Captured adverse reactions to the therapy were evaluated and compared. The women were included in one of two cohorts according to the type of therapy. The first group received spiramycin monotherapy, and the second a double combination of pyrimethamine/sulfadiazine (with the addition of folinic acid).
Both cohorts were patients receiving standard outpatient care for toxoplasmosis in pregnancy. Spiramycin monotherapy was indicated as a reassurance therapy until 15 weeks of gestation, or potentially during other periods of gestation if fetal infection was not suspected. Dual combination therapy was indicated in case of unfavorable serological results and their dynamic, non-physiological ultrasonographic findings of the fetus, or a positive PCR result of Toxoplasma gondii from blood or amniotic fluid. The women consented orally to the proposed therapy. The numbers of women in the two cohorts were limited by the total number of patients treated during the indicated time period of the study
Statistical methods
Continuous variables were presented as medians (range). Categorical variables were compared using Fisher’s exact test and were presented as numbers (%). Differences were considered statistically significant at p < 0.05. All p-values were obtained using two-tailed tests. All statistical analyses were performed using GraphPad Prism for Mac OS (version 9.3.1., GraphPad Software Inc., San Diego, CA, USA).
Results
A total of 112 pregnant women were included in the study in the following two cohorts: patients receiving prophylactic spiramycin therapy (n = 77) and those receiving pyrimethamine/sulfadiazine (n = 35). In the group of pregnant women on spiramycin therapy (n = 77), neurological complications were the most common, with a total of 15 pregnant women (19.5%) reporting paraesthesia of the tongue and/or lips and/or acral parts of the fingers (). These complaints occurred most frequently at week 3 of spiramycin use (median 3, range 2–10). Cutaneous toxic allergic reactions – toxic allergic exanthema – were the second most frequent adverse reaction to the therapy, always requiring discontinuation of the administered therapy. They appeared in a total of 7 pregnant women (9.1%). Complications occurred most frequently at week 2 of therapy (median 2, range 1–6). Gastrointestinal disorders were experienced by 6 clients (7.8%), most commonly in week 4 of the treatment (median 4, range 1–6). In one case, vaginal dysmicrobia occurred (1.3%). Laboratory deviation from the norm was noted in only one case (1.3%), at week 5 of medication, with an increase in liver aminotransferases up to 1.5 times the norm. In no case was there a simultaneous occurrence of multiple complaints or simultaneous laboratory pathology. However, a total of 30 pregnant women (38.9%) showed adverse reactions during spiramycin therapy.
Figure 1. Complications of toxoplasmosis treatment in pregnant women. Frequency of the individual complications is listed as number of patients followed by percentage of total in A) pregnant women treated by spiramycin (n = 77) and B) pregnant women treated by pyrimethamine/sulfadiazine (n = 35).
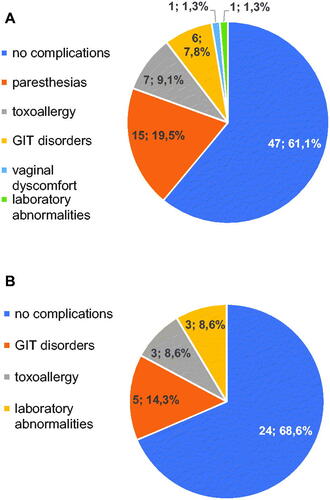
In the group treated with combination therapy of pyrimethamine/sulfadiazine (n = 35), no neurological complications were noted; GIT disorders (nausea, intestinal discomfort) were present in 5 cases (14.3%), most often at week 4 of treatment (median 4, range 2-8), and did not lead to discontinuation of treatment ().
Cutaneous toxic allergic reactions occurred in 3 cases (8.6%), most commonly at week 2 of treatment (median 2, range 1-2), and all cases required modification of therapy. Laboratory deviations from the norm were also recorded in 3 cases (8.6%), most frequently at week 4 with a median of 5 (range 4–6). This was always a limit-exceeding elevation of creatinine up to 1.4 times the norm; no other pathological laboratory values were detected. Adverse reactions to the treatment in both groups were statistically compared using Fisher’s exact test ().
Table 1. Complications of anti-toxoplasma therapy of pregnant women.
Overall, there was no statistically significant difference in adverse reactions between the groups (p = .53). When individual parameters were evaluated, neurological complications (paraesthesia, p = .003) appeared to be the only statistically significant difference to the detriment of spiramycin. The other individual parameters were statistically insignificant. Thus, the superiority/inferiority of any of the treatment regimens in terms of the significance of adverse reactions to treatment was not clearly demonstrated.
Discussion
Any interventional anti-infective therapy is burdened with the risk of adverse reactions. One must always weigh the necessity for and effectiveness of the intervention, and the cost-benefit to the patient. Administration of treatment to pregnant women in whom there is the possibility of T. gondii infection is clearly indicated. However, when administering anti-toxoplasma therapy, the possibility of adverse reactions to the therapy should always be considered. In therapy with spiramycin, which is considered an antibiotic with a good safety profile, up to 38.9% (n = 30) of adverse reactions were observed in the cohort. The fact is that most of them were not serious and did not require discontinuation of the drug (paraesthesia 19.5%, minor GIT disorders 7.8%, vaginal discomfort 1.3%). The only case of elevation (non-significant) of hepatic aminotransferases may not be causally related to the use of spiramycin. Toxic allergic skin reactions (n = 7; 9.1%) were not serious and none was of the severe toxic epidermolysis type. However, they necessitated discontinuation of the drug. In summary, therapy with this antibiotic can be assessed as relatively safe and cannot be refused. Dual combination therapy (pyrimethamine/sulfadiazine) is considered the gold standard treatment for toxoplasmosis. There was a total of 35 patients treated by this combination. Toxic allergic skin reactions (n = 3; 8.6%) were again the only indication for discontinuation of this therapy. This complication is frequently reported in the literature with a wide variation in frequency, and also with a wide variation in severity. In our cohort, it tended to reach a lower percentage and the symptoms were not severe. Other adverse reactions of treatment (GIT disorders and laboratory abnormalities) were not severe enough to require discontinuation. Three women (8.6%) experienced a slight elevation of creatinine during therapy. These cases are described in the context of sulfadiazine therapy concomitant with the finding of crystalluria and with the corresponding sonographic finding of renal parenchymal echo signals, such findings being subsequently corrected with adequate hydration and urine alkalinization. However, crystalluria was not detected in any of our patients. Therefore, the effect of pyrimethamine for which renal elimination was demonstrated may be considered. Frequent monitoring of these laboratory values and emphasis on good preventive hydration is an important part of treatment. Urine alkalinization was not applied; the values improved with increased hydration, and creatinine was normalized with discontinuation of treatment. No hematological abnormalities were noted. We attribute this to the good management of folinic acid administration and its good protective function in terms of the described hematotoxicity of pyrimethamine [Citation8–14]. Statistical processing using Fisher’s exact test did not demonstrate the superiority or inferiority of any of the above treatment regimens in terms of adverse reactions. Spiramycin was shown in isolated cases to be inferior in neurological complications (p = .003). On the basis of these results, it is advisable to prefer the combination therapy of pyrimethamine/sulfadiazin, as its inferiority in terms of adverse reactions has not been statistically demonstrated. Moreover, it is known for its more reliable therapeutic efficacy [Citation9–17].
The meta-analysis of observational studies, that looked at the effectiveness of therapy of different therapeutic regimens [Citation18], has uniformly demonstrated the benefits of treatment with a reduction in the risk of mother-to-fetus transmission. Spiramycin should be administered as soon as possible. This treatment should be changed to a combination of pyrimethamine with sulfadiazin when the fetal infection is suspected or proven These conclusions are in complete agreement with the results of our study, which also support treatment with spiramycin in the initial phase of pregnancy. Another study TOXOGEST [Citation19] reported a higher efficacy of dual combination therapy at the expense of their more frequent adverse effects. Furthermore, this study also discusses the benefits of starting dual combination therapy as early as 14 weeks of gestation in indicated cases. This approach is supported by our results, where in our, admittedly smaller, cohort of women treated with pyrimethamine with sulfadiazine no serious adverse effects of this treatment were noted. Serological testing is an essential part of the care of pregnant women, which is unfortunately not implemented worldwide. The initial therapy with spiramycin also has economic advantages, especially in countries with low health care subsidies. The cost of spiramycin therapy is also lower due to the fact, that it does not require additional laboratory follow-up contrary to the double therapy. The authors are aware of certain limitations of this study caused by a lower number of patients in the individual cohorts. This has been caused by rigorous exclusion criteria, which led to an exclusion of a number of potential subjects. However, the authors believe that the results provide a valuable contribution to the field of therapeutical approaches to toxoplasmosis in pregnant women, which is supported by statistically significant data.
Conclusions
Therapy of toxoplasmosis in pregnant women is essential to achieve a good therapeutic outcome. The treatment is highly effective but requires long-term administration of drugs with anti-protozoal effects and has relatively numerous adverse reactions. Nevertheless, this therapy is quite safe provided there is good clinical and laboratory monitoring.
In controversial cases where individual centers are deciding on the suitability of either therapeutic regimen, consideration should be given to the preference of the combination therapy in indicated cases, due to its known superior therapeutic efficacy and the statistically unproven (or questionable) inferiority of adverse effects.
Acknowledgments
The authors are grateful to Ian McColl M.D., Ph.D. for assistance with the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Data availability statement
Data are available on request due to ethical restrictions.
Additional information
Funding
References
- Remington JS, McLeod R, Wilson CB, et al. Toxoplasmosis. In: Remington JS, editor. Infectious diseases of the fetus and newborn infant. 7th ed. Philadelphia: Elsevier Saunders; 2011. p. 918–1041.
- Montoya JG, Boothryord JC, Kovacs JA, Toxoplasma gondii. In: Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 9th ed. Philadelphia: Elsevier Saunders; 2019. p. 3355–3387.
- Petersen E, Pollak A, Reiter-Owona I. Recent trends in research on congenital toxoplasmosis. Int J Parasitol. 2001; 31(2):115–144.
- Geleneky M, Prasil P, Kodym P. [Guidelines for the diagnosis and therapy of toxoplasmosis]: infekce.cz; 2017. [cited 2022 October 21]. Available from: https://www.infekce.cz/DoporToxo17.htm.
- Dunay IR, Gajurel K, Dhakal R, et al. Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev. 2018;31(4):e00057–17.
- Mandelbrot L, Kieffer F, Wallon M[, et al. Toxoplasmosis in pregnancy: practical management. Gynecol Obstet Fertil Senol. 2021;49(10):782–791.
- Peyron F, L’ollivier C, Mandelbrot L, et al. Maternal and congenital toxoplasmosis: diagnosis and treatment recommendations of a french multidisciplinary working group. Pathogens. 2019;8(1):24.
- Peyron F, Mc Leod R, Ajzenberg D, et al. Congenital toxoplasmosis in France and the United States: one parasite, two diverging approaches. PLOS Negl Trop Dis. 2017;11(2):e0005222.
- Borkowski PK, Brydak-Godowska J, Basiak W, et al. Adverse reactions in Antifolate-Treated toxoplasmic retinochoroiditis. Adv Exp Med Biol. 2018;1108:37–48.
- Caumes E, Bocquet H, Guermonprez G, et al. Adverse cutaneous reactions to pyrimethamine/sulfadiazine and pyrimethamine/clindamycin in patients with AIDS and toxoplasmic encephalitis. Clin Infect Dis. 1995;21(3):656–658.
- Dannemann B, Mccuthan JA, Israelsi D, et al. Treatment of toxoplasmic encephalitis in patients with AIODS- A randomised trial comparing pyrimethamine plus clindamicin to pyrimethamine plus sulfadiazine. Ann Intern Med. 1992;116(1):33–43.
- de la Prada Alvarez FJ, Prados Gallardo AM, Tugores Vazquez A, et al. Acute renal failure due to sulfadiazine crystalluria. An Med Interna. 2007; 24(5):235–238.
- de Sequera P, Albalate M, Hernandez J, et al. Acute renal failure due to sulphadiazine crystalluria in AIDS patients. Postgrad Med J. 1996;72(851):557–558.
- Crespo M, Quereda C, Pascual J, et al. Patterns of sulfadiazine acute nephrotoxicity. Clin Nephrol. 2000;54(1):68–72.
- Midskov C. Rapid gas chromatographic determination of pyrimethamine in human plasma and urine. J Chromatogr. 1984;306:388–393.
- Mouankie JB, Senczuk W, Florek E. Urinary elimination kinetics of pyrimethamine. Eur J Drug Metab Pharmacokinet. 2009; 34(3–4):169–172.
- Ozturk R, Engin A, Ozaras R, et al. Hyperpigmentation due to pyrimethamine use. J Dermatol. 2002;29(7):443–445.
- Montoya JG, Laessig K, Fazeli MS, et al. A fresh look at the role of spiramycin in preventing a neglecterd disease: meta-analyses of observational studies. EJMR. 2021;26:143.
- Mandelbrot L, Kieffer F, Sitta R, et al. Prenatal therapy with pyrimethamine + sulfadiazine vs spiramycin to reduce placental transmission of toxoplasmosis: a multicenter, randomized trial. Am J Obstet Gynecol. 2018;219(4):386.

