Abstract
Objective
Tests capable of accurate prediction of spontaneous preterm birth (sPTB) are crucial to inform clinical decisions to prevent neonatal deaths and reduce the risk of morbidity in surviving infants. A systematic literature review and meta-analysis were performed to assess the utility of the quantitative fetal fibronectin (fFN) test to predict sPTB at different test concentration thresholds.
Methods
Literature searches were conducted in MEDLINE, Embase, and the Cochrane Library in May 2022. Observational studies and clinical trials investigating the clinical utility of the quantitative fFN test in asymptomatic pregnancies prior to 37 weeks of gestation were eligible for inclusion. Meta-analysis quantified the risk of sPTB prior to four gestational age milestones (<28, <30, <34 and <37 weeks) based on quantitative fFN levels. No risk of bias assessment was performed however, clinical and methodological heterogeneity was explored to determine the feasibility of performing analyses.
Results
11 studies showed a quantitative assessment of fFN can differentiate between very high and very low risks of sPTB in asymptomatic pregnancies with <10% of women with very low fFN (<10 ng/mL) versus 37–67% of women with very high fFN (>200 ng/mL) delivering before 34 weeks. A meta-analysis of two studies showed, albeit with a low number of events, the odds of sPTB prior to 28 weeks was nine times higher in women testing positive at ≥50 ng/mL, whereas the odds of sPTB was 25 times higher in women with fFN concentrations >200 ng/mL (versus <50 ng/mL reference). Similarly, pooling three studies showed the odds of sPTB prior to 37 weeks was four times higher in women who tested positive at ≥50 ng/ml whereas the odds of delivery before 37 weeks was seven times higher for women with fFN concentrations ≥200 ng/ml (versus <50 ng/mL reference).
Conclusion
Quantitative fFN testing demonstrates increased predictive capabilities and utility of fFN testing in clinical practice, potentially preventing unnecessary intervention for women at very low risk and allowing an opportunity to optimize the management of asymptomatic patients at high risk of preterm delivery.
Introduction
Preterm birth (PTB) is defined as delivery before 37 weeks and 0 days gestation. It is estimated 15 million PTBs occur globally each year [Citation1], accounting for nearly 16% of neonatal deaths globally [Citation2–4]. PTB is associated with substantial morbidity among surviving infants. Children born preterm are prone to a variety of complications such as developmental disorders, respiratory distress syndrome (RDS), hypoglycemia, sepsis, hyperbilirubinemia, and necrotizing enterocolitis, all of which contribute to significant social, psychological, and economic burden [Citation5,Citation6].
Identification of women who are considered to be at risk of spontaneous PTB (sPTB) is crucial in order to find those who may benefit from treatments that delay the onset of preterm labor, such as tocolytics, cervical cerclage, and progesterone therapy, thereby mitigating the threat of neonatal morbidity and mortality [Citation7], as well as maintaining maternal health. Furthermore, identifying women who are at imminent risk of preterm delivery is critical for optimizing the administration of antenatal corticosteroids (ACS) to aid fetal lung development and prevent RDS. Therefore, tests capable of accurate prediction of the risk of sPTB are critical to ensuring that intervention is given appropriately, to achieve favorable maternal and neonatal health outcomes.
Fetal fibronectin (fFN) is a glycoprotein that plays an integral role in adhering the fetal membrane to the uterine lining [Citation8]. It has also been suggested that fFN facilitates the separation of the placenta from the uterus after delivery [Citation8]. Typically, fFN is not detected in the cervicovaginal secretions collected from the posterior fornix of the vagina between 16- and 22-weeks of gestational age [Citation8]. However, levels of fFN higher than 50 ng/mL can be observed as early as 22 weeks of gestation in women with risk factors for PTB, and high concentrations of fFN during the third trimester are indicative of an increased risk of sPTB [Citation8].
It is well established that a positive result based on the qualitative fFN test, which uses a 50 ng/mL threshold, is associated with an increased risk of PTB [Citation9]. Furthermore, a meta-analysis of randomized controlled trials found that management with knowledge of the qualitative test result was associated with a lower risk of sPTB before 37 weeks in women with symptoms of threatened preterm labor; however, no benefit was observed regarding PTB before 34 weeks or the risk of maternal hospitalization [Citation10]. It is generally accepted that the clinical utility of the qualitative fFN test is attributed to its high negative predictive value, that is, the ability to identify women at very low risk of imminent delivery, while concerns regarding the rate of false-positive test results may limit the utility of the test to exclusively inform clinician decision-making.
In recent years, a quantitative test has been developed as a diagnostic tool to determine the concentration of fFN in cervicovaginal samples collected from pregnant women. Numerous studies have been conducted to demonstrate the predictive capacity of the quantitative fFN test for assessing risk of sPTB in both symptomatic and asymptomatic women [Citation11–20]. Testing, as well as follow-up after the test, is used among women who exhibit symptoms of being in labor. However, in asymptomatic women, the quantitative fFN test can be critical for identifying patients with elevated fFN levels who require close monitoring. In addition, this information may help reduce unnecessary interventions, hospitalizations, and associated costs by identifying women with negative fFN or low fFN levels who are at lower risk of sPTB.
A systematic literature review (SLR) was conducted to collate all evidence on the rates of sPTB among asymptomatic pregnant women based on quantitative fFN test results. We also performed a meta-analysis to assess the prognostic utility of the test based on the concentration of fFN measured in samples.
Methods
The SLR was conducted in accordance with accepted methodological guidelines [Citation21,Citation22], per a prospectively defined protocol (available upon request) which was not registered. Literature searches, conducted on May 5, 2022, were performed in MEDLINE, Embase, and the Cochrane Library. The database searches had no limits regarding publication type to enable emerging research that may not yet be published in peer-reviewed articles (i.e. conference proceedings) to be captured by the search. In addition, we manually hand searched the proceedings from the Annual Meeting for the Society of Maternal-Fetal Medicine (2020–2022), as these materials are not indexed in Embase (SD).
Observational studies and clinical trials investigating the clinical utility of the quantitative fFN test in asymptomatic pregnant women prior to 37 weeks of gestation were eligible for inclusion in the review. One reviewer screened each record at the title and abstract screening stage (MB and SD). As a quality control measure 10% of studies were validated by a second, independent reviewer (MB and SD). All articles considered eligible for full-text review were reviewed by two independent reviewers (MB and SD), and any discrepancies were resolved through discussion and the involvement of a third reviewer when necessary. Data extraction was performed by a single reviewer (SD) and validated by a second independent reviewer (MB).
Studies were considered for meta-analysis if they reported sPTB rates at comparable gestational ages for comparable fFN thresholds. Other sources of heterogeneity across the literature included differences in the study population and timing of fFN testing. Consequently, data from some studies were deemed unsuitable for inclusion in the meta-analysis.
A meta-analysis was performed (BN) to quantify the risk of sPTB prior to various gestational age milestones (<28, <30, <34, and <37 weeks) based on the concentration of fFN measured in cervicovaginal secretions. In the base case, we analyzed high-risk asymptomatic populations. Risk factors included previous PTB, previous cervical surgery, previous miscarriage, uterine anomaly, short cervical length, and multiple gestations. We also performed a sensitivity analysis that included data on all asymptomatic populations, regardless of risk status. The most frequently reported test thresholds were selected for the analysis. Odds ratios (with 95% confidence interval [CI]) were generated to quantify the risk of sPTB in patients with fFN concentrations measured as ≥50 ng/mL, 50–199 ng/mL and ≥200 ng/mL relative to those with fFN concentrations <50 ng/mL and <200 ng/mL.
Classical (frequentist) inverse-variance weighted meta-analyses were performed in R using the metafor (1.9) package using random-effect models. Cochrane’s Q statistic and I2 test, in addition to estimates of τ2, were used to determine whether the level of statistical heterogeneity of study-level effects was substantive.
Results
The literature searches retrieved a total of 668 records. After the removal of duplicates, 429 articles were screened at the title/abstract level to determine eligibility for inclusion in the review, and 139 articles were considered eligible for full-text review. Thirty articles, reporting on 11 unique studies, met the criteria for inclusion in the SLR.
Of the included studies, eight observational studies collected data prospectively [Citation11,Citation14,Citation23–27], one was a retrospective case note review [Citation28], and two were based on randomized controlled trials [Citation16,Citation17]. All studies were conducted either in the United Kingdom [Citation12,Citation16,Citation25,Citation28] or the United States [Citation14,Citation17,Citation23,Citation24,Citation26,Citation27]. The eligibility criteria were based on the perceived risk of PTB in several studies: four enrolled high-risk populations [Citation11,Citation14,Citation26,Citation27], and one study each investigated average [Citation14] and low-risk [Citation24] populations. Four of the studies enrolled only women with singleton pregnancies [Citation14,Citation23,Citation24,Citation27], while another enrolled only twin gestation pregnancies [Citation25]. The sample sizes ranged considerably, from only 43 women [Citation28] up to 10,456 women [Citation14].
.shows the rates of sPTB at different gestational ages (range: <24 weeks up to <37 weeks) for the various fFN test thresholds reported. Across all studies, higher fFN concentrations were associated with an increased risk of preterm delivery, regardless of sPTB risk factors. present the sPTB rates for selected incremental fFN thresholds to illustrate the trend in sPTB rates at two timepoints frequently reported in the literature (<34 and <37 weeks). Outlier fFN values can distinguish very high and very low risk of sPTB; less than 10% of patients with fFN concentrations measured below 10 ng/mL delivered before 34 weeks of gestation, whereas 37% [Citation11] to 67% [Citation29] of women with concentrations above 200 ng/mL delivered before 34 weeks. Higher fFN concentrations were positively correlated with risk of sPTB at all gestational age thresholds assessed. Quantification of fFN has also been shown to help predict imminent sPTB within two weeks of having taken the fFN test in asymptomatic women [Citation28].
Figure 1. (a) Risk of sPTB before 34 weeks based on fFN concentration. This figure does not present data for all thresholds reported because of the substantial heterogeneity observed. The figure presents thresholds that facilitate an assessment of the trend in sPTB based on the incremental fFN thresholds reported. Outcomes for the other thresholds, not shown here, are reported in . Data presented here are reported for the overall cohort enrolled in each study; subgroup data are not presented. fFN: fetal fibronectin; sPTB: spontaneous preterm birth. (b). Risk of sPTB before 37 weeks based on fFN concentration. This figure does not present data for all thresholds reported because of the substantial heterogeneity observed. The figure presents thresholds that facilitate an assessment of the trend in sPTB based on the incremental fFN thresholds reported. Outcomes for the other thresholds, not shown here, are reported in . Data presented here are reported for the overall cohort enrolled in each study; subgroup data are not presented. NuMoM2b collected fFN specimens at three timepoints; data are presented for the final follow-up visit (22–30 weeks). fFN: fetal fibronectin; sPTB: spontaneous preterm birth.
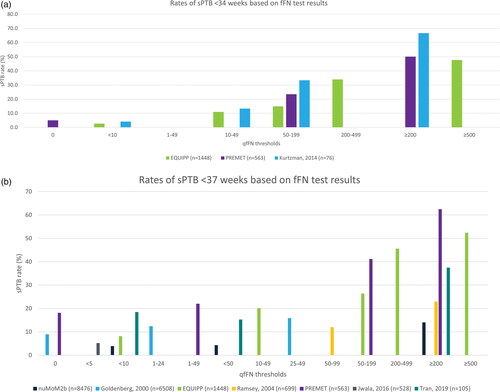
Table 1. Summary of included studies and sPTB rates by fFN levels.
When considering these studies for meta-analysis, heterogeneity regarding the fFN thresholds evaluated and gestational age thresholds for PTB precluded the pooling of most studies in the analysis. Kurtzman 2014 was deemed unsuitable for inclusion as the study investigated only women with twin pregnancies. Three of the remaining studies (EQUIPP, PREMET, and Tran 2019), investigating asymptomatic, high-risk populations, were deemed eligible for inclusion in the meta-analysis to evaluate PTB risk (). The final study, nuMoM2b, investigated women with unspecified sPTB risk who were assumed to be asymptomatic. Given the ambiguous description of the patient population there was uncertainty as to whether this study was suitable for inclusion in the meta-analysis hence, this study was only included in a sensitivity analysis.
The resulting meta-analyses showed that regardless of gestational age, higher fFN levels were associated with a significantly higher risk of sPTB than lower levels in asymptomatic, high-risk women (). The test appeared to have the greatest predictive power to identify sPTB at earlier gestational ages however, due to a low number of events there was greater heterogeneity observed at the earlier timepoints, particularly 28 weeks.
Figure 2. Meta-analysis results: sPTB risk for women with high vs low fFN concentrations. Forest plot showing the results of the random-effects meta-analyses. Results show the likelihood (odds ratio) that patients with higher fFN concentrations will experience sPTB relative to patients with lower fFN concentrations. The higher the odds ratio, the greater risk of sPTB among patients with the higher fFN concentrations relative to the reference group. The 28-week analysis pooled data reported by PREMET and Tran 2019. The 30- and 34-week analysis pooled data reported by EQUIPP and PREMET. The base case 37-week analyses pooled data reported EQUIPP, PREMET and Tran 2019; the sensitivity analysis also adds data from the nuMoM2b study. CI: confidence interval; fFN: fetal fibronectin; sPTB: spontaneous preterm birth
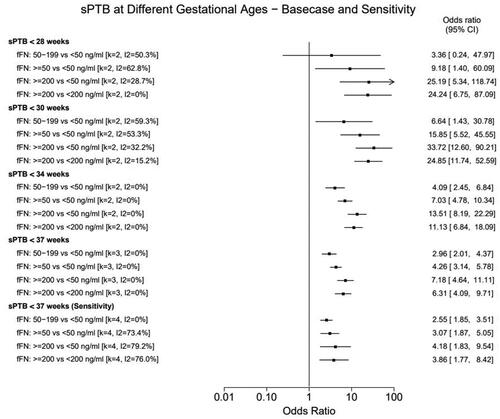
The odds of sPTB by 28 weeks were nine times higher in women who would be considered positive at the qualitative test threshold (≥50 ng/ml) compared to the negative test population at this threshold. In comparison, women with high fFN concentrations (≥200 ng/ml) measured by the quantitative test had 25 times higher odds of delivering <28 weeks compared to the reference population (<50 ng/ml). Similarly, women with the highest measured fFN concentrations (≥200 ng/ml) had 34 times higher odds of delivering prior to 30 weeks compared to those with low fFN concentrations (<50 ng/ml).
The odds of sPTB by 34 weeks were seven times higher in women who would be considered positive at the qualitative test threshold (≥50 ng/ml) compared to the negative test population at this threshold. In comparison, women with high fFN concentrations (≥200 ng/ml) had 13 times higher odds of delivering <34 weeks compared to the reference population (<50 ng/ml).
Similarly, the odds of sPTB by 37 weeks were four times higher in women who were positive at the qualitative threshold (≥50 ng/ml) compared to the negative test population. Whereas the odds of delivery before 37 weeks were seven times higher for women with fFN ≥200 ng/ml compared to the reference population (<50 ng/ml). Trends were similar, albeit less pronounced, in the sensitivity analysis, including the nuMoM2b study.
There was considerable heterogeneity observed for the 28- and 30-week analyses although notably heterogeneity was much lower for the 200 ng/ml threshold analyses compared to the analyses of the conventional qualitative test threshold, as illustrated by the I2 values in . No signs of statistical heterogeneity were observed in the 34- and 37-week base case analyses. In the sensitivity analysis (including nuMoM2b), substantial heterogeneity (I2>75%) was observed for all the comparisons except for 50–199 ng/ml vs. <50 ng/ml, supporting the assumption that this study’s population was meaningfully different from the asymptomatic high-risk studies included in the base case. However, despite this heterogeneity, the overall trends and conclusions from this analysis remained the same as those in the base case.
Subgroup analyses were reported only by the EQUIPP [Citation11,Citation19,Citation30–32] and EQUATE [Citation26] studies, which showed that fFN was predictive of sPTB in patients regardless of cervical length, prior cervical surgery, and prior sPTB. fFN was additively predictive in patients with short cervixes or twin pregnancies. Importantly, low fFN levels may be particularly predictive of low sPTB risk in patients with vaginal blood in the samples, as these patients are more likely to experience false-positive results. Subgroup data are presented in the supplementary materials.
Discussion
Existing research has collated evidence on the accuracy of the quantitative fFN test for predicting PTB in asymptomatic high-risk pregnancies, the findings confirmed the quantitative fFN test can predict the risk of sPTB in asymptomatic women [Citation33]. However, to the best of our knowledge, only narrative synthesis of such data has been published to date. Our objective was to establish whether the additional information provided by the quantitative fFN test may increase the utility of fFN testing in clinical practice and, if feasible, to perform a meta-analysis of the published evidence. This study demonstrates that the risk of sPTB in asymptomatic women can be meaningfully estimated by measuring exact fFN concentrations.
Specifically, the analysis showed that the odds of sPTB were greater when fFN concentration was higher compared to those with lower concentration. A gradient of odds ratios was observed, at both the study-level and in the meta-analyzed results, when comparing across incremental test thresholds (i.e. 50–199, ≥50, and ≥200 ng/mL vs. the reference of <50 ng/mL). This trend was evident at all gestational periods (<28, <30, <34 and <37 weeks), with the relationship being strongest at the earlier gestational ages. While the results showed greater odds for ≥200 vs <50 ng/mL at 30 weeks than at 28 weeks (OR: 33.72 vs 25.19), we should not infer a higher predictive power at 30 weeks compared to 28 weeks, given the low number of events at the earlier gestational age (resulting in the wide credible intervals observed).
The quantitative test can therefore be an important tool in identifying asymptomatic pregnancies at high risk for sPTB that require additional surveillance to ensure optimal maternal and neonatal outcomes. The utility of very low thresholds provides enhanced clarity regarding which patients are unlikely to experience sPTB. Two studies (EQUIPP and NuMoM2b) reported that at the 10 ng/ml threshold the negative predictive value of the test exceeds 95%, showing that the risk of sPTB is extremely low in patients with very low fFN concentrations [Citation11,Citation23]. However, analyses of the EQUIPP study demonstrated that certain risk factors increase the risk of sPTB so dramatically that high rates of sPTB are observed even in patients with low concentrations of fFN. Therefore, fFN concentrations should be considered in conjunction with the clinical assessment of established risk factors, such as short cervical length, prior cervical surgery, prior sPTB, and twin gestations, to guide patient management. The QUIPP app is a validated tool which combines risk factors and quantitative fFN to predict risk of preterm birth [Citation34]. Other biomarker tests are available for the prediction of sPTB including phosphorylated insulin-like growth factor binding protein-1 (phIGFBP-1 or Actim Partus®), or placental alpha macroglobulin-1 (PAMG-1 or Partosure®) [Citation35–38]. Existing research has investigated the utility fFN testing versus other biomarker tests.[Citation39]
We sought to collate all the available literature on the clinical utility of the quantitative fFN test for predicting rates of sPTB in asymptomatic pregnancies. Consequently, the studies included in the review were heterogeneous in terms of study design, patient populations studied, and outcome assessment, which resulted in few studies amenable to pooling in a meta-analysis. Despite these limitations, the results of the meta-analysis showed that statistical significance was observable. Nonetheless, there is insufficient data available to perform a meta-analysis on sPTB risk in particular subgroups of interest, such as twin pregnancies and individuals with shorter cervical length. Furthermore, as with all SLRs there is a possibility that our findings may have been affected by publication bias, to try to mitigate this effect we included evidence from grey literature sources in the review. However, given the small number of studies included in the meta-analyses it was not possible to use statistical methods to assess potential publication biases.
Conclusions
Quantitative estimation of fFN concentrations in the cervicovaginal secretions of pregnant women before 37 weeks’ gestation with risk factors for sPTB, but exhibiting no symptoms of labor, may increase the predictive capabilities and utility of fFN testing in clinical practice. Clinicians can implement personalized care for patients who have higher fFN concentrations, as these patients are at greater risk of undergoing preterm delivery compared to those with lower concentrations of fFN measured in the cervicovaginal secretions. For those who are not at imminent risk of sPTB, the quantitative fFN test may offer reassurance to parents and possibly prevent unnecessary medical intervention, thereby reducing healthcare costs. Future research should investigate the utility of quantitative fFN testing in optimizing management decisions and minimizing medical resource use.
Supplemental Material
Download MS Word (77.7 KB)Acknowledgements
We thank Shruti Nambiar for her contributions to the design and execution of the initial SLR, upon which this study was based. We also thank Allie Cichewicz and Heather Burnett for their contributions to the execution of the initial SLR and interim updates.
Disclosure statement
Sophie Dodman, Marissa Betts, and Binod Neupane are employed by Evidera, which provides consulting and other research services to pharmaceutical, medical device, and related organizations. In their salaried positions, they work with a variety of companies and organizations and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received funding from Hologic, Inc. to participate in the study. Michael Ruma is a consultant and member of the Hologic speaker’s bureau and received funding from Hologic, Inc. to participate in the study. Hologic, Inc. did not participate in the data collection, analysis, nor interpretation of the research outcomes.
Data availability statement
Study protocol available upon request. The data used in the analyses are presented in .
Additional information
Funding
References
- Cao G, Liu J, Liu M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990-2019. JAMA Pediatr. 2022;176(8):787–796. doi: 10.1001/jamapediatrics.2022.1622.
- Valenzuela CP, Gregory E, Martin JA. Mortality in the United States, 2016. NCHS Data Brief. 2022;429:1–8.
- McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312(2):82–90. doi: 10.1056/NEJM198501103120204.
- United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels & trends in child mortality: report 2017, estimates developed by the UN inter-agency group for child mortality estimation. New York: United Nations Children’s Fund; 2017.
- Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. doi: 10.2471/BLT.08.062554.
- Brown HK, Speechley KN, Macnab J, et al. Neonatal morbidity associated with late preterm and early term birth: the roles of gestational age and biological determinants of preterm birth. Int J Epidemiol. 2014;43(3):802–814. doi: 10.1093/ije/dyt251.
- Kiefer DG, Vintzileos AM. The utility of fetal fibronectin in the prediction and prevention of spontaneous preterm birth. Rev Obstet Gynecol. 2008;1(3):106–112.
- Lockwood CJ, Senyei AE, Dische MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325(10):669–674. doi: 10.1056/NEJM199109053251001.
- Leitich H, Egarter C, Kaider A, et al. Cervicovaginal fetal fibronectin as a marker for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 1999;180(5):1169–1176. doi: 10.1016/s0002-9378(99)70612-5.
- Berghella V, Saccone G. Fetal fibronectin testing for prevention of preterm birth in singleton pregnancies with threatened preterm labor: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2016;215(4):431–438. doi: 10.1016/j.ajog.2016.04.038.
- Abbott DS, Hezelgrave NL, Seed PT, et al. Quantitative fetal fibronectin to predict preterm birth in asymptomatic women at high risk. Obstet Gynecol. 2015;125(5):1168–1176. Maydoi: 10.1097/AOG.0000000000000754.
- Abbott DS, Radford SK, Seed PT, et al. Evaluation of a quantitative fetal fibronectin test for spontaneous preterm birth in symptomatic women. Am J Obstet Gynecol. 2013;208(2):122. doi: 10.1016/j.ajog.2012.10.890.
- Bruijn M, Van Baaren GJ, Vis J, et al. Does quantitative fetal fibronectin testing improve the prediction of spontaneous preterm delivery as compared to qualitative fetal fibronectin testing in symptomatic women: a post-hoc analysis. Am J Obstetrics Gynecology. 2014;210(1):S364. doi: 10.1016/j.ajog.2013.10.774.
- Goepfert AR, Goldenberg RL, Mercer B, et al. The preterm prediction study: quantitative fetal fibronectin values and the prediction of spontaneous preterm birth. The national institute of child health and human development maternal-fetal medicine units network. Am J Obstet Gynecol. 2000;183(6):1480–1483. doi: 10.1067/mob.2000.107067.
- Goldenberg RL, Klebanoff M, Carey JC, et al. Vaginal fetal fibronectin measurements from 8 to 22 weeks’ gestation and subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183(2):469–475. doi: 10.1067/mob.2000.106073.
- Kurtzman J, Chandiramani M, Briley A, et al. Quantitative fetal fibronectin screening in asymptomatic high-risk patients and the spectrum of risk for recurrent preterm delivery. Am J Obstet Gynecol. 2009;200(3):263 e1–6. doi: 10.1016/j.ajog.2009.01.018.
- Ramsey P. Relationship of midtrimester fetal fibronectin (FFN) concentration to antibiotic efficacy for the prevention of spontaneous preterm birth in asymptomatic FFN positive women. Am J Obstet Gynecol. 2004;191(6):S11. doi: 10.1016/j.ajog.2004.09.059.
- Vandermolen B, Hezelgrave NL, Seed P, et al. Quantitative fetal fibronectin to predict preterm birth in asymptomatic women with previous cervical surgery. In: BJOG: an international journal of obstetrics and gynaecology. RCOG World Congress 2016; UK. 2016.
- Vandermolen BI, Hezelgrave NL, Smout EM, et al. Quantitative fetal fibronectin and cervical length to predict preterm birth in asymptomatic women with previous cervical surgery. Am J Obstet Gynecol. 2016;215(4):480.e1–480.e10.
- Radford SK, Da Silva Costa F, Araujo JE, et al. Clinical application of quantitative foetal fibronectin for the prediction of preterm birth in symptomatic women. Gynecol Obstet Invest. 2018;83(3):285–289. doi: 10.1159/000480235.
- Page MM, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2020;372:n71.
- Higgins JPT, Thomas J, Chandler J, et al. editors. Cochrane handbook for systematic reviews of interventions. version 6.1. Cochrane; 2020. (cited 2020 September). www.training.cochrane.org/handbook.
- Esplin MS, Elovitz MA, Iams JD, et al. Predictive accuracy of serial transvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047–1056. Mar 14doi: 10.1001/jama.2017.1373.
- Jwala S, Tran TL, Terenna C, et al. Evaluation of additive effect of quantitative fetal fibronectin to cervical length for prediction of spontaneous preterm birth among asymptomatic low-risk women. Acta Obstet Gynecol Scand. 2016;95(8):948–955. doi: 10.1111/aogs.12907.
- Kurtzman J, Hezelgrave N, Abbott D, et al. 790: prediction of preterm birth in asymptomatic patients with twin gestations using a single quantitative fetal fibronectin screen. Am J Obstet Gynecol. 2014;210(1):S386–S387. doi: 10.1016/j.ajog.2013.10.823.
- Norwitz E, Blackwell SC, Adair CD, et al. 545: quantitative fFN for prediction of spontaneous PTB in a high-risk asymptomatic population: the equate trial. Am J Obstet Gynecol. 2019;220(1):S365. doi: 10.1016/j.ajog.2018.11.567.
- Tran TL, Jwala S, Terenna C, et al. Evaluation of additive effect of quantitative fetal fibronectin to cervical length for prediction of spontaneous preterm birth among asymptomatic high-risk women. J Matern Fetal Neonatal Med. 2020;33(15):2628–2634. Aug
- Muzaffar S, Behrens R, Barns F, et al. editors. Quantitative fetal fibronectin and preterm delivery: BJOG-an international journal of obstetrics and gynaecology. Hoboken: Wiley-Blackwell; 2013.
- Kurtzman J, Hezelgrave N, Abbott D, et al. 789: quantitative fetal fibronectin and cervical length screening at 22-27 6/7 weeks’ GA illuminate the spectrum of risk of preterm birth in asymptomatic twin gestations. Am J Obstet Gynecol. 2014;210(1):S386. doi: 10.1016/j.ajog.2013.10.822.
- Hezelgrave N, Kurtzman J, Abbott D, et al. 10: quantitative fetal fibronectin assessment at 22 0/7-27 6/7 weeks’ GA significantly modifies the risk of preterm birth in asymptomatic high risk patients with sonographic cervical shortening. Am J Obstet Gynecol. 2015;212(1):S8–S9. doi: 10.1016/j.ajog.2014.10.056.
- Hezelgrave NK, Cottam K, Seed PT, et al. The effect of blood staining on cervicovaginal quantitative fetal fibronectin concentration and prediction of spontaneous preterm birth. Eur J Obstet Gynecol Reprod Biol. 2017;208:103–108. doi: 10.1016/j.ejogrb.2016.11.027.
- Kuhrt KH-EN, Stock SJ, Tribe R, et al. Quantitative fetal fibronectin for prediction of preterm birth in asymptomatic twin pregnancy. Acta Obstet Gynecol Scand. 2020;99(9):1191–1197. doi: 10.1111/aogs.13861.
- Hezelgrave NL, Shennan AH. Quantitative fetal fibronectin to predict spontaneous preterm birth: a review. Womens Health. 2016;12(1):121–128. doi: 10.2217/whe.15.74.
- Watson HA, Carlisle N, Kuhrt K, et al. EQUIPTT: the evaluation of the QUiPP app for triage and transfer protocol for a cluster randomised trial to evaluate the impact of the QUiPP app on inappropriate management for threatened preterm labour. BMC Pregnancy Childbirth. 2019;19(1):68. doi: 10.1186/s12884-019-2210-1.
- Danti L, Prefumo F, Lojacono A, et al. The combination of short cervical length and phIGFBP-1 in the prediction of preterm delivery in symptomatic women. J Matern Fetal Neonatal Med. 2011;24(10):1262–1266.
- Ness A, Visintine J, Ricci E, et al. Does knowledge of cervical length and fetal fibronectin affect management of women with threatened preterm labor? A randomized trial. Am J Obstet Gynecol. 2007;197(4):426.e1–426.e7. doi: 10.1016/j.ajog.2007.07.017.
- Nikolova T, Bayev O, Nikolova N, et al. Evaluation of a novel placental alpha microglobulin-1 (PAMG-1) test to predict spontaneous preterm delivery. J Perinat Med. 2014;42(4):473–477. doi: 10.1515/jpm-2013-0234.
- Paternoster D, Riboni F, Vitulo A, et al. Phosphorylated insulin-like growth factor binding protein-1 in cervical secretions and sonographic cervical length in the prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2009;34(4):437–440. doi: 10.1002/uog.6428.
- Varley-Campbell JM-MR, Coelho H, Ocean N, et al. Biomarker tests to help diagnose preterm labour in women with intact membranes. Peninsula Technology Assessment Group (PenTAG), University of Exeter Medical School (Report for NICE). 2018.

