Abstract
Objective
To evaluate whether warm povidone-iodine (PI) application before peripherally inserted central catheter (PICC) placement eased pain related to the procedure in premature infants and reduced the duration of the procedure and the number of attempts.
Methods
A prospective randomized controlled trial was conducted with infants born before 32 weeks of gestation who required the first placement of the PICC. Skin disinfection was performed with warm PI before the procedure in the warm PI(W-PI) group, whereas PI kept at room temperature was used in the regular PI(R-PI) group. NPASS scores of the infants were evaluated three times: at baseline(T0), during skin preparation(T1), and during needle insertion(T2).
Results
Fifty-two infants (26 in the W-PI group,26 in the R-PI group) were enrolled in the study. The perinatal and baseline demographic characteristics did not significantly differ between the two groups. While the median NPASS scores at T0 and T2 were similar between the groups, the median T1 score was significantly higher in the R-PI group(p = .019). While the median NPASS scores at T1 and T2 were similar in the R-PI group, there was a significant difference in the W-PI group, with NPASS scores being significantly lower at T1 compared to T2. The results demonstrate that skin disinfection was just as painful as needle insertion in the R-PI group. The duration of the procedure and the number of needle insertions were significantly lower in the W-PI group.
Conclusions
Before invasive interventions, such as PICC insertion, we recommend warm PI as a part of non-pharmacological pain management.
Keywords:
Introduction
Prior to the 1980s, there was a common misconception that neonates did not experience pain. After four decades, the assessment and management of pain in neonates in neonatal intensive care units (NICUs) became standard practice in neonatal care.
A neonate can experience more than 300 painful procedures during their stay in the NICU [Citation1]. Due to their poor pain inhibition mechanisms, preterm infants may even experience pain worse than term infants [Citation2]. Pain management in preterm infants is important as it will also affect their future lives [Citation3]. PICC insertion is a painful and challenging intervention, which almost all very low birth weight (VLBW) infants experience in the early postnatal days of their lives for parenteral nutrition and drug administration [Citation4,Citation5].
Premature infants are very sensitive to environmental temperature changes, especially in the first days of their lives [Citation6]. Their small body masses, relatively large surface areas, and high peripheral circulation cause increased heat loss by evaporation [Citation6,Citation7]. Studies on cutaneous blood flow in preterm infants have demonstrated a decrease in thermal conductance, indicating peripheral vasoconstriction [Citation8,Citation9]. In addition, studies investigating the effect of preterm baths have shown that skin color changes occur with a decrease in water temperature by even 1–1.5 °C [Citation10]. This can also be seen in preterm infants during skin disinfection with local disinfectants stored at room temperature in NICUs.
Many non-pharmacological and pharmacological studies have been conducted to reduce pain experiences and improve the comfort of VLBW infants; methods such as sucrose, swaddling, and non-nutritive sucking have been added to guidelines, while some studies have been discontinued due to adverse effects [Citation11,Citation12]. To reduce pain experiences and improve comfort of VLBW infants, NICU staff should develop non-pharmacological methods and also improve first attempt PICC insertion success rates [Citation13].
In our clinical practice, we often observe changes in skin coloring, cold extremities, and discomfort in preterm infants, as well as difficulty in the visualization of their veins with routine skin disinfection. Therefore, we hypothesize that pre-procedure disinfection with warm povidone iodine (PI) can reduce pain scores and prevent reactive changes in the skin and vasculature, ultimately leading to improved success rates of first attempt PICC insertion with fewer needle insertions and greater patient comfort.
Methods
This single center randomized controlled trial (RCT) (NCT04458441) was conducted between May 2020 and March 2021. The study center is a level IV NICU with 61-bed capacity in a children’s hospital with a perinatology center. The study was approved by the local ethics committee, and informed parental consent was obtained for each infant before enrollment.
Inclusion criteria
Being born before 32 weeks of gestational age and requiring central catheterization for parenteral nutrition according to the routine protocol of the NICU,
First PICC placement after the removal of the umbilical catheter,
Parental consent.
Exclusion criteria
Having received analgesics or sedatives since birth
Being on invasive mechanical ventilation
Major congenital anomalies
Perinatal asphyxia (Fifth-minute APGAR score < 5)
Severe intracerebral hemorrhage and/or neurological disorders
Septic shock and/or unstable vital signs
No parental consent
Study design and randomization
We divided the patients into two groups according to a random number table immediately before the PICC procedure.
Skin disinfection and PICC procedure
The skin disinfection procedure for the area selected for PICC placement was undertaken as described in the literature [Citation14]. 10% PI solution was applied with sterile gauze twice, waited for 10 s to achieve disinfection, and then the area was allowed to dry. This standard application was performed on all infants, and the only difference between the two groups was the temperature of the PI used. Skin was disinfected with appropriate PI according to the randomization groups by a predefined experienced nurse, who also assisted the same neonatologist blind for the groups during all PICC insertions.
As the catheter, 28 G (1 F) VYGON® PREMICATH (Aachen, Germany) with the VYGON® neonatal PICC placement kit (Aachen, Germany) was used in all patients. All procedures were performed under sterile precautions, including hand washing and the use of sterile gloves, gowns, face masks, and hair covers, and the infants were covered by transparent sheets to record their faces and body movements. According to our NICU protocols, the veins (median cubital, basilic and cephalic veins) of antecubital region are preferred for the PICC insertion; therefore, during the study for standardization, right antecubital region was the first choice and left was the second. The procedure was considered successful when the X-ray confirmed that the tip of the catheter was located on the distal superior vena cava (SVC) or right atrium (RA) or at the junction of the SVC and RA.
In the warm PI (W-PI) group, PI was placed in a sterile 100 ml plastic container that was tightly closed and kept in 37 °C water in a bottle warmer for 30 min before the procedure. After checking the temperature of PI with a liquid thermometer, we poured it into the sterile container immediately before skin disinfection to avoid cooling. The temperature of the warm PI was measured to be between 35 °C and 37 °C. In contrast, the R-PI group underwent skin disinfection using standard PI stored at room temperature, which typically ranges between 23–24 °C in our NICU.
In each patient, the Neonatal Pain, Agitation and Sedation Scale (NPASS) was used for the objective evaluation of vital findings [Citation15]. In addition, all the procedures were video-recorded, and their durations were calculated based on the time from the initiation of skin disinfection to the securement of the PICC. Our rationale for incorporating the duration of catheter insertion from the skin preparation to catheter securement was to exclude processes such as catheter preparation and confirmation of catheter tip placement, which may be influenced by varying staff and portable radiography time in our NICU. After PICC placement, the disinfected skin area was cleaned with warm sterile saline to avoid iodine absorption through the skin.
The demographic data of all preterm infants, vital signs, body temperatures, the duration of the procedures, and the number of needle attempts were recorded bedside. The infants were kept in isolated Drager Isolette ® C2000 incubators (Draeger, USA), and their oxygen saturation was monitored using Masimo SET pulse oximetry (Masimo Corporation, Irvine, CA), and heart rate using the electrocardiogram lead with the GE CARESCAPE™ monitor (GE Healthcare, Waukesha WI, USA). To prevent heat loss, the front doors of the incubator are opened while the mattress is not pulled out during PICC procedures in our unit, taking advantage of the air curtain technology. Additionally, the body temperatures of all infants are servo-controlled and documented.
Pain scoring
Special attention was paid to ensure that the patients were calm and relaxed, especially in the last 30 min before the PICC procedure. None of the patients received any systemic or topical analgesia or sedation since birth as the clearances of these drugs are of question in different gestational ages. Two minutes before the procedure, all the enrolled infants received non-nutritive sucking or breast milk, according to the recommendations of the Turkish Neonatal Society [Citation16] and positioned in midline flexion position within the nest.
NPASS was used for the objective evaluation of pain [Citation15,Citation16]. In our study, two blinded neonatologists performed NPASS scoring using the unmuted video records at three different times: baseline (immediately before the procedure) (T0), during skin preparation (T1), and during needle insertion (T2). The needle insertion was initiated when the patient showed no signs of discomfort after skin disinfection. The scores were adjusted according to the gestational age by the principal investigator at the time of data entry. Definition of CRBSI is given in [Citation17].
Table 1. Demographic data of the neonates included in the study.
Primary outcome
The primary outcome measures were differences in pain scores between the two different PI application temperatures at the three evaluation times.
Secondary outcome
The secondary outcome measures were the number of total needle attempts, the duration of the procedure, and changes in body temperature during PICC placement.
Sample size and power analysis
Based on our previous data from our NICU, we hypothesized that warm PI would reduce the NPASS scores by 25% (absolute reduction of 20%). With a two-sided alpha error of 0.05 and a beta error of 0.2 at 80% power, the estimated sample size was 32 infants (16 in each group).
Ethics approval
The trial was approved by the Ethics Committee of University of Health Sciences Umraniye Research and Training Hospital (approval number: 2021/342). Written informed consent was obtained from the parents or legal guardians of the newborns.
Statistical analyses
Statistical analyses were performed using SPSS statistical software for Windows (ver. .21.0; SPSS Inc., Chicago, IL, USA). Continuous values were presented as mean ± standard deviation or median (range) values according to the homogeneity of the distribution, which was evaluated by the Kolmogorov-Smirnov test. Student’s t-test was used to analyze continuous variables with a normal distribution, and the Mann-Whitney U and Kruskal-Wallis tests for analyzing those that did not exhibit a normal distribution. Categorical data were presented as numbers (n) and frequencies (%) and analyzed with the χ2 test. A p-value of <0.05 was considered statistically significant. To assess interrater reliability between the two neonatologists, we used the intraclass correlation coefficient (ICC).
Results
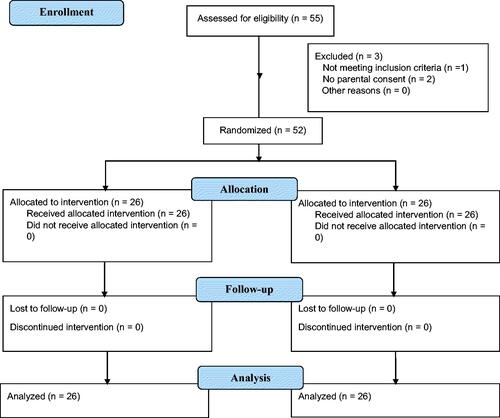
During the study period, a total of 55 preterm neonates were evaluated for eligibility, but one infant did not meet the inclusion criteria, and the parents of two infants did not provide consent (). As a result, 52 preterm infants were enrolled in the study and randomly allocated to the W-PI and R-PI groups (26 infants each). The mean gestational age of the W-PI and R-PI groups were 27.7 ± 2.3 and 28.7 ± 2.7 weeks, respectively, and their mean birth weight values were 968 ± 321 g and 1,124 ± 284 g, respectively, with no statistically significant significance in terms of gender, antenatal steroid exposure, APGAR scores, and other demographic data. Patients included in both groups were either on noninvasive ventilation or spontaneous breathing with no statistical difference. There were no significant differences observed in the CRBSI scores between the two groups ().
presents the NPASS scores of the groups at different evaluation times. Accordingly, the NPASS scores were similar between the groups at T0 and T2 but significantly higher at T1 in the R-PI group than in the W-PI group [7.5 (3–9) vs. 4 (2,75–5,5), p = .019]. When the pain scores were compared within the groups, there was no significant difference between T1 and T2 in the R-PI group (). In the W-PI group, T2 was significantly higher than T1 (p < .01). The results demonstrate that skin disinfection was just as painful as needle insertion in the R-PI group.
Table 2. NPASS is evaluated by two observers at different time points in both groups.
Table 3. Comparison of the pain scores within the groupsTable Footnotea.
The ICC values of the two neonatologists were similar for the evaluation of the NPASS scores at all evaluation times ().
Table 4. Interrater reliability between the observers.
Concerning the secondary outcomes, the duration of the procedure was significantly shorter (p = .02), and the number of needle insertions was significantly lower in the W-PI group (p = .017) (). In one infant from the R-PI group, it was only possible to insert the PICC after two sessions with six needle insertions on each. When we excluded this extreme patient from the statistical analysis, the mean procedure time [9.8 ± 6.2 min in R-PI group vs. 6.7 ± 3.8 min in W-PI group (p = .04)] and number of needle attempts [median number 1 (1–9) in R-PI group vs. 1 (1–3) in W-PI group, (p = .027)] were found to be significantly lower in the W-PI group. The final body temperature after the PICC procedure was higher in the W-PI group, but this was not statistically significant.
Table 5. Secondary outcomes.
Discussion
In our study, we demonstrated that skin disinfection was a painful procedure with relatively cold PI for the preterm infants and this resulted in a higher pain experience than the needle insertion even though it did not cause any tissue damage. Taddio et al. conducted an RCT study that evaluated pain response during PICC placement in ventilated infants. Their results were similar to ours, as they found that the pain response during skin preparation was significantly higher in the group without analgesics and the group with local analgesics compared to the group with parenteral analgesics [Citation18]. However, in their study, the study sample included both preterm and full-term infants, and the methods used for skin preparation and pain scoring were not specified. In the present study, we demonstrated that skin disinfection was a painful procedure, and that this pain can be effectively reduced by using warm PI in non-ventilated preterm infants. We chose to conduct our study on infants with either spontaneous breathing or noninvasive ventilation, as preterm infants on invasive ventilation support receive sedo-analgesia, which precludes proper pain scoring. We also used an objective pain scoring system evaluated by two blinded neonatologists.
In our study we also demonstrated that warm PI reduced the number of needle insertions and shortened the procedure time. To standardize this, we performed the procedure through the right antecubital region in all patients. In only one patient in the R-PI group, the first attempt of PICC insertion was not successful and ultimately required a total of 12 needle insertions across two attempts to successfully insert the PICC. Skin disinfection was performed with regular PI in both attempts. The lower number of needle insertions observed in the warm PI group during PICC insertion in this study can be attributed to the decreased venous constriction associated with warm PI, as well as lack of skin discoloration caused by cold. We suppose that these factors increase vessel selection and visibility, which aligns with the findings of Paulson et al. and leads to greater success rates in PICC placement [Citation19].
The Turkish Neonatal Society recommends limiting the duration of diagnostic and therapeutic management of painful and stressful procedures and the total amount of noxious stimuli applied [Citation16]. As of our current knowledge, there is no existing literature on the duration of PICC insertion. We aimed to investigate this aspect in our study to reduce patient discomfort caused by longer procedure times and demonstrate the efficiency of time management in units with a high patient volume and relatively low healthcare personnel.
In the literature, there is limited data, especially in terms of the pain management of non-ventilated preterm infants before painful procedures. The Turkish Neonatal Society and the American Academy of Pediatrics recommend non-pharmacologic interventions before PICC line placement in non-ventilated preterm infants, as many of the pharmacologic methods suppress spontaneous breathing effort [Citation16,Citation20]. Hulse et al. reported that skin disinfection with a warm PI during local anesthesia in adult patients increased patient comfort [Citation21]. Therefore, we hypothesized and demonstrated that the use of warm antiseptic solutions during PICC insertion was an effective option in non-pharmacologic pain management in non-ventilated preterm infants.
Although there are many studies comparing the performance of various disinfectants in preventing catheter-related infections without damaging the skin’s integrity, none of these studies have provided data on the temperature of the disinfectant solutions used in neonates [Citation22]. The manufacturer of PI states that it should be kept at room temperature to preserve its antiseptic properties [Citation23]. However, Leung et al. showed that PI was as effective at 32 °C as at 25 °C and recommended warm PI for the patient’s comfort [Citation24]. Maloney et al. also suggested that PI might be stored at temperatures of up to 37 °C for a maximum of six months without the loss of available iodine [Citation25]. In another study conducted by Gezer et al. to prevent surgical site infections in adults, warmed PI (10%) (37 °C) and chlorhexidine gluconate with alcohol (37 °C) were compared to PI and chlorhexidine gluconate with alcohol kept at 25 °C. The results of this study demonstrate a decrease in surgical site infections with warm PI [Citation26]. In the current study, we used PI at 35–37 °C and did not find any significant difference in the rate of CRBSI between the two groups.
Since this is the first study in which warm PI was used for skin disinfection in preterm infants, it was important to show that this application did not increase infection rates. Similar studies can be conducted to investigate other disinfectants. The use of warm PI was found to be safe in preterm infants, as no skin lesions were detected in either group, suggesting that warm PI can be safely used in neonates, particularly preterm infants, without increasing the risk of infection.
Pharmacological pain management and the assessment of pain in neonates have been established, and analgesics and sedatives have become a part of routine neonatal care. On the other hand, non-pharmacological techniques, such as sweet-tasting substances, kangaroo care, breast milk and breastfeeding, non-nutritive sucking, swaddling, and facilitated tucking alone or in combination have been also shown to be effective in soothing infants undergoing painful/stressful procedures [Citation1,Citation16,Citation27]. In a review conducted by Mangat et al. it is noted that although there are many publications in the literature on non-pharmacological pain treatments in neonates, there is insufficient data regarding superiority of these treatments over each other and their long-term outcomes [Citation11]. The review suggests that studies should use an objective pain scoring system for comparison between studies.
In addition to decreased pain scores, our study had several strengths that enhance its validity. First, we standardized the PICC insertion technique and the personnel who performed the procedure. Moreover, the personnel were blinded to the intervention arm, which eliminated any potential bias in the evaluation of pain. Second, we employed an objective pain scoring system that was video-recorded and evaluated by two blinded neonatologists to demonstrate its accuracy, and a statistically strong agreement was found between the two evaluators. Finally, we included all infants who met the inclusion criteria in our study during the study period.
However, our study also had some limitations, including the lack of existing comparative data on neonates in the literature, the single-center design, and the absence of temperature measurements of the regular PI.
Conclusion
While much progress has been made in the understanding of neonatal pain, the pain experience of newborns still has many unknowns due to the lack of evidence and data in neonatal pain assessment and management, which may lead to challenges in clinical practice. We suggest that warm PI can be safely used to provide comfort to neonates before invasive procedures, such as small surgical interventions, paracentesis, lumbar puncture, central or umbilical catheterization, and thorax tube insertion. This highlights the potential of simple interventions to have significant effects on newborns. To our knowledge, this is the first study in which this evaluation was performed as a prospective randomized clinical trial using an objective pain scoring system.
Ethical approval
This study was performed with the approval of the Clinical Research Ethical Committee.
This article does not contain any studies with animals performed by any of the authors.
Disclosure statement
No conflict of interest was declared by the authors.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Perry M, Tan Z, Chen J, et al. Neonatal pain: perceptions and current practice. Crit Care Nurs Clin North Am. 2018;30(4):549–561.
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7(3):246–257.
- Grunau RE, Holsti L, Haley D, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113(3):293–300.
- Govindan S, Jobe A, O’Malley ME, et al. To PICC or not to PICC? A cross-sectional survey of vascular access practices in the ICU. J Crit Care. 2021;63:98–103.
- Gullo G, Colin A, Frossard P, et al. Appropriateness of replacing fuoroscopic guidance with ECG-electromagnetic guidance for PICC insertion: a randomized controlled trial. AJR Am J Roentgenol. 2021;216(4):981–988.
- Loring C, Gregory K, Gargan B, et al. Tub bathing improves thermoregulation of the late preterm infant. J Obstet Gynecol Neonatal Nurs. 2012;41(2):171–179.
- Finn M, Meyer A, Kirsten D, et al. Swaddled bathing in the neonatal intensive care unit. Neoreviews. 2017;18(8):e504–e506.
- Hammarlund K, Sedin G, Strömberg B. Transepidermal water loss in newborn infants. VII. Relation to post-natal age in very pre-term and full-term appropriate for gestational age infants. Acta Paediatr Scand. 1982;71(3):369–374.
- McCall EM, Alderdice F, Halliday HL, et al. Interventions to prevent hypothermia at birth in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2018;2(2):CD004210.
- Fernández D, Antolín-Rodríguez R. Bathing a premature infant in the intensive care unit: a systematic review. J Pediatr Nurs. 2018;42:e52–e57.
- Mangat AK, Oei JL, Chen K, et al. A review of Non-Pharmacological treatments for pain management in newborn infants. Children (Basel). 2018;5(10):130.
- Monk V, Moultrie F, Hartley C, et al. Oral morphine analgesia for preventing pain during invasive procedures in non-ventilated premature infants in hospital: the poppi RCT. Southampton: NIHR Journals Library. 2019.
- Xiaoli S, Weiyan H, Li D. Construction of neonatal PICC nursing quality evaluation system. Appl Bionics Biomech. 2022;2022:8290526.
- O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-193–e193.
- Batton DG, Barrington KJ, Wallman C. Prevention and management of pain in the neonate: an update. American academy of pediatrics committee on fetus and newborn; American academy of pediatrics section on surgery; Canadian paediatric society fetus and newborn committee. Pediatrics. 2006;118(5):2231–2241.
- Yiğit Ş, Ecevit A, Altun Köroğlu Ö. Turkish neonatal society guideline on the neonatal pain and its management. Turk Pediatri Ars. 2018;53(Suppl 1):S161–S171.
- Miller DL, O'Grady NP, Society of Interventional Radiology Guidelines for the prevention of intravascular catheter-related infections: recommendations relevant to interventional radiology for venous catheter placement and maintenance. J Vasc Interv Radiol. 2012;23(8):997–1007.
- Taddio A, Lee C, Yip A, et al. Intravenous morphine and topical tetracaine for treatment of pain in preterm neonates undergoing Central line placement. JAMA. 2006;295(7):793–800.
- Paulson PR, Miller KM. Neonatal peripherally inserted Central catheters: recommendations for prevention of insertion and postinsertion complications. Neonatal Netw. 2008;27(4):245–257.
- AAP Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine Prevention and management of procedural pain in the neonate: an update. Pediatrics. 2016;137(2):e20154271.
- Hulse N, Paul AS. Warm povidone-iodine for surgical skin preparation. Ann R Coll Surg Engl. 2005;87(6):476.
- Lai NM, Taylor JE, Tan K, et al. Antimicrobial dressings for the prevention of catheter-related infections in newborn infants with Central venous catheters. Cochrane Database Sys Rev. 2016;3(3):CD011082.
- Johnson SB. Warming povidone-iodine solution for skin preparation[letter. Anesth Analg. 1978;57(1):142.
- Leung MP, Bishop KD, Monga M. The effect of temperature on bactericidal properties of povidone-iodine solution (10. %)Am J Obstet Gynecol. 2002;186(5):869–871.
- Maloney T, O'Neill B. Stability of povidone-iodine antiseptic solution stored at 37 degrees C. Med J Aust. 1986;144(7):389–389.
- Gezer S, Yalvaç HM, Güngör K, et al. Povidone-iodine vs chlorhexidine alcohol for skin preparation in malignant and premalignant gynaecologic diseases: a randomized controlled study. Eur J Obstet Gynecol Reprod Biol. 2020;244:45–50.
- Shen Q, Huang Z, Leng H, et al. Efficacy and safety of non-pharmacological interventions for neonatal pain: an overview of systematic reviews. BMJ Open. 2022;12(9):e062296.