Abstract
Background/aim of the study
L-Arginine (L-Arg)/Nitric Oxide (NO) system is involved in the pathophysiology of relevant Obstetric conditions. This review aims at summarizing the effects of L-Arg supplementation in pregnancy looking at safety and efficacy.
Methods
We conducted a systematic review of the literature utilizing PubMed for studies published from inception to September 2022. The search included human and animal studies where L-Arg was supplemented pre-conceptionally or during pregnancy, by either oral or intravenous route. The main perinatal outcomes were focused.
Results
Among 1028 publications, 51 studies were eligible for inclusion, 25 were performed in women, and the remnant in animals. Compared to controls/placebo, the supplementation with L-Arg reduced the development of pre-eclampsia (four studies), decreased blood pressure, and reduced the need for antihypertensive drugs in women with Hypertensive Disorders of Pregnancy (HDP, eight studies). In women carrying growth retarded fetuses, L-Arg improved fetoplacental circulation, birth weight and neonatal outcomes (five studies), while in the case of threatened preterm birth, L-Arg reduced uterine contractions (two studies). In several animal species, L-Arg supplementation in pregnancy improved reproductive performance by increasing the litter number and size. Moreover, in pre-eclamptic and metabolic syndrome experimental models, maternal hypertension and fetal growth were improved.
Conclusion
L-Arg displays biological activities in pregnancies complicated by HDP and growth restriction, both in women and animal models. L-Arg administration is safe and could be a candidate as an intervention beneficial to maternal and fetal outcomes, at least in moderate clinical disorders.
Introduction
Hypertensive disorders of pregnancy (HDP) remain one of the major causes of pregnancy-related maternal and fetal morbidity and mortality, worldwide. Affected women are also at increased risk for cardiovascular disease later in life, independently from traditional cardiovascular risks [Citation1]. Pre-eclampsia (PE) is the most severe HDP and represents one of the main causes of maternal death [Citation2]. Being the most important reason for iatrogenic prematurity, PE is a major contributor to perinatal mortality and fetal growth restriction [Citation3].
Placental insufficiency (or uteroplacental vascular insufficiency) is another important issue in pregnancy that compromises fetal growth and increases the risks of low birth weight, IUGR, pre-term birth, and stillbirth [Citation4–7].
A recent systematic review and meta-analysis by Goto [Citation8], conducted on human studies, confirmed a pathophysiological role of L-Arg in placental function and vascular compliance, on which pregnancy outcomes may be dependent [Citation5] and reported the favorable effects of prenatal oral l-arginine on birth outcomes.
L-Arg has the potential to improve birth outcomes in pre- and peri-conceptional strategies, being also beneficial for pregnant women, their families, health professionals and policy makers. However, despite arginine being a topic studied by several researchers, there are still many unexplored areas in which it may play a role, such as the metabolic profile during pregnancy.
This systematic review aims to summarize the results of main human and animal studies supplementing L-Arginine either orally or intravenously during pregnancy in terms of pregnancy and perinatal outcomes, to finally underline the future research prospective of this semi-essential amino acid.
Materials and methods
Literature search and data extraction
This systematic review followed the PRISMA guidelines [Citation9].
A comprehensive literature search was conducted in PubMed (www.ncbi.nlm.nih.gov) for studies published from inception to September 2022, using as keywords: L-Arginine OR Arginine OR Nitric Oxide donor OR Arginine supplementation AND preeclampsia OR high-risk pregnancy OR fetal growth restriction OR hypertension OR hypertension in pregnancy OR perinatal outcomes). In addition to database searches, we performed a full-text review of studies included in meta-analyses investigating the effects of L-Arg supplementation in high-risk pregnancies on perinatal outcomes, selecting 4 more trials from two metanalysis [Citation8,Citation10].
This systematic review included both human and animal studies. We restricted our search to studies published in English. Review articles were excluded after a search of the reference lists.
Study selection
After the primary records were retrieved from PubMed, duplicates were removed. The remaining records’ titles and abstracts were screened, and irrelevant studies were excluded. Full texts of studies deemed relevant were obtained and reviewed in detail for eligibility according to the inclusion criteria. Reviews, Letters to the Editor, meeting précis and other articles reporting studies that did not provide the primary data were excluded.
Publications were included in the final analysis if they met the following inclusion criteria: studies evaluating supplementation of L-Arg during pregnancy or it the pre-gestational and postnatal period. Studies with unclear treatment details or with a combination of several amino acids/supplements were excluded. At least one of the following outcomes was used as a primary or secondary endpoint: maternal hypertension, pre-eclampsia, fetal growth restriction/retardation, feto/placental hemodynamic, birthweight, perinatal outcomes. In case of human treatment, only randomized controlled trials and prospective or retrospective cohort studies were considered.
Studies were excluded according to the following criteria: narrative reviews, systematic reviews and meta-analyses, studies evaluating pathophysiology rather than clinical outcomes, studies published in other languages than English.
Analysis
The outcomes evaluated included maternal hypertensive disorders, pre-eclampsia, fetal growth restriction/retardation, and perinatal outcomes. We defined maternal hypertensive disorders as blood pressure values above 140/85 mmHg, pre-eclampsia as elevated blood pressure associated with proteinuria and/or the presence of kidney or liver function alterations, neurological signs, hemolysis or thrombocytopenia and/or fetal underdevelopment [Citation11].
Fetal growth restriction as an estimated fetal weight <10th percentile [Citation12], while perinatal outcomes included birthweight, Apgar score, delivery mode, NICU admission.
We also explored the association between maternal blood pressure levels during pregnancy and offspring outcomes whenever it was possible.
Results
Flow chart
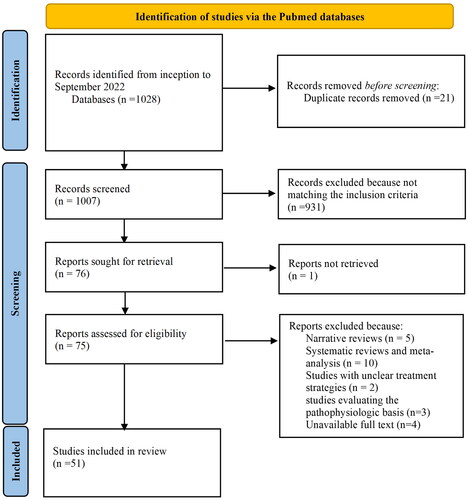
The literature search identified 1028 articles, of which 21 duplicate records were removed. The remaining 1007 records were screened and 75 of them matched the inclusion criteria. These studies were then assessed for eligibility: five narrative reviews, 10 systematic reviews and meta-analyses, two studies with unclear treatment strategies, three studies evaluating the pathophysiologic basis of disease, and four studies with unavailable full text were excluded. Thus, our review was restricted to 51 studies ().
Human studies
Doses and timing of administration
Among the 25 human studies, L-Arg was administered orally in 16, intravenously in eight, and a mixed treatment was done in the remnant as reported in . Oral treatment doses ranged from 1 g/day [Citation13] to 16 g/day [Citation14, Citation15], lasting from 8 to 10 days [Citation15] to the entire duration of pregnancy [Citation16]. Intravenous administrations were mainly utilized in the acute treatment of hypertension, PE or severe IUGR, doses ranging 15 g (in 500 ml of 5% glucose) [Citation17] to 30 g (in 100 ml of saline) [Citation18]. Treatments usually lasted one day, except for the study by Xiao et al. [Citation19] which administered L-Arg for 7 days to women carrying IUGR fetuses.
Table 1. Main human study characteristics.
L-Arginine and assisted reproductive technology
L-Arg was administered in three studies to women undergoing assisted reproductive technology (ART) [Citation14, Citation15, Citation20]; medications were taken orally in addition to gonadotrophin-releasing hormone analogue (GnRHa) and pure follicle stimulating hormone (pFSH) [Citation14, Citation15], or in addition to folate and vitamin E [Citation20]. L-Arg led to less cycle cancelation, more oocytes and transferred embryos, increased plasma and follicular fluid nitrite/nitrate, as well as to Doppler flow improvement in one study [Citation15]. A detrimental role of L-Arg was reported on embryo quality and pregnancy rate during controlled ovarian hyperstimulation cycles, due to the inverse correlation between follicular fluid concentration of nitrite/nitrate and embryo quality [Citation14]. On the contrary, So [Citation20] reported that, especially in cases of male infertility, both biochemical and clinical pregnancy rate were significantly increased by L-Arg ().
Table 2. Main findings of human studies.
L-Arginine supplementation and fetal growth restriction
L-Arg was administered in seven studies to women carrying IUGR fetuses: in three of them the administration occurred intravenously while in the remnant 4 it was oral. Overall, the studies showed that L-Arg infusion affects utero-placental circulation by lowering the uterine artery pulsatility index [Citation18], improving birthweight [Citation19, Citation21, Citation22], reducing placental apoptosis, and improved placental function and fetal development [Citation17]. L-Arg also reduced the incidence of abnormal umbilical artery blood flow and uterine artery early—diastolic notching [Citation23], being not effective for severe vascular IUGR in possibly due to the severity of the growth retardation (3rd percentile) and to the early course of gestation (28 weeks) [Citation24] ().
L-Arginine and hypertensive disorders of pregnancy
In 11 studies L-Arg was used to treat women with hypertensive disorders of pregnancy (HDP), chronic hypertension (CH), or preeclampsia (PE). Three studies administered L-Arg intravenously while seven orally and one either orally or intravenously when oral medication could not be taken [Citation25].
Five studies included women diagnosed with PE [Citation25–29], and treatments lasted from 2 days (acute treatment) [Citation27] to 3 weeks [Citation29]. L-Arg supplementation lowered systolic blood pressure (SBP), diastolic blood pressure (DPB), mean arterial pressure (MAP) and increased 24-h urinary excretion of NOx as well as plasma levels of L-citrulline [Citation26]. Moreover, L-Arg markedly improved fetal growth [Citation29] fetal well-being, neonatal outcome and prolonged pregnancy [Citation28]. L-Arg also significantly improved blood pressure and kidney function the 10th day postpartum without hasten the recovery of women with PE [Citation25]. An acute treatment of PE patients with gestational length varying from 28 to 36 weeks (4 g of L-Arg for 2 days orally) with L-Arg supplementation did not reduce mean diastolic blood pressure compared with placebo (Staff AC).
Three studies were conducted on women with HDP [Citation30–32] and L-Arg was administered intravenously (20 g/day, for a maximum of 5 days). L-Arg infusion showed an acute hypotensive effect both on systolic and diastolic values [Citation30, Citation31], without affecting fetal movements, while prolonging pregnancy [Citation31].
The remnant studies included women at high risk for PE [Citation33, Citation34] or suffering CH [Citation16, Citation35]. Oral prophylactic treatments started in the first trimester and lasted from 10 weeks to the entire duration of pregnancy (more than 30 weeks). Although L-Arg did not change BP a lower percentage of women required antihypertensive drugs [Citation35] and improved uterine artery impedance [Citation16]. PE was lesser developed and the incidence of superimposed PE indicating early delivery <34 weeks show a trend to be reduced [Citation33–35]. L-Arg treatment was also associated with higher birthweight and less preterm births [Citation34].
Two studies were conducted in women with threatened preterm birth (PTB), one using 3 g/day of orally since admission until delivery [Citation28], while the other acutely infusing L-20 g/500 ml in 3 h of L-Arg [Citation36]. Oral supplementation increased feto-placental blood flow while i.v. infusion reduced uterine contractions, increasing both serum growth hormone and nitrates levels.
Transgenerational/metabolic effects of L-Arg
A single study evaluated L-Arg/NO system and its role in insulin signaling and endothelial function in pregnant women of different BMI categories [Citation37]. NO availability was found impaired in overweight/obese women, and this deranged endothelial function and insulin regulation. L-Arg reduced insulin level in the first trimester, only in normal-weight in the second trimester.
Animal studies
Doses and timing of administration
In 23 out of 26 animal studies, L-Arg was administered orally, either dissolved in drinking water or introduced with the diet, whereas in the remnant 3 studies it was administered intravenously [Citation38–40] ().
Table 3. Main characteristics of basic science studies.
Studies focused on the effects of L-Arg on reproductive performances (five studies), fetal growth (16 studies), hyperinsulinemia and/or hypertensive disorders (four studies) while immune response was outcome of the remnant study.
L-Arginine and reproductive performance
Pigs [Citation41–43], mares [Citation44], and ewes [Citation38], were the target of L-Arg. In mares L-Arg reduced uterine fluid accumulation, without altering follicular development, representing a breeding management tool in postpartum period to increase reproductive success [Citation44]. Moreover, L-Arg markedly increased live-born piglets by two per litter [Citation41] and increased birthweight [Citation43], also improving lactation performance of first-parity sows [Citation42]. No effects were found in lambing rates [Citation38] ().
Table 4. Main findings of the basic science studies.
L-Arginine supplementation and fetal growth
Supplementation (mainly via drinking water or through diet) was done in undernourished sheep (six studies), pigs (eight studies), rats (two studies).
Generally, L-Arg increased birth weight and muscle weight as well as maturation [Citation39, Citation45, Citation46] possibly through altered mTOR protein abundance [Citation39]. No effect on the stillbirth rates was found in sows [Citation47], while L-Arg supplementation enhanced fetal survival [Citation48, Citation49] in swine. In sheep L-Arg (and NCG) supplementation decreased IUGR by improving metabolic homeostasis and through the expression of fetal somatotropic axis genes [Citation50, Citation51]. Zhang et al. 2016 [Citation52] confirmed the promotion of fetal-placental development through the improvement of antioxidation capability.
Placental growth and vascularity were enhanced by L-Arg [Citation53] also in pigs, although Li et al. [Citation54] reported a decreased litter size in gilts.
Once supplemented late in pregnancy, L-Arg had no effect on piglet birth weight or lactation performance [Citation55], being unable to mitigate consequences of restricted maternal nutrition [Citation56] ().
L-Arg supplementation and hyperinsulinemia/hypertensive disorders
Three studies were conducted on murine models of chronic exogenous hyperinsulinemia, leading to hypertension and heart failure [Citation57–59]. Another study used L-NAME in rats creating a model of preeclampsia [Citation60].
L-Arg reversed the endothelial lesion due to the L-NAME exposure, and lowered blood pressure in late pregnancy [Citation60], decreased the degree of proteinuria and the proportion of injured [Citation58, Citation60, Citation61].
The supplementation with L-Arg increased birthweight, without any changes in the levels of plasma insulin or serum glucose [Citation59, Citation60], but stimulating NO system in the placenta [Citation58] ().
These data, obtained in different experiment models of insulin-induced rat hypertension, suggest a direct reversal effect of L-Arg administration on hypertension and fetal weight changes, activating NO systems in placenta and kidney.
Immune response and L-Arginine
In Trypanosoma cruzi infected pregnant Wistar rats, which allowed Chagas disease development [Citation62], L-Arg decreased the levels of corticosterone and parasitemia and increased fetal and placental weigh, and reduced amastigote burdens. L-Arg supplementation might improve the host immune response during the acute phase ().
Safety and tolerability of dietary supplementation of L-Arginine
None of the human studies reported adverse events associated with the supplementation with L-Arg during pregnancy, neither for a long period of administration (3 months) [Citation20] nor by using high doses as for the acute treatments (20 or 30 g/100 ml of saline for 1 day or 1 week) [Citation18, Citation19]. Among animal studies, only one study conducted on sows reported an increase in stillbirth rates, in the group receiving the combination of Arg and Ractopamine (Rac) from day 25 to 53 of gestation [Citation47] (). However, it is unknown to which supplement such effect could be ascribed.
Discussion
This systematic review of the literature found human and animal studies over a large period of time, witnessing the still actual interest toward L-Arg administration for reproductive purposes.
Overall, the results demonstrated that L-Arg supplementation during pregnancy could be beneficial in several circumstances, especially on maternal hypertension and fetal growth, by reducing blood pressure levels, the onset of preeclampsia, and improving vascularity as well as placental function. However, it is worth emphasizing that many of the RCTs included were conducted more than 15 years ago and had weak power and a heterogeneous population. Also, no data are available on severest outcomes (i.e. stillbirth, placental abruption or severe IUGR), unlike other studies with NO donors [Citation63, Citation64] which however failed in demonstrating efficacy on the development of FGR, preterm delivery, and perinatal mortality and morbidity.
While pharmacological NO donors have been associated with poor efficacy (Cochrane), L-Arg which is the physiological substrate of endothelial NO synthase seems to show a better risk/benefit profile. Possibly, the production of peroxynitrites as a result of an excess NO bioavailability does not occur when administering the amino acid [Citation65].
However, short-term supplementation of L-Arg, especially late in pregnancy, resulted insufficient to improve maternal hemodynamics and did not mitigate the effects of severe IUGRs [Citation25]. This suggests that L-Arg should be initiated early and continued over the course of the entire pregnancy in order to positively affect blood pressure or placental vascular insufficiency, via the arginine–NO pathway [Citation66].
These findings confirm what has been reported in a recent meta-analysis by Goto [Citation8] (based upon solely 10 eligible articles) which concluded that the supplementation with L-Arg should be recommended in women with a history of poor pregnancy outcomes, both in those at high-risk of pre-eclampsia, as well as in those with already established HDP. However, we agree with the authors stating that more trials are required to provide stronger conclusions, since the small study effects.
The partial efficacy of L-Arg may be related to the splanchnic extraction and metabolism of arginine which often precluded its efficacy with possible degradation by arginase. Indeed, animal studies supplementing citrulline (direct NO donor without splanchnic degradation), reported an enhanced placental function and fetal growth in rat models of IUGR through the involvement of insulin-like growth factor 2 and angiogenic factors [Citation67] and improved perinatal and postpartum maternal vascular function in a mouse model of preeclampsia [Citation68]. Citrulline effectively raised fetal arginine availability, although it failed to increase the concentrations of essential amino acids in fetal plasma of murine models of IUGR [Citation69]. Unfortunately, no human trials are available about Citrulline and/or combined supplementation.
Another systematic review [Citation70] analyzed the role of Arginine synthesis and metabolism in pregnancy and provided evidence for the link between an impaired arginine metabolic pathway and the pathogenesis of compromised pregnancy and fetal programming. Interestingly, the Authors presented L-Arg supplementation as a potential reprogramming strategy during pregnancy, in order to prevent non-communicable diseases (NCDs) in the offspring. Many of the evidence supporting such an idea stay on the capacity of improving fetal growth, also in cases where placenta function is compromised. Accordingly, it has to be remembered that the Barker’s observation correlating low than normal weight at birth with the later adult development of cardiovascular diseases was the milestone of epigenetic hypotheses [Citation71].
The fetal somatotrophic stimulation by L-Arg is evident also in several animal species through the exploitation of the amino acid as a booster of NO availability in the placenta vasculature. In addition, we understand from rats that L-Arg display its effects also in cases where hypertension and growth restriction are sustained by chronic hyperinsulinemia allowing insulin resistance [Citation57–59]. Indeed, besides cardiovascular benefits, serum glucose and free fatty acids concentration was reduced in overweight/obese females in fertile period [Citation72] and type 2 diabetes [Citation73]. Although reported only in a single study, the ability of L-Arg to activate endothelium-dependent vasodilation in obese pregnant women reducing circulating insulin levels suggests the possible employment of supplementation in such a condition, well characterized as being insulin-resistant [Citation37]. Interestingly, among possible target of L-Arg administration there are also women with a reduced pre-pregnancy BMI. They showed low circulating Arginine levels which seem related to poor pregnancy outcome [Citation79].
Finally, in ovine, swine and equines, L-Arg supplementation also improved the reproductive performances becoming one among the interventions able to increase animal production.
Among the studies included, in this review none reported serious adverse reactions to L-Arg supplementation thus confirming a previously reported safety profile in pregnancy [Citation72]. Severe adverse events were reported only in a population of patients with a recent coronary heart disease over a long-term treatment (6 months) at high dose (9 g/day) [Citation74]. Moreover, in a controlled study, L-Arg has been found safe in subjects ingesting 15–30 g/day for 90 days, with no impact on the intake of energy, protein, carbohydrates, vitamins, or minerals. However, it is worth mentioning that NO donor supplementation in pregnancy is still debated, as not all NO donors are considered safe in pregnancy. Sildenafil, for example, when administered for severe early-onset fetal growth retardation, not only did not reduce the risk of perinatal mortality or severe neonatal morbidity, but actually increased the risk of neonatal pulmonary hypertension [Citation75].
Arginine is a non-essential amino acid whose intake from the diet (meats, dairy products, nuts, …) has been estimated to be >4 g/day in western countries [Citation76] while it seems much less available in people living in low resource settings [Citation77]. For a lot of micronutrients, such as iron, iodine, calcium, Vitamin D, and so on, pregnancy represents a status of relative deficiency [Citation78]. Thus, although we lack standard of Arg intake in pregnancy, it seems not unlikely that during gestation women require more intake due to both major needs (fetal growth) and to cover changes in eating behavior (i.e. less intake of meat).
Furthermore, L-Arg administered intravenously was associated with important fetal and maternal vascular effects [Citation18, Citation32]. However, the effects were not long-lasting, and the clinical feasibility was poor allowing oral Arginine be preferred in clinical trials.
However, the evidence highlighted in this systematic review indicate that L-Arg displays biological activities supporting its potential as a “therapeutic agent.” Moreover, neither human, nor animal ingestion of L-Arg have been associated to side effects and/or adverse reactions, at the given doses.
The “novelty” of this systematic review is that it examined not only the vascular but also the metabolic effect of arginine, including both animal and human studies. In particular, the L-NAME model was evaluated to target the well-consolidated, NO-mediated L-Arg action. The hyperinsulinemia animal model, on the other hand, allows for the first time to transfer the possible metabolic effects to pregnancy, which have not yet been well studied, contrary to non-pregnant conditions where various evidence of the metabolic impact of arginine is already available both in experimental [Citation80, Citation81] and human studies [Citation81–83].
Nitric oxide (NO) is a key regulator of both maternal and fetal homeostasis during pregnancy nevertheless strategies involving supplementation with NO precursors, NO donors, natural derivatives or pharmacological modulators of the NO system need to be more evaluated and randomized trials are yet warranted. This review suggests that the supplementation of a certain kind of L-Arg (i.e. in intravenous form or in oral vial as salt free form at a dose of 3 g/day) may not simply be used as a replacement for a transitory deficiency. Instead, through its cardiovascular and metabolic effects, L-Arg could be candidate as an intervention beneficial to maternal and fetal outcomes, at least in moderate clinical disorders.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Garovic VD, Dechend R, Easterling T, et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association. Hypertension. 2022;79(2):e21–e41.
- Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. BJOG Int J Obstet Gynaecol. 2011;118:1–203.
- Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195(6):1557–1563.
- Morgan T. Role of the placenta in preterm birth: a review. Am J Perinatol. 2016;33(3):258–266.
- Goto E. Meta-regression analysis to evaluate relationships between maternal blood levels of placentation biomarkers and low delivery weight. Int J Gynaecol Obstet. 2018;142(2):148–155.
- Gaccioli F, Lager S. Placental nutrient transport and intrauterine growth restriction. Front Physiol. 2016;7:40.
- Nigam J, Misra V, Singh P, et al. Histopathological study of placentae in low birth weight babies in India. Ann Med Health Sci Res. 2014;4(Suppl 2):S79–S83.
- Goto E. Effects of prenatal oral l-arginine on birth outcomes: a meta-analysis. Sci Rep. 2021;11(1):22748.
- PRISMA transparent reporting of systematic reviews and meta-analyses; 2021 [accessed 2022 Nov 18]. Available from: https://prisma-statement.org/
- Zhu Q, Yue X, Tian Q-Y, et al. Effect of l-Arginine supplementation on blood pressure in pregnant women: a meta-analysis of placebo-controlled trials. Hypertens Pregnancy. 2013;32(1):32–41.
- SIGO. I Disordini Ipertensivi in Gravidanza: Classificazione, Diagnosi e Terapia. Raccomandazioni di Buona Pratica Clinica AIPE (Associazione Italiana Preeclampsia); 2020 [accessed 2022 Dec]. Available from: https://www.sigo.it/wp-content/uploads/2020/11/RaccomandazioniAIPE-Disordini_Ipertensivi_Gravidanza.pdf
- Fetal growth restriction. Obstet Gynecol. 2021;137(2):e16–e28.
- Borisova LV, Martinussen PE, Rydland HT, et al. Public evaluation of health services across 21 European countries: the role of culture. Scand J Public Health. 2017;45(2):132–139.
- Battaglia C, Regnani G, Marsella T, et al. Adjuvant L-arginine treatment in controlled ovarian hyperstimulation: a double-blind, randomized study. Hum Reprod. 2002;17(3):659–665.
- Battaglia C, Salvatori M, Maxia N, et al. Adjuvant L-arginine treatment for in-vitro fertilization in poor responder patients. Hum Reprod. 1999;14(7):1690–1697.
- Monari F, Menichini D, Pignatti L, et al. Effect of L-arginine supplementation in pregnant women with chronic hypertension and previous placenta vascular disorders receiving aspirin prophylaxis: a randomized control trial. Miner Obstet Gynecol. 2021;73(6):782–789.
- Shen S, Hua C. Effect of L-arginine on the expression of bcl-2 and bax in the placenta of fetal growth restriction. J Matern Neonatal Med. 2011;24(6):822–826.
- Neri I, Mazza V, Galassi MC, et al. Effects of L-arginine on utero-placental circulation in growth-retarded fetuses. Acta Obstet Gynecol Scand. 1996;75(3):208–212.
- Xiao XM, Li LP. L-Arginine treatment for asymmetric fetal growth restriction. Int J Gynaecol Obstet. 2005;88(1):15–18.
- So S, Yamaguchi W, Murabayashi N, et al. Beneficial effect of L-arginine in women using assisted reproductive technologies: a small-scale randomized controlled trial. Nutr Res. 2020;82:67–73.
- Sieroszewski P, Suzin J, Karowicz-Bilińska A. Ultrasound evaluation of intrauterine growth restriction therapy by a nitric oxide donor (L-arginine). J Matern Neonatal Med. 2004;15(6):363–366.
- Singh S, Singh A, Sharma D, et al. Effect of L-Arginine on nitric oxide levels in intrauterine growth restriction and its correlation with fetal outcome. Indian J Clin Biochem. 2015;30(3):298–304.
- Ropacka M, Szymanski P, Kowalska J, et al. Effect of oral supplementation with nitric oxide donor on fetomaternal hemodynamic. Ultrasound Obstet Gynecol. 2007;30(4):632–632.
- Winer N, Branger B, Azria E, et al. L-Arginine treatment for severe vascular fetal intrauterine growth restriction: a randomized double-bind controlled trial. Clin Nutr. 2009;28(3):243–248.
- Hladunewich MA, Derby GC, Lafayette RA, et al. Effect of L-Arginine therapy on the glomerular injury of preeclampsia. Obstet Gynecol. 2006;107(4):886–895.
- Rytlewski K, Olszanecki R, Korbut R, et al. Effects of prolonged oral supplementation with L-arginine on blood pressure and nitric oxide synthesis in preeclampsia. Eur J Clin Invest. 2005;35(1):32–37.
- Staff AC, Berge L, Haugen G, et al. Dietary supplementation with L-arginine or placebo in women with pre-eclampsia. Acta Obstet Gynecol Scand. 2004;83(1):103–107.
- Rytlewski K, Olszanecki R, Lauterbach R, et al. Effects of oral L-arginine on the pulsatility indices of umbilical artery and Middle cerebral artery in preterm labor. Eur J Obstet Gynecol Reprod Biol. 2008;138(1):23–28.
- Valdivia-Silva JE, López-Molina K, Macedo R. Efecto de la terapia temprana con L-arginina en el crecimiento intrauterino restringido en la preclampsia. Estudio aleatorizado en mujeres latinoamericanas. Prog Obstet Ginecol. 2009;52(2):89–98.
- Neri I, Blasi I, Facchinetti F. Effects of acute L-arginine infusion on non-stress test in hypertensive pregnant women. J Mat Fet Neonat Med. 2004;16(1):23–26.
- Facchinetti F, Saade GR, Neri I, et al. L-Arginine supplementation in patients with gestational hypertension: a pilot study. Hypertens Pregnancy. 2007;26(1):121–130.
- Neri I, Jasonni VM, Gori GF, et al. Effect of L-arginine on blood pressure in pregnancy-induced hypertension: a randomized placebo-controlled trial. J Matern Neonatal Med. 2006;19(5):277–281.
- Vadillo-Ortega F, Perichart-Perera O, Espino S, et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ. 2011;342:d2901.
- Camarena Pulido EE, García Benavides L, Panduro Barón JG, et al. Efficacy of L-arginine for preventing preeclampsia in high-risk pregnancies: a double-blind, randomized, clinical trial. Hypertens Pregnancy. 2016;35(2):217–225.
- Neri I, Monari F, Sgarbi L, et al. L-Arginine supplementation in women with chronic hypertension: impact on blood pressure and maternal and neonatal complications. J Matern Neonatal Med. 2010;23(12):1456–1460.
- Facchinetti F, Neri I, Genazzani AR. L-Arginine infusion reduces preterm uterine contractions. J Perinat Med. 1996;24(3):283–285.
- Petrella E, Pignatti L, Neri I, et al. The L-arginine/nitric oxide pathway is impaired in overweight/obese pregnant women. Pregnancy Hypertens. 2014;4(2):150–155.
- Crane AR, Redden RR, Van Emon ML, et al. Impacts of supplemental arginine on the reproductive performance of fall lambing ewes. J Anim Sci. 2016;94(8):3540–3549.
- Sales F, Sciascia Q, van der Linden DS, et al. Intravenous maternal L-arginine administration to twin-bearing ewes, during late pregnancy, is associated with increased fetal muscle mTOR abundance and postnatal growth in twin female lambs. J Anim Sci. 2016;94(6):2519–2531.
- De Boo HA, Van Zijl PL, Smith DEC, et al. Arginine and mixed amino acids increase protein accretion in the growth-restricted and normal ovine fetus by different mechanisms. Pediatr Res. 2005;58(2):270–277.
- Mateo RD, Wu G, Moon HK, et al. Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. J Anim Sci. 2008;86(4):827–835.
- Mateo RD, Wu G, Bazer FW, et al. Dietary L-Arginine supplementation enhances the reproductive performance of gilts. J Nutr. 2007;137(3):652–656.
- Li J, Xia H, Yao W, et al. Effects of arginine supplementation during early gestation (day 1 to 30) on litter size and plasma metabolites in gilts and sows. J Anim Sci. 2015;93(11):5291–5303.
- Kelley DE, Warren LK, Mortensen CJ. Oral L-arginine supplementation impacts several reproductive parameters during the postpartum period in mares. Anim Reprod Sci. 2013;138(3–4):233–240.
- Madsen JG, Mueller S, Kreuzer M, et al. Milk replacers supplemented with either L-arginine or L-carnitine potentially improve muscle maturation of early reared low birth weight piglets from hyperprolific sows. Animal. 2018;12(1):43–53.
- Quesnel H, Quiniou N, Roy H, et al. Supplying dextrose before insemination and L-arginine during the last third of pregnancy in sow diets: effects on within-litter variation of piglet birth weight. J Anim Sci. 2014;92(4):1445–1450.
- Garbossa CAP, Júnior FMC, Silveira H, et al. Effects of ractopamine and arginine dietary supplementation for sows on growth performance and carcass quality of their progenies. J Anim Sci. 2015;93(6):2872–2884.
- Bérard J, Bee G. Effects of dietary L-arginine supplementation to gilts during early gestation on foetal survival, growth and myofiber formation. Animal. 2010;4(10):1680–1687.
- Li X, Bazer FW, Johnson GA, et al. Dietary supplementation with L-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids. 2014;46(2):375–384.
- Sun L, Zhang H, Fan Y, et al. Metabolomic profiling in umbilical venous plasma reveals effects of dietary rumen-protected arginine or N-carbamylglutamate supplementation in nutrient-restricted Hu sheep during pregnancy. Reprod Domest Anim. 2017;52(3):376–388.
- Sun L, Zhang H, Wang Z, et al. Dietary rumen-protected arginine and N-carbamylglutamate supplementation enhances fetal growth in underfed ewes. Reprod Fertil Dev. 2018;30(8):1116–1127.
- Zhang H, Sun L, Wang Z, et al. N-carbamylglutamate and L-arginine improved maternal and placental development in underfed ewes. Reproduction. 2016;151(6):623–635.
- Gao K, Jiang Z, Lin Y, et al. Dietary l-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids. 2012;42(6):2207–2214.
- Li X, Bazer FW, Johnson GA, et al. Dietary supplementation with 0.8% L-Arginine between days 0 and 25 of gestation reduces litter size in gilts. J Nutr. 2010;140(6):1111–1116.
- Bass BE, Bradley CL, Johnson ZB, et al. Influence of dietary-arginine supplementation of sows during late pregnancy on piglet birth weight and sow and litter performance during lactation. J Anim Sci. 2017;95(1):248–256.
- Peine JL, Jia G, Van Emon ML, et al. Effects of maternal nutrition and rumen-protected arginine supplementation on ewe performance and postnatal lamb growth and internal organ mass. J Anim Sci. 2018;96(8):3471–3481.
- Bursztyn M, Podjarny E, Dahan R, et al. Insulin‐induced hypertension, L‐arginine, and endothelial nitric oxide synthase in pregnant rats. Hypertens Pregnancy. 2003;22(3):267–274.
- Sharkey LC, McCune SA, Yuan O, et al. Spontaneous pregnancy-induced hypertension and intrauterine growth restriction in rats. Am J Hypertens. 2001;14(10):1058–1066.
- Podjarny E, Bursztyn M, Rashed G, et al. Chronic exogenous hyperinsulinaemia-induced hypertension in pregnant rats: effect of chronic treatment with l-arginine. Clin Sci. 2001;100(6):667–671.
- Helmbrecht GD, Farhat MY, Lochbaum L, et al. L-arginine reverses the adverse pregnancy changes induced by nitric oxide synthase inhibition in the rat. Am J Obstet Gynecol. 1996;175(4 Pt 1):800–805.
- Altun ZS, Uysal S, Guner G, et al. Effects of oral L‐arginine supplementation on blood pressure and asymmetric dimethylarginine in stress‐induced preeclamptic rats. Cell Biochem Funct. 2008;26(5):648–653.
- da Costa CMB, de Freitas MRB, Brazão V, et al. Does l-arginine availability during the early pregnancy alters the immune response of Trypanosoma cruzi infected and pregnant Wistar rats? Exp Parasitol. 2014;142:59–66.
- Groten T, Lehmann T, Städtler M, et al. Effect of pentaerythritol tetranitrate (PETN) on the development of fetal growth restriction in pregnancies with impaired uteroplacental perfusion at midgestation—a randomized trial. Am J Obstet Gynecol. 2023;228(1):84.e1–84.e12.
- Duckitt K, Thornton S. Nitric oxide donors for the treatment of preterm labour. Cochrane Database of Syst. Rev. 2002;(3):CD002860.
- Nanetti L, Giannubilo S, Raffaelli F, et al. Nitric oxide and peroxynitrite platelet levels in women with small-for-gestational-age fetuses. BJOG. 2008;115(1):14–21.
- McDonald CR, Cahill LS, Gamble JL, et al. Malaria in pregnancy alters l-arginine bioavailability and placental vascular development. Sci Transl Med. 2018;10(431):eaan6007.
- Tran N-T, Amarger V, Bourdon A, et al. Maternal citrulline supplementation enhances placental function and fetal growth in a rat model of IUGR: involvement of insulin-like growth factor 2 and angiogenic factors. J Matern Neonatal Med. 2017;30(16):1906–1911.
- Gemmel M, Sutton EF, Brands J, et al. L-Citrulline supplementation during pregnancy improves perinatal and postpartum maternal vascular function in a mouse model of preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2021;321(3):R364–R376.
- Bourdon A, Hannigsberg J, Misbert E, et al. Maternal supplementation with citrulline or arginine during gestation impacts fetal amino acid availability in a model of intrauterine growth restriction (IUGR). Clin Nutr. 2020;39(12):3736–3743.
- Hsu C-N, Tain Y-L. Impact of arginine nutrition and metabolism during pregnancy on offspring outcomes. Nutrients. 2019;11(7):1452.
- Barker D. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111.
- McNeal CJ, Meininger CJ, Wilborn CD, et al. Safety of dietary supplementation with arginine in adult humans. Amino Acids. 2018;50(9):1215–1229.
- Ivy JL. The use of L-arginine supplements for cardiovascular disease and related disorders is questionable. Nov Tech Nutr Food Sci. 2020;5(1):406–412.
- Schulman SP, Becker LC, Kass DA, et al. L-Arginine therapy in acute myocardial infarction. JAMA. 2006;295(1):58–64.
- Pels A, Derks J, Elvan-Taspinar A, et al. Maternal sildenafil vs placebo in pregnant women with severe early-onset fetal growth restriction. JAMA Netw Open. 2020;3(6):e205323.
- Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): The Natlional Academies Press; 2005.
- Schönfeldt HC, Gibson Hall N. Dietary protein quality and malnutrition in Africa. Br J Nutr. 2012;108(S2):S69–S76.
- Gernand AD, Schulze KJ, Stewart CP, et al. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. 2016;12(5):274–289.
- Kurpad AV, Kao C, Dwarkanath P, et al. In vivo arginine production and nitric oxide synthesis in pregnant Indian women with normal and low body mass indices. Eur J Clin Nutr. 2009;63(9):1091–1097.
- de Castro Barbosa T, Jiang LQ, Zierath JR, et al. L-Arginine enhances glucose and lipid metabolism in rat L6 myotubes via the NO/c-GMP pathway. Metabolism. 2013;62(1):79–89.
- Hu S, Han M, Rezaei A, et al. L-Arginine modulates glucose and lipid metabolism in obesity and diabetes. Curr Protein Pept Sci. 2017;18(6):599–608.
- Hadi A, Arab A, Moradi S, et al. The effect of L-arginine supplementation on lipid profile: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. 2019;122(9):1021–1032.
- Jobgen WS, Fried SK, Fu WJ, et al. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17(9):571–588.