Abstract
Objective
Surgery for placenta accreta spectrum disorders is known to be associated with urologic morbidity. Although previous studies have shown preoperative ureteral stent placement might be useful for preventing the urologic morbidity, the patient’s discomfort caused by it should not be ignored. Whether there is an alternative management strategy remains unknown. This study was to evaluate the effectiveness of ureteral stents and catheters in preventing urologic injury in patients with placenta accreta spectrum undergoing surgery.
Methods
We conducted a retrospective cohort study. All cases with diagnosed placenta accreta spectrum who underwent surgery at Peking University Third Hospital between January 2018 and December 2020 were collected and reviewed. They were divided into two groups according to the different management strategies for preoperative placement of ureteral catheters or stents. The primary outcome was urologic injury, which was defined as the presence of ureteral or bladder injury during and after surgery. Secondary outcomes included urologic complications within the first three months after surgery. The median (interquartile range) or proportions were reported for variables. The Man Whitney U test, chi-square test and multivariate logistic regression were used for analysis.
Results
Ultimately, 99 patients were included in this study. Ureteral catheters were placed in 52 patients and ureteral stents were placed in 47 patients. Placenta accreta, placenta increta, and placenta percreta were diagnosed in three, 19, and 77 women, respectively. The hysterectomy rate was 52.53%. Overall, urologic injuries occurred in three patients (3.03%), including one case of combined bladder and ureteral injury (1.01%) and two cases of bladder injuries (2.02%). Only one ureteral injury occurred in a patient with a ureteral stent, which was recognized postoperatively (p = .475). All bladder injuries were vesical rupture which were recognized and repaired intraoperatively; one patient in the catheter group and two patients in the stent group (p = .929). After adjusting for confounding variables, multinomial regression analysis revealed no significant differences between the two groups in the incidence of bladder injuries(aOR: 0.695, 95% CI: 0.035–13.794, p = .811). A lower risk of urinary irritation (aOR: 0.186, 95% CI: 0.057–0.605, p = .005), hematuria (aOR: 0.011, 95% CI: 0.001–0.136, p < .001), and lower back pain (aOR: 0.075, 95% CI: 0.022–0.261, p < .001) was found in patients with ureteral catheters than in those with ureteral stents.
Conclusion
The ureteral stents didn’t confer a protective benefit in the surgical management for placenta accreta spectrum compare with catheters; however, they did result in a higher incidence of postoperative urologic complications. Ureteral temporal catheters may be an alternative strategy for placenta accreta spectrum cases suspected with urinary tract involved prenatally. Moreover, clearly and explicitly reporting “double J stent” or “temporal catheter” is necessary for future researches.
Introduction
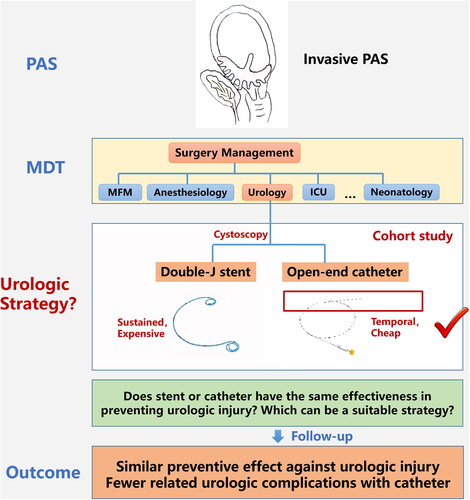
Placenta accreta spectrum (PAS) refers to a group of diseases in which the placental tissue invades the myometrium, and in severe cases, other adjacent organs such as the bladder and rectum can be involved [Citation1,Citation2]. PAS is known to be associated with significant maternal morbidity and mortality due to severe hemorrhage [Citation3]. In addition, urologic injury is also an important complication of concern because of direct invasion and surgical dissection of tissues [Citation4].
The incidence of unintentional urologic injury in hysterectomy for PAS was reported to be 29% in a systematic review, of which 78% involved the bladder and 17% involved the ureter [Citation5]. Currently, guidelines generally agree that multidisciplinary management of PAS, including urologist involvement in the placement of ureteral stents or catheters, can reduce the incidence of adverse outcomes [Citation6–9]. Ureteral stenting in PAS surgery originated from the strategy of radical surgery for gynecological cervical cancer and was also an empirical practice in the early stage of PAS treatment. Double-J stent is a common method in urologic management [Citation10]. Due to its special design, it can be placed for a longer period of time to avoid delayed ureteral injury but may also be associated with higher postoperative urologic complications [Citation11]. Catheter is another method that can be used for intraoperative identification of the ureter. It is often temporary and removed immediately after surgery, with relatively fewer postoperative complications [Citation12]. Whether ureteral catheters can be used as an alternative to stents in the perioperative management of PAS is unknown.
Therefore, we conducted a retrospective cohort study to evaluate the effectiveness of ureteral stents and catheters in preventing urologic injury in patients with PAS undergoing surgery to provide a reference for clinicians.
Materials and methods
This retrospective cohort study included patients with PAS who underwent cesarean section at the Department of Obstetrics and Gynecology of Peking University Third Hospital from January 2018 to December 2020.
Patients suspected of having PAS were subjected to prenatal ultrasonic evaluation using a specific scoring system [Citation13]. The scoring system is a combined set of generally recognized ultrasonic signs that can be used to assess the severity of placental invasion. A previous study indicated that when an ultrasonic score is greater than or equal to 10 points, the risk of blood loss equal to or greater than 1500 mL or performing hysterectomy increases significantly [Citation14]. Based on the prenatal assessment, an individualized surgical plan was developed. Cystoscopy was performed preoperatively to assess bladder invasion, followed by placement of a ureteral catheter or stent. The management of invasive PAS in our center mainly has two plans: one is hysterectomy with placenta in situ which is for some severe cases, and the other is uterus preservation. For the latter plan, we often remove the placenta during the operation, and various hemostatic techniques will be applied.
The diagnosis was confirmed based on intraoperative findings or pathological examination. Intraoperative findings included: (1) the placenta does not detach normally from the uterus after delivery of the fetus; thus, manual removal is needed; and (2) obvious signs of placenta percreta with thin myometrium and uterovescical hypervascularity. (3) Pathological confirmation was invasion of placental villi into the myometrium on the placenta or hysterectomy specimen. The inclusion criteria were as follows: (1) preoperative ultrasonic score ≥ 10 points, (2) delivery by cesarean section in the aforementioned hospital, (3) preoperative ureteral catheter or stent placement, and (4) diagnosis of PAS confirmed by intraoperative findings or pathological examination.
This study was approved by the Human Ethics Committee of Peking University Third Hospital. All participants provided informed consent to participate in the study, and all methods were carried out in accordance with relevant guidelines and regulations.
Patients were divided into two groups according to the different management strategy for preoperative placement of ureteral catheters or stents, which could be obtained through the electronic medical record system. The stents were Perc flex plus double-J stents, and the catheters were open-end catheters.
The primary outcome was urologic injury, which was defined as the presence of ureteral or bladder injury during and after surgery. Secondary urologic outcomes included urologic complications such as urinary tract infection, urinary irritation, dysuria, hematuria, lower back pain, urinary calculus within the first 3 months after surgery, and readmission for urologic complications. Data were collected from medical records and was supplemented with telephone follow-up.
Demographic and clinical characteristics included maternal age, gestational age at delivery, gravidity, parity, PAS type, PAS ultrasonic score, intraoperative estimated blood loss, surgical procedure, and ureteral stent placement duration.
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) v.22.0. The median and interquartile range (IQRs) were calculated for continuous variables (age, gestational age, gravidity, ultrasonic score, and estimated blood loss) and compared between the two groups using the Mann-Whitney U test. We used proportions to describe the distribution of categorical variables (parity, type of PAS, hysterectomy rate) and examined the differences between the two groups using the chi-square test. Outcomes were further evaluated using multivariate logistic regression models adjusted for potential confounding factors, which showed significant differences in univariate analysis. Adjusted odds ratios (aORs) with 95% confidence intervals (CIs) were also calculated. A subgroup analysis of urologic complications based on the duration of ureteral stent placement was also performed to compare stents removed within 30 days after surgery to those removed > 30 days after surgery. A two-tailed p value of < .05 was considered statistically significant.
Results
In total, 113 women met the inclusion criteria. Of all the cases, 14 were lost to follow-up. Ultimately, 99 patients were included in this study. Ureteral catheters were placed in 52 patients, and ureteral stents were placed in 47 patients ().
Figure 1. Process of clinical data collection and selection. Women meeting the inclusion criteria were selected and then followed up. PAS: placenta accreta spectrum.

The baseline clinical characteristics of each group are shown in . Placenta accreta, placenta increta, and placenta percreta were diagnosed in three, 19, and 77 women, respectively. The median estimated blood loss was 2000 mL. The hysterectomy rate was 52.53% (52/99). The median ureteral stent placement duration was 38 days.
Table 1. Characteristics of the included women at baseline.
There were no significant differences in maternal age, gestational age at delivery, parity, estimated blood loss during the operation, or hysterectomy rate between the two groups. However, women who underwent preoperative placement of ureteral stents had more pregnancies, higher ultrasonic scores for predicting the risk of PAS, and more severe types of PAS.
The details of the urologic injuries and complications are presented in . Overall, three of 99 patients (3.03%) experienced urologic injuries, including one case of combined bladder and ureteral injury (1.01%) and two cases of bladder injuries (2.02%). Only one ureteral injury occurred in a patient with a ureteral stent, which was recognized to have ureteral stricture postoperatively due to the patient’s persistent lower back pain and repaired by secondary operation of ureteral reimplantation (p = .475). All bladder injuries were vesical rupture which were recognized and repaired intraoperatively; one patient (1.92%) was in the catheter group and the other two patients (4.26%) were in the stent group (p = .929). After controlling for potential confounding factors of gravidity, PAS ultrasonic score, and PAS type, no significant differences were found between the two groups in the incidence of bladder injuries (aOR: 0.695, 95% CI: 0.035–13.794, p = .811).
Table 2. Primary and secondary outcomes.-n (%).
The overall rate of urologic complications was 28.85% for patients with catheters and 89.36% for those with stents (p < .001). Univariate analysis showed that there were significant differences in urinary tract infection (1.92% vs. 19.15%), urinary irritation (15.38% vs. 59.57%), dysuria (3.85% vs. 19.15%), hematuria (1.92% vs. 42.55%), and lower back pain (21.15% vs. 78.72%) between the stent and catheter groups (p < .05). In the multivariate analyses, a lower risk of urinary irritation (aOR: 0.186, 95% CI: 0.057–0.605, p = .005), hematuria (aOR: 0.011, 95% CI: 0.001–0.136, p < .001), and lower back pain (aOR: 0.075, 95% CI: 0.022–0.261, p < .001) was found in patients with ureteral catheters than in those with ureteral stents. In addition, six patients in the stent group were readmitted for urinary complications (two with urinary tract infection, two with hematuria, one with dysuria, and one with lower back pain), while no readmission occurred in the catheter group (p = .025).
A subgroup analysis of urologic complications based on the duration of ureteral stent placement is shown in . Stents were removed within 30 days after surgery in 12 patients (25.53%) and > 30 days after surgery in 35 patients (74.47%). There were no significant differences in the rates of urologic complications between the two groups.
Table 3. Subgroup analysis of urologic complications.-n(%).
Discussion
In this retrospective cohort study, the preoperative placement of ureteral catheters and stents showed no significant difference in preventing urologic injuries in patients with PAS undergoing surgery. However, ureteral stent placement was associated with a higher risk of postoperative urologic complications.
The overall rate of unintentional urologic injury during peripartum hysterectomy for PAS was reported to be 29% (83/285) [Citation5], which was higher than the rate for hysterectomies for other gynecological indications, which was 1.0–4.3% [Citation15,Citation16]. In cesarean delivery with PAS, urologic injury was described as 8.3% vs 0.2% comparing with non-PAS. Moreover, 5.2% for accreta, 11.8% for increta, 24.5% for percreta, which showed the more severe the invasion, the higher the rate of injury [Citation1] Citation7 .Intraoperative identification of the ureters can be difficult in some cases of cesarean hysterectomy for PAS because of the placental mass effect or massive bleeding. Although direct invasion is uncommon, surgical extirpation of the invasive retroperitoneal placental tissue may also result in urologic injury. In our study, the rate of urologic injury was 3.03%, which is lower than the reported rate. This might be due to the different characteristics of the included populations and operation procedures. In addition, some surgeons prefer deliberate cystotomy and bladder excision to bladder dissection in cases of deep invasion to avoid massive blood loss [Citation18,Citation19]. However, this procedure was not performed routinely in our management strategies.
Moreover, the rate of ureteral injury was only 1.01% (1/99). Similarly, Felice et al. reported that the rate of ureteral injury was zero (0/44) in cesarean hysterectomy for placenta accreta in 2021 [Citation20]. The result was inconsistent with the reported rate of 5% in the systematic review in 2012. This result may be attributed to precise preoperative evaluation and improved surgical skills over the past decade. In addition, ureteral catheters or stents were used preoperatively in invasive cases of our study, which may also contribute to the low rate of ureteral injury.
Ureteral catheters and stents facilitate the palpation and identification of the ureters in cases in which the placenta has invaded the parametrium. In addition, double-J ureteral stents can be placed during surgery and left in situ for a longer time, which may prevent sustained or delayed injury. Current evidence indicates that prophylactic ureteral catheter placement has the advantage of reducing ureteral injury in laparoscopic gynecological surgery, especially with pelvic adhesions [Citation12,Citation21,Citation22]. Therefore, ureteral catheterization serves as one of the strategies for PAS preoperative management. Both ureteral catheters and stents are used clinically, and their choice depends on physician preference. The 2018 International Federation of Gynecology and Obstetrics (FIGO) guidelines report that in the United States, 26.2–35% of maternal-fetal medicine specialists and 26.3% of American College of Obstetricians and Gynecologists (ACOG) fellows use ureteral catheters or stents in the perioperative management of placenta accreta spectrum and recommend cystoscopy and the use of ureteral stents when bladder invasion is considered in prenatal imaging [Citation6]. The 2018 ACOG and Society for Maternal-Fetal Medicine (SMFM) updated guidelines for placenta accreta spectrum note that the role of ureteral stenting remains unclear for placenta accreta with significant bladder invasion assessed preoperatively, and individual case assessment and management are recommended [Citation8]. The 2018 Royal College of Obstetricians and Gynecologists (RCOG) and Society of Obstetricians and Gynecologists of Canada (SOGC) guidelines state that there is insufficient evidence to recommend the routine use of ureteral stents in patients with PAS; however, their use may be useful in cases of bladder invasion and should be considered for selective use [Citation7,Citation9]. The Perinatal Medicine Branch of the Chinese Medical Association suggest in the 2015 guidelines for the diagnosis and treatment of PAS disorders that the use of ureteral stents during hysterectomy in PAS can reduce the risk of ureteral injury, admission to the intensive care unit, and massive bleeding, but will increase the incidence of complications such as hematuria, lower back pain, and urinary irritation; therefore, benefits and risks should be weighed when making a decision [Citation23]. Generally speaking, the role of ureteral stents in preventing urologic injury is controversial. When we place prophylactic ureteral stents in selective cases of PAS, we have to consider whether there are alternative methods to assist in the identification of the ureter in surgery with lower postoperative urologic complications. The ureteral catheter is a temporal device with a lower cost that can also be placed preoperatively to indicate the position of the ureter. It is removed immediately after surgery without a second cystoscopy, which may lower the incidence of urologic complications related to stent placement.
In addition, it should be noted that ureteral ‘stents’ or ‘catheters’ are both used in the current literatures without clear descriptions, which might be ambiguous for readers. It is suggested that the terms of ureteral stents (double J tube) and temporal catheters should be clarified more clearly in the studies, which is conducive to the future researches on urinary system strategies.
Ureteral catheters with similar function but fewer complications and lower costs have not been investigated. To date, no study has compared the benefits and risks of ureteral catheters and stents in PAS surgery. Our study aimed to explore the choice of stents and catheters in perioperative urologic management strategies for patients with suspected invasive placenta accreta, suggesting that ureteral catheters perform similarly to stents in preventing intraoperative urologic injuries. The preoperative ultrasonic evaluation score was higher in the stent group than that in the catheter group, possibly because physicians were more likely to use stents in severely invasive cases to ensure that postoperative injuries could be detected. Higher ultrasonic scores suggest more severe cases, such patients may have more complicated surgery and may be at greater risk of urologic injury. To avoid confounding factors, we adjusted for ultrasonic scores and the invasion type in the multivariate analysis, which still showed no difference in the rate of urologic injury between the stent and catheter groups.
Due to the concern of delayed injuries, we kept the stents in situ for 1–3 months. In the subgroup analysis, there was no significant difference in the risk of urologic complications between stent placement times of more than 1 month and within 1 month; however, the overall incidence of postoperative urologic complications in the stent group was significantly higher than that in the catheter group. Since ureteral catheters are removed immediately after the operation, stents are placed for a long time in our center, and the high rate of postoperative urinary complications may also be related to long-term indwelling. Therefore, we suggest that catheters may be used as an alternative for intraoperative identification of the ureter in cases of suspected bladder or parametrium invasion. If a double-J stent is used, early stenting removal is also recommended. Ureteral temporal catheters may be a safe and economical alternative to ureteral stents and can be considered as a choice for patients suspected of having PAS to enable shared decision-making.
Few studies have investigated the urologic management strategies for PAS. A systemic review [Citation2] summarized the approaches which may reduce the urologic injury. The data showed that preoperative bilateral ureteral stent placement significantly reduced the risk of urologic complications at hysterectomy for PAS but did not entirely eliminate it. No similar study was conducted to explore the choice of stent or catheter. To the best of our knowledge, this is the first study to address the benefits and risks of ureteral stents and temporal catheters in surgery for PAS, with a relatively large sample size and comprehensive outcome assessment. The results of this study can provide a reference for urologic assistants chosen for the surgical management of PAS.
Limitations
Nevertheless, this study has some limitations, such as its retrospective, single-center design. This observational study had uncontrolled confounders. Generalization of our study is probably limited due to specific characteristics of patients and clinical skills of obstetricians in a single tertiary hospital. The secondary outcomes of postoperative urologic complications are mainly self-reported by patients, which may lead to recall bias. In addition, the criteria for diagnosing PAS are not uniform because of the absence of histological confirmation in some cases without hysterectomies. However, the lack of histological confirmation affects all studies investigating strategies other than hysterectomy. Therefore, our results should be cautiously interpreted.
Acknowledgements
We would like to thank the patients who participated in the study. Thanks to the multidisciplinary team of PAS for their meticulous work. Also, thanks to Editage (www.editage.cn) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [LC], upon reasonable request.
Additional information
Funding
References
- Silver RM, Branch DW. Placenta accreta spectrum. N Engl J Med. 2018;378(16):1529–1536. doi: 10.1056/NEJMcp1709324.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218(1):75–87. doi: 10.1016/j.ajog.2017.05.067.
- Jauniaux E, Bunce C, Gronbeck L, et al. Prevalence and main outcomes of placenta accreta spectrum: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221(3):208–218. doi: 10.1016/j.ajog.2019.01.233.
- Hoffman MS, Karlnoski RA, Mangar D, et al. Morbidity associated with nonemergent hysterectomy for placenta accreta. Am J Obstet Gynecol. 2010;202(6):628.e1–628.e5. doi: 10.1016/j.ajog.2010.03.021.
- Tamtam KB, Dozier J, Martin JN, Jr. Approaches to reduce urinary tract injury during management of placenta accreta, increta, and percreta: a systematic review. J Matern Fetal Neonatal Med. 2012;25(4):329–334. doi: 10.3109/14767058.2011.576720.
- Allen L, Jauniaux E, Hobson S, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: nonconservative surgical management. Int J Gynaecol Obstet. 2018;140(3):281–290. doi: 10.1002/ijgo.12409.
- Hobson SR, Kingdom JC, Murji A, et al. No. 383-Screening, diagnosis, and management of placenta accreta spectrum disorders. J Obstet Gynaecol Can. 2019;41(7):1035–1049. doi: 10.1016/j.jogc.2018.12.004.
- Society of Gynecologic Oncology; American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine, et al. Placenta accreta spectrum. Am J Obstet Gynecol. 2018;219:b2–B16.
- Jauniaux E, Alfirevic Z, Bhide AG, et al. Placenta praevia and placenta accreta: diagnosis and management: green-top guideline no. 27a. BJOG. 2019;126(1):e1–e48. doi: 10.1111/1471-0528.15306.
- Matsubara S, Kuwata T, Usui R, et al. Important surgical measures and techniques at cesarean hysterectomy for placenta previa accreta. Acta Obstet Gynecol Scand. 2013;92(4):372–377. doi: 10.1111/aogs.12074.
- Koprowski C, Kim C, Modi PK, et al. Ureteral stent-associated pain: a review. J Endourol. 2016;30(7):744–753. doi: 10.1089/end.2016.0129.
- Feng D, Tang Y, Yang Y, et al. Does prophylactic ureteral catheter placement offer any advantage for laparoscopic gynecological surgery? A urologist’ perspective from a systematic review and meta-analysis. Transl Androl Urol. 2020;9(5):2262–2269. doi: 10.21037/tau-20-674.
- Chong Y, Zhang A, Wang Y, et al. An ultrasonic scoring system to predict the prognosis of placenta accreta a prospective cohort study. Medicine. 2018;97(35):e12111. doi: 10.1097/MD.0000000000012111.
- Chen L, Shi Hui F, Jiang H, et al. Correlation of an ultrasonic scoring system and intraoperative blood loss in placenta accreta spectrum disorders: a retrospective cohort study. Biomed Environ Sci. 2021;34:163–169.
- Ibeanu OA, Chesson RR, Echols KT, et al. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10. doi: 10.1097/AOG.0b013e31818f6219.
- Merritt AJ, Crosbie EJ, Charova J, et al. Prophylactic pre-operative bilateral ureteric catheters for major gynaecological surgery. Arch Gynecol Obstet. 2013;288(5):1061–1066. doi: 10.1007/s00404-013-2853-5.
- Matsuzaki S, Mandelbaum RS, Sangara RN, et al. Trends, characteristics, and outcomes of placenta accreta spectrum: a national study in the United States. Am J Obstet Gynecol. 2021;225(5):534.e1–34.e38. doi: 10.1016/j.ajog.2021.04.233.
- Erfani H, Salmanian B, Fox KA, et al. Urologic morbidity associated with placenta accreta spectrum surgeries: single-center experience with a multidisciplinary team. Am J Obstet Gynecol. 2022;226(2):245.e1–245.e5.
- Woldu SL, Ordonez MA, Devine PC, et al. Urologic considerations of placenta accreta: a contemporary tertiary care institutional experience. Urol Int. 2014;93(1):74–79. doi: 10.1159/000356064.
- Crocetto F, Esposito R, Saccone G, et al. Use of routine ureteral stents in cesarean hysterectomy for placenta accreta. J Matern-Fetal Neonatal Medi. 2021;34(3):386–389. doi: 10.1080/14767058.2019.1609935.
- Tanaka Y, Asada H, Kuji N, et al. Ureteral catheter placement for prevention of ureteral injury during laparoscopic hysterectomy. J Obstet Gynaecol Res. 2008;34:67–72.
- Manoucheri E, Cohen SL, Sandberg EM, et al. Ureteral injury in laparoscopic gynecologic surgery. Rev Obstet Gynecol. 2012;5:106–111.
- Perinatal Medicine Branch of Chinese Medical Association: guidelines for the diagnosis and treatment of placenta accreta spectrum. Chin J Perinat Med. 2015;(7):481–485.