Abstract
Objective
Interleukin 22 (IL-22) plays a role in inflammatory diseases. However, whether IL-22 affects the function of ovarian granulosa cells (GCs) and its relationship with Polycystic Ovary Syndrome (PCOS)remains unclear.
Methods
We investigated the level of IL-22 in human follicular fluid using ELISA. The expression and localization of the IL-22 receptor 1 (IL-22R1) in GCs were investigated by RT-PCR and immunofluorescence staining, respectively. The proliferation of KGN cells (human GCs line) was assessed by CCK-8 assay and EdU assay after treatment with recombinant human IL-22 (rhIL-22) and lipopolysaccharide (LPS). Apoptosis was assessed using flow cytometry. Apoptotic proteins and steroidogenic genes were detected by western blotting.
Results
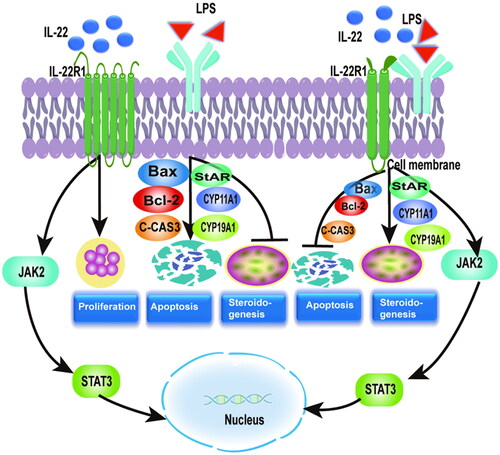
ELISA's results showed that compared with the control group, PCOS patients showed lower expression of IL-22 in follicular fluid. Immunofluorescence showed that IL-22R1 is expressed and localized in human granulosa cell membranes. IL-22 promoted cell proliferation and reversed LPS-induced inhibition of cell proliferation. IL-22 alone did not affect apoptotic or steroidogenic protein expression, however, it reversed LPS-induced apoptosis via downregulation of Bcl-2, upregulation of Bax and cleaved caspase-3, and restoration of LPS-downregulated StAR, CYP11A1, and CYP19A1 expression. Western blotting confirmed that IL-22 activated the JAK2/STAT3 signaling.
Conclusion
IL-22 promotes cell proliferation, inhibits apoptosis, and regulates KGN cell steroidogenesis confronted with LPS, and decreased IL-22 may be involved in the development of PCOS.
1. Introduction
Polycystic ovary syndrome (PCOS) is an endocrine and metabolic disorder characterized by anovulation, hyperandrogenemia and polycystic ovarian morphology (PCOM). Although PCOS affects 5–10% of women of reproductive age globally, its precise pathogenesis remains unclear [Citation1,Citation2]. In recent years, low-grade inflammation has indeed been pointed out as a serious risk factor for PCOS [Citation3–5], however, its role in cell survival remains controversial. Kaipia et al. suggested that inflammatory signaling promotes follicle apoptosis [Citation6], whereas Sasaki et al. found that proinflammatory cytokines promoted granulosa cells (GCs) survival [Citation7]. In addition, studies have demonstrated that abnormal steroidogenesis was closely associated with inflammation [Citation8]. GCs are steroidogenic cells that surround oocytes and produce hormones to maintain a suitable microenvironment for oocyte maturation, which is essential for folliculogenesis [Citation9,Citation10]. Aberrant function of GCs is implicated in the pathogenesis of PCOS.
Interleukin-22 (IL-22), a cytokine that belongs to the IL-10 family, is primarily secreted by hematopoietic cells such as T helper 17 (Th17) and innate lymphoid cells (ILCs) [Citation11]. By connecting to the IL-22 receptor, IL-22 initiates the signal transduction cascades that quickly activate several transcription factors, including the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) proteins [Citation12,Citation13]. IL-22 is crucial for mediating inflammation, cell growth, and host defense [Citation14]. This cytokine plays a complex and diverse role in inflammation. It has been demonstrated to exert an anti-inflammatory role in chronic inflammatory states such as diabetes and insulin resistance [Citation15,Citation16], which share endocrinological features with polycystic ovary syndrome. Further, exogenous IL-22 treatment has been demonstrated to reduce tissue damage and cell death caused byinflammation [Citation17–20]. In contrast, IL-22 has been associated with the etiology of inflammatory diseases such as rheumatoid arthritis, and psoriasis [Citation21,Citation22]. A recent study revealed that reduced IL-22 levels were observed in the follicular fluid of patients with PCOS and were associated with abnormal levels of amino acids and glycolipid metabolites [Citation23]. These findings indicate that decreased IL-22 may contribute to the etiology of PCOS. However, whether IL-22 affects GCs function is still unknown. Therefore, we aimed to confirm the alteration of IL-22 levels in follicular fluid of PCOS and investigate the effects of IL-22 on ovarian GCs proliferation, apoptosis, and steroidogenesis and its role confronted with inflammation caused by LPS.
2. Materials and methods
2.1. Patient selection Sample Collection
This study was approved by the Ethics Committee of the First Hospital of Chongqing Medical University. And informed consent was obtained from all participants. Thirty participants were recruited from the reproductive medicine center of the First Affiliated Hospital of Chongqing Medical University. All participants were undergoing in vitro fertilization/intracytoplasmic sperm injection and embryo transfer (IVF/ICSI-ET). This included 15 PCOS patients and 15 healthy women as controls. All patients with PCOS were diagnosed based on the presentation of any two of the three essential Rotterdam diagnostic criteria [Citation24], clinical/biochemical hyperandrogenism, polycystic ovarian morphology, and chronic anovulation. The control group included women with infertility caused by tubal or male factors. Women with gynecological or other systemic diseases such as premature ovarian failure, endometriosis, diabetes, thyroid dysfunction, hypertension, and other manifestations of hyperandrogenism were excluded. Before collecting participant follicular fluid and ovarian GCs samples, basic clinical information on the participants was collected.
2.2. Controlled ovarian hyperstimulation and sample collection
All subjects underwent controlled ovarian hyperstimulation using the fixed GnRH antagonist stimulation regimen protocol. Ovulation was promoted with recombinant follicle stimulating hormone (Merck, Switzerland) 150–225 U/d starting on day 2 of menstruation, and cetrorelix acetate (Merck, Switzerland) 0. 25 mg/d was added on day 5 of ovarian stimulation until the trigger day. When three or more follicles ≥17 mm in diameter were observed, 6000 IU of urine human chorionic gonadotrophin (uHCG, Lizhu Pharmaceuticals, China) was injected if the estrogen level was less than 5000 pg/ml, 10,000 IU of uHCG was injected if the estrogen level was between 5000 and 10,000 pg/ml, and was excluded if the estrogen level was greater than 10,000 pg/ml. The egg retrieval procedure was performed after 36 h. At the time of oocyte retrieval, follicular fluid was collected in 15 ml sterile tubes and centrifuged at 1500 × g for 10 min. The upper layer of clear and clarified liquid was the follicular fluid. And add 1 ml of hyaluronidase (Solarbio-Life-Sciences,Beijing,China) to the sterile tube, resuspend the cells at the bottom of the tube, and bathe in water at 37 °C for 20 min. Then, the luteinized granulosa cells were separated from red blood cells with Ficoll-Percoll (Solarbio-Life-Sciences, Beijing, China), centrifuged at 1500 × g for 10 min, aspirated the cloudy cell mass in the sterile tube. Follicular fluid and ovarian granulosa cells without blood contamination were collected and stored at −80 °C.
2.3. Enzyme-Linked immunosorbent assay (ELISA)
A human IL-22 ELISA kit (Bo Pei Science, Chongqing, China) was used to determine the concentration of IL-22 in follicular fluid samples in accordance with the manufacturer’s instructions. Follicular fluid samples were retrieved from patients with PCOS (n = 15) and healthy controls (n = 15). We first performed a pre-experiment using samples from the PCOS group (n = 3) control group (n = 3) to verify that the ELISA kit for IL-22 can be used for follicular fluid. The measured concentrations were within the range of the human IL-22 kit (10 pg/ml–320pg/ml) and the results were like those reported articles [Citation25,Citation26], we continued the experiment using samples from the PCOS group (n = 15) control group (n = 15). The intra- and inter-assay coefficients of variation for the IL-22 assay were 4.15% and 5.21%, respectively. The minimum detectable amount is 10 pg/ml.
2.4. Reverse transcription-polymerase chain reaction (RT-PCR)
Following the manufacturer’s recommendations, total RNA was isolated from GCs samples using TRIZOL reagent (Roche, Basel, Switzerland). cDNA was generated using a first Strand cDNA Synthesis Kit from 1 g of RNA (Takara Bio, Dalian, China). The reaction was conducted under the following conditions: 5 min at 95 °C, 40 cycles of 10 s at 95 °C, 20 s at 64 °C, and 20 s at 76 °C. After each endpoint amplification, melting curves were produced for 10 s at 95 °C, then the temperature was increased by 0.5 °C every 30 s from 65 to 95 °C. GAPDH and IL-22R1 fragments were amplified using specific primers (Takara Bio, Dalian, China). On a 3% agarose gel, the amplified PCR products were visible.
GAPDH:
Forward primer (5′->3′) AAGGCTGAGAACGGGAAGC
Reverse primer (5′->3′) TCGCCCCACTTGATTTTGGA
IL22R1:
Forward primer (5′->3′) TGTTCATCCTACCCCCACGC
Reverse primer (5′->3′) TCTCTGCTTCCCTCCAAGGTG
2.5. Cell Culture and treatment
The human ovarian granulosa cell line, KGN, was purchased from the American Type Culture Collection (ATCC, Manassas, VA). The cells were maintained in DMEM/F12 medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 g/mL streptomycin (both from Beyotime, Shanghai, China) at 37 °C in a humid environment with 5% CO2. Every two to three days, cells were digested with 0.05% trypsin and 0.02% EDTA in a trypsin-EDTA solution (Beyotime, Shanghai, China), and then resuspended and inoculated into 6 cm culture dishes. KGN cells were separated into four groups at random and used for western blotting and EdU tests.
Control group: KGN cells were left untreated.
Lipopolysaccharide (LPS) group: after reaching 60-70% confluence, KGN cells were administered with 1μg/mL LPS (Sigma-Aldrich, St Louis, MO) for 24 h.
The IL-22 group was given 10 ng/mL rhIL-22 (R&D Systems, Minneapolis, MN) for 24 hours to treat KGN cells.
IL-22 + LPS group: KGN cells were treated with 10 ng/mL rhIL-22 for 24 hours after being pretreated with 1ug/mL LPS for 24 hours.
2.6. Immunofluorescence staining
Immunofluorescence assay was performed on KGN cells to determine the localization of IL-22R1. Cells were fixed and permeabilized on slides with 4% paraformaldehyde and 0.5% Triton-X100 for 5 min, blocked with 5% BSA at room temperature (18-21 °C), and then incubated with anti-IL-22R1 antibody (Abcam, Cambridge, UK) at a 1:100 dilution overnight at 4 °C. The cells were then stained with Slow Fade Gold Anti-Fade Reagent (Beyotime, Shanghai, China), which contains DAPI, the next day after being incubated with fluorescein-conjugated rabbit IgG secondary antibody for 1 h. Images were captured at a magnification of about 200x using a laser scanning confocal microscope (Nikon, Tokyo, Japan)
2.7. Cell counting kit-8 (CCK-8) assay
KGN cells were planted at a density of 5 × 103 cells per well in 96 well plates. Each group of cells (control, IL-22, LPS, IL-22 + LPS) was prepared in replicates of six. Cells were treated with increasing doses of rhIL-22 (0, 1, 2, 5, and 10 ng/mL) (R&D Systems, Minneapolis, MN) for different durations (3, 6, 12, and 24h). Cells were treated with 10 l of CCK8 solution (Beyotime, Shanghai, China) per the manufacturer’s instructions for 2 h at 37 °C. At 450 nM, absorbance was measured. Six replicates per group were used in each of the three independent experiments.
2.8. 5-ethynyl-20-deoxyuridine (EdU) assay
A 5-ethynyl-20-deoxyuridine (EdU) assay kit was used to determine the proliferation of KGN cells following treatment with 10 ng/mL rhIL-22 and/or 1 g/mL LPS for each group (Beyotime, Shanghai, China). KGN cells were treated with 50 mol/l EdU according to the manufacturer’s instructions, incubated for 1 h, and then fixed in 4% paraformaldehyde for 10 min. The cells were then subjected to three PBS washes, a 10-min treatment with 0.5% Triton X-100 in phosphate buffer, a 5-min treatment with 2 mg/mL glycine, three PBS washes, and a 30-min staining with Apollo dye at room temperature without exposure to light. DAPI was used as a counterstained for nuclei. Zeiss fluorescence microscopy and Image J software were used to capture images and evaluate cellular EdU binding. Three independent experiments were performed, with three replicates per group. Scale bar: 100 µm.
2.9. Flow cytometry analysis
Flow cytometry was used to assess cell apoptosis using an Annexin V-FITC kit (Beyotime, Shanghai, China). First, KGN cells were washed three times and suspended in a phosphate buffer solution. 100 ul of cell suspension was then mixed with 10 ul of protein V-FITC solution, and the mixture was incubated for 20 min at room temperature (18–21 °C). Propidium iodide (PI) dye was then applied and left to sit for 20 min. In order to identify apoptotic cells, fluorescent signals were detected using a flow cytometer (BD Biosciences, San Jose, CA). Three independent experiments were performed, with three replicates per group.
2.10. Western blotting
After treatment with rhIL-22 and/or LPS, KGN cells were lysed on ice using RIPA lysis solution containing protease and phosphatase inhibitors (Beyotime, Shanghai, China) for 20 min. A bicinchoninic acid (BCA) protein assay kit was used to quantify the total protein content in accordance with the manufacturer’s instructions (Beyotime, Shanghai, China). Subsequently, 20ul of protein was then placed into the SDS-PAGE gel’s wells and electrophoretically transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Boston, MA). Antibodies against β-actin, caspase-3, cleaved caspase-3, Bcl-2-associated X protein (Bax), B-cell lymphoma 2 (Bcl-2), StAR (Abcam, 1:1000), CYP11A1, CYP19A1, STAT3, phosphorylated (P)-STAT3, JAK2, and phosphorylated (P)-JAK2 were incubated on the membranes for an overnight period at 4 °C (Cell Signaling, Beverly, MA, 1:1000).
The membranes were then treated with horseradish peroxidase-labeled secondary rabbit anti-mouse IgG or goat anti-rabbit IgG (Zhongshan jin qiao, 1;5000) antibody for 1 h after being washed at least three times with PBS (Beyotime, Shanghai, China). Bands were then developed using enhanced chemiluminescence reagents (Millipore) and quantified using ImageJ. Target protein's relative expression was scaled to the density of the β-actin band.
2.11. Statistical analysis
Normality of the distribution of clinical characteristics was obtained by the Kolmogorov-Smirnov test using SPSS software (version 26 SPSS Software, Chicago, IL, USA. The t-test is used for normally distributed data and the Mann-Whitney test for non-normally distributed data. The experimental data were analyzed using Graph Pad Prism software (version 9.0 Graph Pad Software, San Diego, CA). The mean and standard error of the mean are displayed for all data (SEM). Student’s t-test was used to compare two groups, while one-way ANOVA and Tukey’s multiple comparison test were used to compare multiple groups. Statistical significance was defined as p-value < 0.05.
3. Results
3.1. Clinical and biochemical characteristics of subjects
Thirty participants (15 PCOS patients and 15 healthy women as controls) were recruited for this study. displays the major clinical characteristics of the PCOS patients and the control group.
Table 1. Clinical and biochemical characteristics of PCOS and control women.
Between PCOS and controls, there were no appreciable differences in age, body mass index (BMI), follicle-stimulating hormone (FSH), estrogen (E2), or testosterone (T) between PCOS and controls. However, the PCOS patients had higher levels of the hormones luteinizing hormone (LH) (p = 0.004), anti-Müllerian hormone (AMH) (p = 0.001), and antral follicle count (AFC) (p = 0.001) ().
3.2. IL-22 was decreased in the follicular fluid of PCOS patients and may exert biological effects on ovarian GCs through IL-22R1
We compared the level of IL-22 in follicular fluid between the PCOS and control groups using ELISA. According to the findings, individuals with PCOS had significantly lower levels of IL-22 than those in the control group (25.85 ± 0.97 pg/mL vs. 29.47 ± 1.11 pg/mL, p < 0.05) (). Using RT-PCR, the mRNA expression of IL-22R1 in ovarian GCs of PCOS patients was determined (). The protein expression of IL-22R1 was localized in the cell membrane of KGN cells, according to immunofluorescence (IF) (). These data suggest that decreased levels of IL-22 in the follicular fluid are associated with PCOS.
Figure 1. Patients with PCOS had lower levels of IL-22 in their follicular fluid, and IL-22R1 protein localization was detected in ovarian GCs. (a) IL-22 protein expression in follicular fluid of the control (n = 15) and PCOS (n = 15) groups as determined via ELISA assay. *p < 0.05. (b) RT-PCR findings demonstrating IL-22R1 mRNA expression in human GCs (n = 3). (c) Immunofluorescence staining indicating the location of IL-22R1 in KGN cells. The positive expression of IL-22R1 in cell membranes is represented by green fluorescence, and the DAPI-labelled nuclei in blue indicate that IL-22R1 is localized in the cell membrane of human ovarian GCs. Scale bar: 100 μm.

3.3. IL-22 promoted the proliferation of KGN cells and reversed the inhibitory effect of LPS
We performed CCK8 and EdU cell assays to determine the influence of IL-22 on the proliferation of KGN cells. For 3, 6, 12, and 24 h, the cells were pretreated with rhIL-22 at various doses (0, 1, 2, 5, and 10 ng/mL). CCK8 assay revealed that IL-22 administration enhanced the proliferation of KGN cells in a dosage- and time-dependent manner. Proliferation levels were highest at concentrations of 5 ng/mL and 10 ng/mL IL-22, which were considerably greater than those in the control group at 12 and 24 h (p < 0.0001, p < 0.0001) (). For subsequent experiments, we selected 10 ng/mL as the effective dose of rhIL-22. EdU results showed that IL-22 treatment alone promoted the proliferation of KGN cells in comparison to the control group (IL-22 vs. Control:0.32 ± 0.009 vs. 0.27 ± 0.009, p < 0.05), which was consistent with the CCK8 assay results. LPS treatment alone inhibited the proliferation of KGN cells as compared with the control group (LPS vs. Control: 0.21 ± 0.006 vs. 0.27 ± 0.009, p < 0.01). Moreover, IL-22 reversed the inhibition of proliferation by LPS (LPS vs. LPS + IL-22: 0.21 ± 0.006 vs. 0.25 ± 0.008, p < 0.05) ().
Figure 2. IL-22 promoted proliferation in KGN cells and reversed the inhibitary effect of LPS. There were four treatment groups for cells: Control; LPS treated; rhIL-22 treated; LPS and rhIL-22 treated. (a) Graph showing the viability of KGN cells treated with different concentrations (0, 1, 2, 5, and 10 ng/mL) of IL-22 measured using CCK8 assay at different time points (3, 6, 12, and 24 h). ****p < 0.0001. (b) Representative images showing the proportion of proliferating KGN cells treated with LPS and/or IL-22 in the EdU assay. Cell nuclei were detected by Hoechst staining (blue) and proliferating cells by EdU staining (red). the results were analyzed using ImageJ. Scale bar: 50 μm. (c) Statistical graph showing the proportion of EdU-positive cells. Values are expressed as the mean ± SEM, n = 3. All experiments were performed in triplicate. *p < 0.05, **p < 0.01.

3.4. IL-22 reversed the pro-apoptotic effects of LPS in KGN cells
Flow cytometry and western blotting were employed to investigate how IL-22 influenced the apoptosis of KGN cells (). Flow cytometry indicated that, in comparison to the control group, LPS treatment alone vastly improved the ratio of apoptotic KGN cells. (LPS vs. Control: 16.07%±1.15 vs. 6.06%±0.68, p < 0.001). There was a tendency for rhIL-22 treatment to decrease the percentage of apoptotic cells in comparison to the control group, but the difference was not statistically significant (IL-22 vs. Control: 3.89%±0.84 vs6.06%±0.68, (p > 0.05). Cell apoptosis caused by LPS was significantly decreased by rhIL-22 pretreatment. (LPS vs. LPS + IL-22: 16.07%±1.15 vs. 10.66% ±1.15, (p < 0.05) (). western blotting was performed to further confirm the expression of Bax, Bcl-2, caspase-3, and cleaved caspase-3 to ascertain the impact of IL-22 on apoptosis-related proteins. As shown in , the LPS group had significantly higher levels of Bax and cleaved caspase-3 expression than the control group, while Bcl-2 expression was lower. (LPS vs. Control: Bax: p < 0.01; Bcl-2: p < 0.01; cleaved caspase-3: p < 0.01). IL-22 did not affect the expression of Bcl-2, Bax, and cleaved caspase-3 individually. However, Bcl-2 expression was up-regulated in the rhIL-22 pretreated LPS group, while Bax and cleaved caspase-3 were significantly downregulated (LPS vs. LPS + IL-22: Bax: p < 0.05; Bcl-2: p < 0.05; cleaved caspase-3: p < 0.01) (). These findings imply that IL-22 reverses the pro-apoptotic signaling of LPS in KGN cells.
Figure 3. IL-22 reversed the pro-apoptotic role of LPS in KGN cells. There were four treatment groups for cells: Control; LPS treated; rhIL-22 treated; LPS and rhIL-22 treated. (a) Representative images of flow cytometry in each group. (b) Graph showing statistical analysis using flow cytometry (). Values are expressed as the mean ± SEM, n = 3. * p < 0.05, *** p < 0.001. (c) The expression levels of apoptosis-related proteins Bax, Bcl-2, caspase-3 and cleaved caspase-3 were evaluated by western blotting in the different groups. (d–f) Graph showing statistical analysis of western blotting (). Values are expressed as the mean ± SEM, n = 3. *p < 0.05, **p < 0.01, ns: nonsignificant. All experiments were performed in triplicate. Values are expressed as the mean ± SEM, n = 3.

3.5. IL-22 reversed LPS-mediated inhibition of steroidogenic genes in KGN cells
GCs are critical for the development and selection of follicles, due to their crucial function in steroidogenesis. Therefore, we investigated the involvement of IL-22 in the regulation of steroidogenic genes in KGN cells. According to our data, LPS administration decreased the expression of StAR, CYP11A1, and CYP19A1 proteins in KGN cells (LPS vs. Control StAR: p < 0.0001; CYP11A1: p < 0.01; CYP19A1: p < 0.05). rhIL-22 administration had no discernible impact on StAR, CYP11A1, or CYP19A1 expression (IL-22 vs. Control StAR: ns; CYP11A1: ns; CYP19A1: ns). However, it significantly restored the LPS-induced downregulation of StAR, CYP11A1, and CYP19A1 (LPS vs. LPS + IL-22 StAR: p < 0.001; CYP11A1: p < 0.05; CYP19A1: p < 0.05, ).
Figure 4. IL-22 reversed LPS-mediated inhibition of steroidogenic genes in KGN cells. There were four treatment groups for cells: Control; LPS treated; rhIL-22 treated; LPS and rhIL-22 treated. (a) The expression levels of steroidogenesis-related proteins StAR, CYP11A1, and CYP19A1 were assessed by western blotting in the different groups. (b–d) Graph showing the statistical analysis of western blotting results in . Values are expressed as the mean ± SEM, n = 3. *p < 0.05, **p < 0.01 ***p < 0.001, ****p < 0.0001 ns: nonsignificant. All experiments were performed in triplicate.

3.6. IL-22 activated p-JAK2 and p-STAT3 in KGN cells
Previous studies have implicated aberrant IL-22 signaling in many disorders, mostly through activation of the JAK/STAT3 pathway [Citation27,Citation28]. We subsequently studied the signaling pathways potentially responsible for the effect of IL-22 on KGN cells using western blotting (). LPS treatment did not affect the phosphorylation of JAK2 and STAT3. (LPS vs. Control: p-JAK2: ns; p-STAT3: ns). However, IL-22 administration either alone or as a pretreatment with LPS significantly induced the phosphorylation of STAT3 and JAK2 (IL-22 vs. Control: p-JAK2: p < 0.01; p-STAT3: p < 0.01) (LPS vs. LPS + IL-22: p-JAK2: p < 0.05; p-STAT3: p < 0.001) (). These data suggest that IL-22 activates the JAK/STAT3 signaling pathway in KGN cells.
Figure 5. IL-22 activated p-JAK2 and p-STAT3 in KGN cells. There were four treatment groups for cells: Control; LPS treated; rhIL-22 treated; LPS and rhIL-22 treated. (a) The expression levels of p-JAK2/JAK2 and p-STAT3/STAT3 were assessed by western blotting in the different groups. (b-c) Graph showing the statistical analysis of western blotting for p-JAK2/JAK2 and p-STAT3/STAT3, respectively. Values are expressed as the mean ± SEM, n = 3. *p < 0.05, **p < 0.01 ***p < 0.001 ns: nonsignificant. All experiments were performed in triplicate.

4. Discussion
Chronic inflammation is an underlying factor in the etiology of PCOS. Numerous research on PCOS shown that hyperinsulinemia, obesity, hyperandrogenism, and inflammatory status have synergistic roles in PCOS etiology. Recent studies suggest that chronic inflammation underlies the development of metabolic abnormalities and ovarian dysfunction in PCOS [Citation29]. Inflammatory cytokines regulate ovarian function through paracrine or autocrine regulation and contribute to the development of PCOS through steroidogenesis dysfunction and promotion of GCs apoptosis [Citation30]. In our previous work, we found that decreased pro-inflammatory factors resulted in elevated metformin-mediated ovulation rates in PCOS, supporting an association between inflammation and ovarian functions [Citation31].
IL-22 acts as an important immunomodulatory cytokine in inflammatory disease and acts mainly on non-leukocytes. This property is carried on the selective expression of IL-22 receptor chain-1(IL-22R1).IL-22R1 is an IL-22-specific cytokine receptor subunit [Citation32]. Our study identified IL-22R1 in human ovarian GCs for the first time, which implies that IL-22 may affect GCs by binding to IL-22R1. Moreover, we found reduced concentrations of IL-22 in the follicular fluid of PCOS patients as compared to the control group, which is consistent with a previous report [Citation26]. These findings shed light on the potential function of IL-22 in follicle development and therefore in PCOS pathogenesis. However, the cellular origins of IL-22 and the mechanism behind its reduced levels in follicular fluid of PCOS patients remain unknown. In this study, the BMI and serum testosterone of recruited PCOS patients were not different from controls, which may be attributed to the non-classical phenotype of PCOS recruited in our study. There is a limitation in this study we measured total testosterone, which did not represent the actual activity of testosterone. Physiologically active free testosterone in circulating blood and its relationship between IL-22 expression and the metabolic characteristics of PCOS, such as inflammation, insulin resistance, and hyperandrogenism need to be studied further.
Given its anti-apoptotic, pro-proliferative, and regenerative healing properties, IL-22 has received a lot of attention as a protective agent [Citation33]. Numerous studies have reported that IL-22 inhibited apoptosis and encouraged cell proliferation in various inflammatory diseases [Citation34–37]. Supporting this, molecular studies found that IL-22 suppressed cell apoptosis by triggering the STAT3 pathway and its downstream anti-apoptotic proteins Bcl-2 and Bcl-xL [Citation38,Citation39]. However, whether IL-22 modulates proliferation and apoptosis in human ovarian GCs remains unclear. Our findings demonstrated that IL-22 significantly and dose-dependently promoted the proliferation of KGN cells. Furthermore, IL-22 reversed the LPS-induced inhibition of proliferation in KGN cells. Although rhIL-22 administration alone had no discernible impact on KGN apoptosis, IL-22 inhibited LPS-induced apoptosis by upregulating the anti-apoptotic protein Bcl-2 and downregulating the pro-apoptotic proteins Bax and cleaved caspase-3. Taken together, our results imply that IL-22 might be protective for cell growth in inflammatory states.
GCs are steroidogenic cells surrounding oocytes that produce hormones and maintain the microenvironment for oocyte maturation, which is essential for folliculogenesis [Citation9,Citation10]. Steroidogenesis involves relatively complex molecular mechanisms mediated by differing enzymes in GCs at various stages of the menstrual cycle [Citation40] Reduced expression of cytochrome P450 aromatase in a dynamic follicular and inflammatory environment has been proposed as a potential reason for poor oocyte quality [Citation41]. StAR, CYP11A1, and CYP19A1 are steroidogenic enzymes that have been linked to aberrant steroid synthesis in GCs of PCOS patients [Citation42,Citation43]. To assess the effect of IL-22 in steroidogenesis, the expression levels of StAR, CYP11A1, and CYP19A1 in KGN cells were assessed using western blotting. Our findings agree with earlier research which shows LPS-mediated inhibition of steroidogenesis-associated genes (StAR, CYP11A1, and CYP19A1) in GCs [Citation44,Citation45]. IL-22 attenuated the LPS-induced inhibition of these proteins, suggesting that IL-22 may play a protective role in GCs steroidogenesis during inflammatory states. However, we did not evaluate estrogen or progesterone production to confirm our results. Further experiments are needed to explore whether alterations in steroidogenic genes influence the endocrinological function of GCs and follicle development.
IL-22 regulates cellular biological functions, primarily via the STAT3 signaling pathway. STAT3 activation prevents apoptosis by upregulating anti-apoptotic Bcl-2 and Bcl-XL proteins [Citation46]. STAT3 inhibits apoptosis and promotes cell proliferation through the increased transcription of the cell cycle proteins D1 and c-Myc [Citation47,Citation48]. Depending on whether Socs3 is expressed, STAT3 can play a dual role in supporting pro-inflammatory or anti-inflammatory responses [Citation49,Citation50]. To verify the role of IL-22 in the JAK2/STAT3 signaling pathway, we measured the expression of STAT3, P-STAT3, JAK2, and P-JAK2. The expression of P-STAT3 and P-JAK2 increased after treatment with rhIL-22, showing that IL-22 activates the STAT3 and JAK2 pathways. However, LPS treatment had no effect on STAT3 or JAK2 phosphorylation. We therefore speculate that the observed antagonism of LPS by IL-22 in proliferation, apoptosis, and steroidogenic genes of KGN cells may not be through the JAK2 and STAT3 pathways. Furthermore, we did not verify the contribution of the JAK2/STAT3 pathway in IL-22-regulated GCs proliferation, apoptosis, and steroidogenesis. Other critical signaling pathways and their interaction with the STAT3 and JAK2 pathways are required to be further investigated.
We acknowledge our study had further limitations. Instead of primary GCs, we utilized the KGN cells in vitro experiments. KGN cells maintain the physiological characteristics of normal GCs and have been extensively used to study the function and regulatory mechanisms of GCs [Citation51–54]. Since the relatively stable cell culture environment in vitro may differ from the fluctuating microenvironment in vivo, the data shown in this study need to be confirmed using in vivo models. In addition, we used LPS treatment as a model of inflammation; although it is a widely used model, the state of inflammation was different from the metabolic inflammation in PCOS.
5. Conclusion
In conclusion, we demonstrated that IL-22 enhanced cell proliferation and reversed LPS-induced inhibition of cell proliferation in an LPS model of inflammation. IL-22 had no effect on apoptosis and steroidogenesis-related proteins individually, but it could reverse LPS-induced apoptosis of GCs by upregulating Bax and cleaved caspase-3 and downregulating Bcl-2 and LPS inhibited StAR, CYP11A1, and CYP19A1 expression in GCs. In addition, IL-22 also stimulated JAK/STAT3 activation in GCs. These findings offer further indication that GCs proliferation, apoptosis, and steroidogenesis are coordinated by IL-22. Furthermore, we determined that PCOS patients had lower levels of IL-22 in their follicular fluid than did controls. Abnormal follicular IL-22 expression may be a pathological factor of PCOS. Further studies are needed to investigate the pathogenic mechanism of PCOS.
Ethical approval
The First Affiliated Hospital of Chongqing Medical University’s Ethics Committee approved all procedures used in studies involving individuals.
Acknowledgment
The authors are grateful to everyone who contributed to this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
Data availability on request from the corresponding author.
Additional information
Funding
References
- Rotterdam ESHER/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2003;81(1):19–25.
- Lizneva D, Suturina L, Walker W, et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. doi:10.1016/j.fertnstert.2016.05.003.
- Kelly CC, Lyall H, Petrie JR, et al. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86(6):2453–2455. doi:10.1210/jcem.86.6.7580.
- Möhlig M, Spranger J, Osterhoff M, et al. The polycystic ovary syndrome per se is not associated with increased chronic inflammation. Eur J Endocrinol. 2004;150(4):525–532. doi:10.1530/eje.0.1500525.
- Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2011;95(3):1048–1058.e2. e1041-1042. doi:10.1016/j.fertnstert.2010.11.036.
- Kaipia A, Chun SY, Eisenhauer K, et al. Tumor necrosis factor-alpha and its second messenger, ceramide, stimulate apoptosis in cultured ovarian follicles. Endocrinology. 1996;137(11):4864–4870. doi:10.1210/endo.137.11.8895358.
- Soboloff J, Sasaki H, Tsang BK. Follicular stage-dependent tumor necrosis factor alpha-induced hen granulosa cell integrin production and survival in the presence of transforming growth factor alpha in vitro. Biol Reprod. 2001;65(2):477–487. doi:10.1095/biolreprod65.2.477.
- Thackray VG. Sex, microbes, and polycystic ovary syndrome. Trends Endocrinol Metab. 2019;30(1):54–65. doi:10.1016/j.tem.2018.11.001.
- Karuputhula NB, Chattopadhyay R, Chakravarty B, et al. Oxidative status in granulosa cells of infertile women undergoing IVF. Syst Biol Reprod Med. 2013;59(2):91–98. doi:10.3109/19396368.2012.743197.
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122(6):829–838. doi:10.1530/rep.0.1220829.
- Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33(1):747–785. doi:10.1146/annurev-immunol-032414-112123.
- Lindemans CA, Calafiore M, Mertelsmann AM, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528(7583):560–564. doi:10.1038/nature16460.
- Wolk K, Witte E, Witte K, et al. Biology of interleukin-22. Semin Immunopathol. 2010;32(1):17–31. doi:10.1007/s00281-009-0188-x.
- McGee HM, Schmidt BA, Booth CJ, et al. IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol. 2013;133(5):1321–1329. doi:10.1038/jid.2012.463.
- Wang X, Ota N, Manzanillo P, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514(7521):237–241. doi:10.1038/nature13564.
- Abadpour S, Halvorsen B, Sahraoui A, et al. Interleukin-22 reverses human islet dysfunction and apoptosis triggered by hyperglycemia and LIGHT. J Mol Endocrinol. 2018;60(3):171–183. doi:10.1530/JME-17-0182.
- Kolumam G, Wu X, Lee WP, et al. IL-22R ligands IL-20, IL-22, and IL-24 promote wound healing in diabetic db/db mice. PLOS One. 2017;12(1):e0170639. doi:10.1371/journal.pone.0170639.
- Feng D, Park O, Radaeva S, et al. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci. 2012;8(2):249–257. doi:10.7150/ijbs.3967.
- Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13(1):21–38. doi:10.1038/nrd4176.
- Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines–from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14(12):783–795. doi:10.1038/nri3766.
- Xie Q, Huang C, Li J. Interleukin-22 and rheumatoid arthritis: emerging role in pathogenesis and therapy. Autoimmunity. 2015;48(2):69–72. doi:10.3109/08916934.2014.959165.
- Kim J, Krueger JG. The immunopathogenesis of psoriasis. Dermatol Clin. 2015;33(1):13–23. doi:10.1016/j.det.2014.09.002.
- Qi X, Nie Q, Pang Y, et al. IL-22 and its interaction with amino acid and glycolipid metabolite in polycystic ovary syndrome (PCOS) patients. Chin Med J. 2022;135(10):1258–1260. doi:10.1097/CM9.0000000000001915.
- Rotterdam ESHER/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2003;19(1):41–47.
- Yan MQ, Wang Y, Wang Z, et al. Mitoguardin2 is associated with hyperandrogenism and regulates steroidogenesis in human ovarian granulosa cells. J Endocr Soc. 2023;7(5):bvad034.
- Qi X, Yun C, Sun L, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25(8):1225–1233. doi:10.1038/s41591-019-0509-0.
- De Simone V, Franzè E, Ronchetti G, et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34(27):3493–3503. doi:10.1038/onc.2014.286.
- Aggor FEY, Break TJ, Trevejo-Nuñez G, et al. Oral epithelial IL-22/STAT3 signaling licenses IL-17-mediated immunity to oral mucosal candidiasis. Sci Immunol. 2020;5(48):eaba0570. doi:10.1126/sciimmunol.aba0570.
- González F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77(4):300–305. doi:10.1016/j.steroids.2011.12.003.
- Huang J, Chen P, Xiang Y, et al. Gut microbiota dysbiosis-derived macrophage pyroptosis causes polycystic ovary syndrome via steroidogenesis disturbance and apoptosis of granulosa cells. Int Immunopharmacol. 2022;107:108717. doi:10.1016/j.intimp.2022.108717.
- Zhang Y, Ran Y, Kong L, et al. Decreased SFRP5 correlated with excessive metabolic inflammation in polycystic ovary syndrome could be reversed by metformin: implication of its role in dysregulated metabolism. J Ovarian Res. 2021;14(1):97. doi:10.1186/s13048-021-00847-4.
- Hines IN, Kremer M, Isayama F, et al. Impaired liver regeneration and increased oval cell numbers following T cell-mediated hepatitis. Hepatology. 2007;46(1):229–241. doi:10.1002/hep.21674.
- Mühl H, Scheiermann P, Bachmann M, et al. IL-22 in tissue-protective therapy. Br J Pharmacol. 2013;169(4):761–771. doi:10.1111/bph.12196.
- Zhuang L, Ma W, Yan J, et al. Evaluation of the effects of IL‑22 on the proliferation and differentiation of keratinocytes in vitro. Mol Med Rep. 2020;22(4):2715–2722. doi:10.3892/mmr.2020.11348.
- Lejeune D, Dumoutier L, Constantinescu S, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277(37):33676–33682. doi:10.1074/jbc.M204204200.
- Yu J, Xiao Z, Zhao R, et al. Paeoniflorin suppressed IL-22 via p38 MAPK pathway and exerts anti-psoriatic effect. Life Sci. 2017;180:17–22. doi:10.1016/j.lfs.2017.04.019.
- Li H, Zhang Q, Wu Q, et al. Interleukin-22 secreted by cancer-associated fibroblasts regulates the proliferation and metastasis of lung cancer cells via the PI3K-Akt-mTOR signaling pathway. Am J Tran Re. 2019;11(7):4077–4088.
- Zhang W, Chen Y, Wei H, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14(20):6432–6439. doi:10.1158/1078-0432.CCR-07-4401.
- Wang B, Han D, Li F, et al. Elevated IL-22 in psoriasis plays an anti-apoptotic role in keratinocytes through mediating Bcl-xL/Bax. Apoptosis. 2020;25(9–10):663–673. doi:10.1007/s10495-020-01623-3.
- Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Mol Cell Endocrinol. 2004;228(1-2):67–78. doi:10.1016/j.mce.2004.04.018.
- Sanchez AM, Vanni VS, Bartiromo L, et al. Is the oocyte quality affected by endometriosis? A review of the literature. J Ovarian Res. 2017;10(1):43. doi:10.1186/s13048-017-0341-4.
- Das M, Djahanbakhch O, Hacihanefioglu B, et al. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(3):881–887. doi:10.1210/jc.2007-1650.
- Wang M, Sun J, Xu B, et al. Functional characterization of microRNA-27a-3p expression in human polycystic ovary syndrome. Endocrinology. 2018;159(1):297–309. doi:10.1210/en.2017-00219.
- Mehta, Anu, Onteru, Suneel Kumar, Singh, Dheer Ravinder,. HDAC inhibitor prevents LPS mediated inhibition of CYP19A1 expression and 17β-estradiol production in granulosa cells. Mol Cell Endocrinol. 2015; 414:73–81. doi:10.1016/j.mce.2015.07.002.
- Qu X, Yan L, Guo R, et al. ROS-Induced GATA4 and GATA6 downregulation inhibits StAR expression in LPS-treated porcine granulosa-lutein cells. Oxid Med Cell Longev. 2019;2019:5432792–5432714. doi:10.1155/2019/5432792.
- Stephanou A, Brar BK, Knight RA, et al. Opposing actions of STAT-1 and STAT-3 on the Bcl-2 and Bcl-x promoters. Cell Death Differ. 2000;7(3):329–330. doi:10.1038/sj.cdd.4400656.
- Masuda M, Suzui M, Yasumatu R, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62(12):3351–3355.
- Bowman T, Broome MA, Sinibaldi D, et al. Stat3-mediated myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci U S A. 2001;98(13):7319–7324. doi:10.1073/pnas.131568898.
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178(5):2623–2629. doi:10.4049/jimmunol.178.5.2623.
- El Kasmi KC, Smith AM, Williams L, et al. Cutting edge: a transcriptional repressor and corepressor induced by the STAT3-regulated anti-inflammatory signaling pathway. J Immunol. 2007;179(11):7215–7219. doi:10.4049/jimmunol.179.11.7215.
- Wang Y, Yang Q, Wang H, et al. NAD + deficiency and mitochondrial dysfunction in granulosa cells of women with polycystic ovary syndrome‡. Biol Reprod. 2021;105(2):371–380. doi:10.1093/biolre/ioab078.
- Li M, Zhao H, Zhao SG, et al. The HMGA2-IMP2 pathway promotes granulosa cell proliferation in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(4):1049–1059. doi:10.1210/jc.2018-00544.
- Zhou R, Li S, Liu J, et al. Up-regulated FHL2 inhibits ovulation through interacting with androgen receptor and ERK1/2 in polycystic ovary syndrome. EBioMedicine. 2020;52:102635. doi:10.1016/j.ebiom.2020.102635.
- Nishi Y, Yanase T, Mu Y, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142(1):437–445. doi:10.1210/endo.142.1.7862.