Abstract
Objective
This study aimed to analyze the effect of low-molecular-weight heparin (LMWH) on the decidualization of stromal cells in early pregnancy and explore the effect of LMWH on pregnancy outcomes.
Methods
Recurrent spontaneous abortion (RSA) mouse model (CBA/J × DBA/2) and normal pregnant mouse model (CBA/J × BALB/c) were established. The female mice were checked for a mucus plug twice daily to identify a potential pregnancy. When a mucus plug was found, conception was considered to have occurred 12 h previously. The pregnant mice were divided randomly into a normal pregnancy control group, an RSA model group, and an RSA + LMWH experimental group (n = 10 mice in each group). Halfway through the 12th day of pregnancy, the embryonic loss of the mice was observed; a real-time quantitative polymerase chain reaction was used to detect the messenger ribonucleic acid (mRNA) expressions of prolactin (PRL) and insulin-like growth factor-binding protein 1 (IGFBP1) in the decidua of the mice. Additionally, the decidual tissues of patients with RSA and those of normal women in early pregnancy who required artificial abortion were collected and divided into an RSA group and a control group. Decidual stromal cells were isolated and cultured to compare cell proliferation between the two groups, and cellular migration and invasion were detected by membrane stromal cells. Western blotting was used to detect the protein expressions of proliferating cell nuclear antigen (PCNA), cyclin D1, matrix metalloproteinase- (MMP) 2, and MMP-7 in stromal cells treated with LMWH.
Results
Compared with the RSA group, LMWH significantly reduced the pregnancy loss rate in the RSA mice (p < 0.05). Compared with the RSA group, the LMWH + RSA group had significantly higher expression levels of PRL and IGFBP1 mRNA (p < 0.01). LMWH promoted the proliferation, migration, and invasion of human decidual stromal cells; compared with the control group, the expression levels of MMP-2, MMP-7, cyclin D1, and PCNA proteins in the decidual stromal cells of the LMWH group increased (p < 0.05).
Conclusions
The use of LMWH can improve pregnancy outcomes by enhancing the proliferation and migration of stromal cells in early pregnancy and the decidualization of stromal cells.
Introduction
Recurrent spontaneous abortion (RSA) refers to two or more spontaneous abortions occurring within 24 weeks in the same couple [Citation1]. At present, the incidence of RSA in China is increasing year on year, affecting around 2%–5% of reproductive women [Citation2,Citation3]. Patients with RSA experience multiple pregnancies and miscarriages, seriously affecting reproductive health and causing signficiant distress to patients and their families. The main causes of RSA include genetic factors, abnormal uterine anatomy, endocrine disorders, infectious diseases, environmental factors, and immunological issues [Citation4]. Despite a comprehensive examination of couples with RSA, 50% of patients still have an unknown cause of miscarriage, which is known as unexplained RSA (URSA) [Citation5,Citation6]. Recurrent spontaneous abortion is a major problem in the diagnosis and treatment of obstetric and gynecological diseases, and it seriously affects the physical and mental health of patients.
Domestic and foreign experts have made some progress in researching the etiology of RSA, but its pathogenesis has not yet been fully elucidated. A large proportion of patients with recurrent spontaneous abortion are considered to be associated with immune abnormalities, and anticardiolipin antibody syndromes account for 20% of immune abnormalities. It has been suggested [Citation7] that antiphospholipid antibodies (aPL) may contribute to placental vascular thrombosis, trophoblast dysfunction at the time of implantation, or imbalances in maternal hormone secretion. In addition to aPL, several other autoantibodies have been associated with unexplained RSA, including anti-membrane link protein V and anti-nuclear antibodies (ANA). It has been shown [Citation8] that ANA may impair oocyte quality and embryo development, leading to reduced pregnancy and implantation rates. Tissue deposition of immune complexes and local complement activation as well as chemotaxis of the inflammatory infiltrate may be one of the mechanisms of ANA-mediated pregnancy failure. Antiphospholipid antibodies reduce placental hormone production as well as fusion and invasion between trophoblasts, and the likelihood of a successful pregnancy is greater after treatment of patients with anticardiolipin antibody syndrome with heparin and low-dose aspirin. Recent studies have shown [Citation9] that anti-membrane link protein V antibodies are usually closely associated with an elevated incidence of RSA. Anti-membrane link protein V antibodies induce syncytiotrophoblast apoptosis by inducing trophoblast apoptosis, affecting trophoblast invasiveness and trophoblast gonadotropin secretion. The prevention of RSA and the exploration of its possible pathological mechanism have important clinical significance for protecting the reproductive health of women of childbearing age.
Early pregnancy is a particularly important stage. The decidua allows the semi-allogeneic embryo to implant, grow, and be delivered by providing a fertile substrate and an immune-privileged microenvironment on the maternal–fetal interface [Citation10]. Stromal cells are the main cells in decidualization; a stromal cell that is not fully decidualized will continue to undergo decidualization after pregnancy to receive embryonic development and growth [Citation11]. Completely decidualized stromal cells secrete and accumulate a variety of cytokines, hormones, and transcription factors in the maternal–fetal interface layer to form a local immune environment, regulate the phenotypic remodeling of immune cells, and induce immune tolerance formation [Citation12]. The decidual tissue of early pregnancy involves extensive tissue remodeling of the endometrial interstitium stimulated by decidualization-inducing factors, including stromal cell proliferation and differentiation, glandular epithelium secretory transformation, invasion of special immune cell groups, and vascular remodeling, in preparation for embryonic implantation [Citation13]. Decidualization plays an extremely important role in regulating fetal trophoblast invasion, resisting immune and oxidative stress responses during pregnancy, and regulating immune cell function [Citation14]. Since it appears that decidualization (rather than defective/dysregulated) is a causative factor of pregnancy loss [Citation15,Citation16]. Therefore, it is imperative to study in depth the molecular mechanism of the decidualization of stromal cells in early pregnancy and develop effective treatment methods.
Current studies show that the decidualization of stromal cells in early pregnancy is a complex process initiated by progesterone and affected by many factors, including cytokines, signal transducers, hypophyseal hormones, transcription factors, and immune cells [Citation17,Citation18]. Cyclic adenosine monophosphate, insulin-like growth factor-binding protein 1 (IGFBP1), and prolactin (PRL) are widely recognized as key components for the decidualization process, However, their exact role in the decidualization process, differently from cAMP, is still uncompletely determined [Citation19–21]. The changes in the proliferation, migration, and movement of stromal cells can indicate the degree of decidualization in early pregnancy. The degraded decidualization of stromal cells participates in the occurrence and development of URSA [Citation16].
Low-molecular-weight heparin (LMWH)—a glycosaminoglycan composed of 12–18 glucose units—is produced from unfractionated heparin by enzymatic or chemical depolymerization. Its molecular weight is one-third that of unfractionated heparin, and its average molecular weight is 4000–5000 Da [Citation22,Citation23]. Low-molecular-weight heparin is used for anticoagulation purposes by inhibiting the activities of thrombin IIa and coagulation factor Xa [Citation24]. In addition, LMWH can stimulate endothelial cells to synthesize and release a tissue-type plasminogen activator and reduce plasminogen activator inhibitor-1 to transform plasminogen into plasmin and promote fibrinolysis [Citation25]. In recent years, LMWH has been widely and effectively used in RSA cases caused by a prethrombotic state to prevent miscarriage [Citation26,Citation27]. Research has shown that LMWH also effectively prevents URSA, indicating that it may have other functions in embryonic protection besides being an anticoagulant and antithrombotic agent [Citation27,Citation28]. However, it is unclear whether LMWH can improve the decidualization of stromal cells in early pregnancy.
Materials and methods
Laboratory animals and treatment
This study was conducted following the guidelines of the laboratory animal center of the School of Medicine, Kunming University of Science and Technology, as well as the principles of animal protection, animal welfare, and ethics. The laboratory animals were 60-day-old female CBA/J mice, male DBA/2 mice, and male BALB/c mice, which were purchased from Beijing HFK Bioscience Co., Ltd. All the laboratory mice were reared in the animal laboratory of Kunming University of Science and Technology in a 12-h light/12-h dark cycle, under constant temperature (25 °C–27 °C) and humidity (50%). To establish the RSA mouse models, the female CBA/J mice were housed with the male DBA/2 mice at a ratio of 2:1, and to establish normal pregnancy mouse models, the female CBA/J mice were housed with the male BALB/c mice at a ratio of 2:1 [Citation29,Citation30]. The female mice were checked for a mucus plug twice daily to identify a potential pregnancy. When a mucus plug was found, conception was considered to have occurred 12 h previously. The pregnant mice were divided randomly into a normal pregnancy control group, an RSA model group, and an RSA + LMWH experimental group (n = 10 mice in each group). The mice in the RSA + LMWH group were injected intraperitoneally with 100 µl of 20-IU/l LMWH (Changshan Biochemical Pharmaceutical Co., Ltd., Jiangsu., China) every day after the confirmation of pregnancy. The RSA model group and the normal pregnancy control group were injected with 100 µl of normal saline.
Embryo resorption rate
The CBA/J pregnant mice were euthanized 12.5 days after the observation of a vaginal plug. The size of the normal embryos, placenta, and resorption site, as well as the appearance of necrosis and hemorrhage, were observed. The number of resorbed and normal embryos was recorded. The embryo resorption rate = the number of resorbed embryos/(the number of resorbed embryos + the number of viable embryos) × 100%.
Separation and identification of patients’ samples and cells
Decidual tissues were collected from healthy women in early pregnancy who required an induced abortion due to unwanted pregnancy and women with RSA; the samples were collected from both groups at 7–9 gestational weeks. The patients were between 20 and 35 years old, without pregnancy comorbidity or complication; genetic, endocrine, or anatomical abnormalities, or infection-induced abortion. In addition, patients taking contraceptive drugs or steroid hormones during the 6 months before the operation were excluded from the study.
The obtained fresh decidual tissues were washed with Phosphate Buffered Saline (PBS) within 30 min of the abortion and cut into pieces. Decidual stromal cells were isolated and extracted by the differential-time adherence method. Briefly, decidual tissue was placed in Dulbecco’s modified Eagle’s medium/F12 in a digestion solution (0.5-mg/ml deoxyribonuclease (Dnase), 1.5-mg/ml type-II collagenase, 1-mg/ml Bovine Serum Albumin (BSA) and shaken in a water bath at 37 °C for 40–60 min. The supernatant liquid was collected, the digestion was terminated, and the liquid was centrifuged at 1 g for 3 min to pellet the undigested tissue. The supernatant was collected, and the cells were pelleted by centrifugation at 2 g for 10 min. Then, the supernatant was discarded. The cell pellets were resuspended and filtered through a 40-mesh cell strainer. Then, the filtrate was inoculated into a 25-cm cell culture flask and cultured at 37 °C in a 5% CO2 incubator. After 30–40 min of adherence, the culture medium was changed to remove non-adherent glandular epithelial cells and blood cells, and it was changed again after 48 h. When the cells adhered and grew to 70%–80% of the bottom of the bottle, immunofluorescence was used to identify decidual stromal cells. The decidual stromal cells were fixed with 4% paraformaldehyde for 30 min, washed three times with PBS, permeabilized with 0.1% Triton™ for 20 min, washed with PBS, and finally washed with 2% BSA solution at room temperature (25 °C to 27 °C, the temperature range was the same during all stages of the experiment). The cells were blocked for 1 h, and a primary antibody of rabbit monoclonal vimentin (1:500, ab92547, Abcam,USA) was added. The cells were incubated overnight at 4 °C; then, they were washed three times with PBS and mixed with goat secondary antibody with a fluorescent dye (1:500, ab150115, Abcam,USA). They were incubated at room temperature for 1 h in the dark and rinsed with PBS three times before 4′,6-diamidino-2-phenylindole was added to stain the nuclei for 3 min. The sections were observed under a Nikon inverted fluorescence microscope, and images were obtained. The fluorescence images were analyzed using Image J software (1.48 version).
Cell counting kit-8 assays
The primary decidual stromal cells from the patients with and without RSA were purified into cell suspensions and seeded onto 96-well plates at a density of 8000 cells per well. When cell adhesion was observed, 10 µl of cell counting kit-8 (CCK-8) reagent was added to the media at different times. After developing for 1–2 h in a dark place, cell proliferation was measured by detecting the optical density (OD) at a wavelength of 450 nm (OD450). The normal primary cell suspension was seeded similarly on a 96-well plate. When cell adhesion was observed, various concentrations of LMWH (0, 0.25, 0.50, 1, 2.50, 5, and 10 IU/ml) were added, and cell proliferation was measured after 24 h.
Migration and invasion
After dissociation and centrifugation, the cells were resuspended in the media and counted. The decidual stromal cell suspension was seeded on a 6-well plate to 2.5 × 105 cells/ml and cultured for 24 h. The plate was scratched along a marked straight line with a sterile 10-µl pipette tip. Cell debris was removed by washing three times with PBS, and serum-free various concentrations LMWH media (0, 0.25, 0.50, 1, 2.50, and 5 IU/ml)) was added. The cells were incubated at 37 °C in a 5% CO2 incubator for 48 h. The cell regrowth in the scratched area was observed, and three areas were selected randomly and photographed.
The cell suspensions were prepared with the cells treated with different concentrations of LMWH, and 200 µl of single-cell suspension was transferred into a Transwell chamber; this was placed in a 24-well plate containing 700 µl of complete media (with 20% serum) and cultured at 37 °C for 24 h. Then, the chamber was removed, and the media was discarded from the well; it was washed twice with calcium-free PBS and fixed with methanol for 30 min. The chamber was thoroughly air dried. The cells were stained with 0.1% crystal violet solution for 20 min in the dark; then, the unmigrated cells at the upper layer were removed gently with swabs. The chamber was washed three times with PBS and photographed under a microscope.
Real-time quantitative polymerase chain reaction
Ribonucleic acid (RNA) was extracted from the tissues using TRIzol™ (Sigma-Aldrich, USA) as a reagent, according to the manufacturer’s instructions. The RNA concentration and purity were measured with a nucleic acid analyzer. An OD260/OD280 ratio of 1.8–2.2 indicated high RNA purity. Nucleic acids up to 1 μg were reverse transcribed into complementary DNA(cDNA) using a cDNA kit (R223-01, Vazyme, China), and a real-time quantitative polymerase chain reaction (RT-qPCR) was performed on a Roche ABI Fluorometer using ChamQ SYBR qPCR Master Mix (Q711, Vazyme, China) for RT-qPCR detection. The primers used in this study are listed in . The reaction program for each RT-qPCR was as follows: pre-denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. The melting curve was 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s. At the end of each extension step (60 °C), SYBR Green fluorescence was detected. The melting curves were analyzed to check the specificity of the PCR primers, and the relative expression levels of the target genes were calculated by normalizing them to the expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the endogenous control group using the 2−△△Ct method.
Table 1. Primer sequence.
Western blot analysis
The cells were removed, and the medium was discarded. After washing the cells once with PBS, an appropriate amount of radioimmunoprecipitation assay protein lysate buffer was added, and the cells were spread evenly. A petri dish was placed on ice to collect the cells using a scraper, and the cells were incubated on ice and lysed for 30 min. The lysate was centrifuged at 4 °C at 23 g for 10 min; then, the supernatant was collected and transferred to a 1.5-ml centrifuge tube. The protein concentration was detected using an enhanced bicinchoninic acid protein concentration analysis kit (Beyotime, Shanghai, China). About 30 μg of protein per well was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skimmed milk powder at room temperature (25 °C–27 °C) for 2 h; then, they were transferred into the primary antibody and incubated overnight on a shaker at 4 °C. The primary antibody dilution concentration was as follows: rabbit monoclonal cyclin D1 antibody (1:200 dilution, ab16663, Abcam,USA), rabbit monoclonal PCNA antibody (1:5000 dilution, ab92552, Abcam,USA), rabbit monoclonal MMP-2 antibody (1:5000 dilution, ab92536, Abcam,USA), rabbit monoclonal MMP-7 antibody (1:1000 dilution, ab207299, Abcam,USA), and rabbit polyclonal GAPDH antibody (1:2500 dilution, ab9485, Abcam,USA) as a control. Next, the membranes were incubated with goat anti-rabbit IgG H & L (1:10,000 dilution, ab6721, Abcam,USA), and proteins in the bands were detected using an enhanced chemiluminescence kit (Beyotime, Haimen, China). The levels of protein expression were analyzed using Image J software.
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM) and were analyzed using GraphPad Prism 8.0 and SPSS 22.0 software. The Wilcoxon rank–sum test was used to compare two independent samples of the original data, and the Kruskal–Wallis test was used for non-normally distributed data. Multiple-group comparisons were evaluated using a one-way analysis of variance with post hoc testing. A value of p < 0.05 was considered statistically significant.
Results
Low-molecular-weight heparin reduces the resorption rate of embryos
In embryonic development, resorbed and surviving embryos can be clearly distinguished by observation, and there is a significant difference in color between the two. In our study, the resorbed embryos were dull in color, accompanied by ischemic necrosis or brown clotted material, with obviously smaller volumes, and there was no placental formation, whereas the surviving embryos were ruddy, plump, and large, with a complete amniotic sac, placenta, and formed mouse embryos ().
Figure 1. Embryonic development of the pregnant mice in each group (n = 10, scale: 1:1, a representative figure for each group was selected). A: Control group (normal pregnant mice + normal saline). B: RSA group (RSA model mice + normal saline). C: LMWH group (RSA model mice + LMWH).
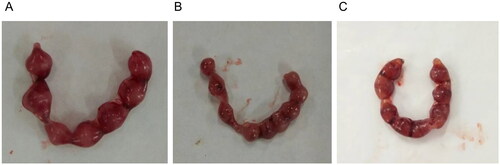
We investigated the effect of LMWH on pregnancy and embryo loss rates in RSA mice (). The pregnancy rates in the control group, RSA group, and LMWH + RSA group were 81.4%, 30.3%, and 60.7%, respectively. shows that the embryo resorption rate of the RSA group was significantly higher than that of the control group (Χ2 = 6.424, p = 0.015), while the rate of the LMWH group was significantly lower than that of the RSA group (X2 = 3.875, p = 0.037). The above results revealed that the resorption rate and embryo loss rate of the RSA model pregnant mice were significantly higher than the rates of the control group.
Table 2. Comparison of embryo resorption rate in pregnant mice among different groups.
In summary, LMWH increased the pregnancy rate in the mice and reduced the embryo resorption rate, thereby improving the pregnancy outcomes of the RSA pregnant mice.
Low-molecular-weight heparin promotes the expression of insulin-like growth factor-binding protein 1, prolactin, and messenger ribonucleic acid
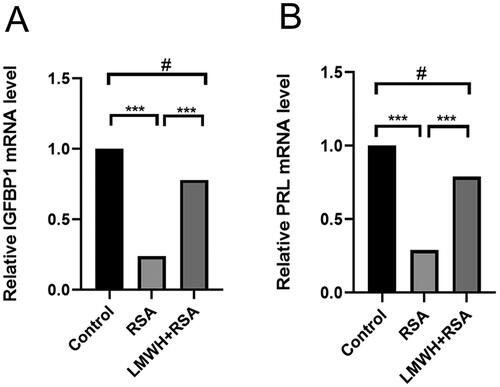
To further confirm that the effect of LMWH on the resorption rate of embryos is related to the decidualization of uterine stromal cells, we detected the mRNA expression levels of decidualization marker molecules IGFBP1 and PRL using RT-qPCR. As presented in , the mRNA expressions of IGFBP1 and PRL in the RSA group were lower than those in the control group (p < 0.001), while the mRNA expressions of IGFBP1 and PRL in the LMWH + RSA group were higher than those in the RSA group (p < 0.001). Comparing the two groups, the mRNA expression levels of IGFBP1 and PRL were similar (p > 0.05). The results revealed that LMWH may attenuate the rate of embryonic loss in early pregnancy in RSA mice by increasing the level of endometrial decidualization.
Figure 2. Expression levels of IGFBP1and PRL mRNA in the decidual tissues of the mice in each group. A: Expression level of IGFBP1 mRNA in the decidual tissues of mice. B: Expression level of PRL mRNA in the decidual tissues of mice. All experiments were conducted independently in triplicate, and the data were presented as mean ± SEM (n = 9) (*p < 0.05, **p < 0.01, ***p < 0.001, #p > 0.05, not significant).
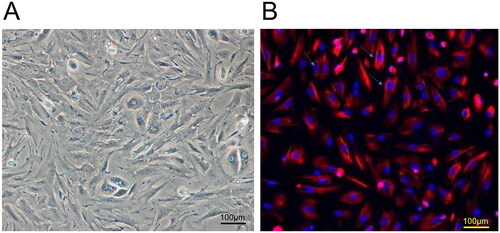
Identification of human decidual stromal cells in early pregnancy
Primary human decidual stromal cells in early pregnancy were separated and cultivated. shows the morphologies of the decidual stromal cells under a light microscope and primary cells identified by fluorescence staining.
The effect of recurrent spontaneous abortion on the proliferation rate of decidual stromal cells
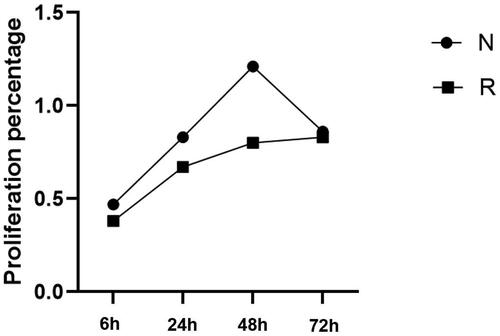
The proliferation rates were compared by analyzing the purified primary decidual stromal cells in patients with and without RSA. The proliferation rate of the decidual stromal cells in patients without RSA in early pregnancy was higher than in patients with RSA. This indicates that endometrial stromal cells in patients with RSA may not be able to decidualize effectively, which has always led to poor pregnancy outcomes ().
Low-molecular-weight heparin can promote the proliferation of human decidual stromal cells
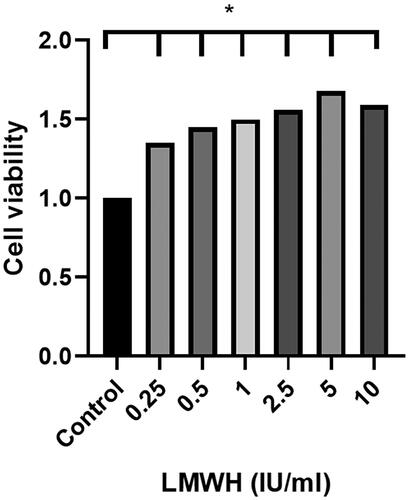
Different concentrations (0, 0.25, 0.50, 1, 2.50, 5, and 10 IU/ml) of LMWH were co-cultured with normal human decidual stromal cells in early pregnancy, and the proliferation of stromal cells was detected by CCK-8 assay. Compared with the control group, the addition of different concentrations of LMWH significantly increased the proliferation of stromal cells. There was a statistically significant difference between the two groups (p < 0.05); however, this proliferation did not correlate with the concentration of LMWH (p > 0.05) ().
Figure 5. Proliferation level of decidual stromal cells under the action of LMWH. All experiments were conducted independently in triplicate, and the data were presented as mean ± SEM (n = 6) (*p < 0.05, **p < 0.01, ***p < 0.001, #p > 0.05, not significant).
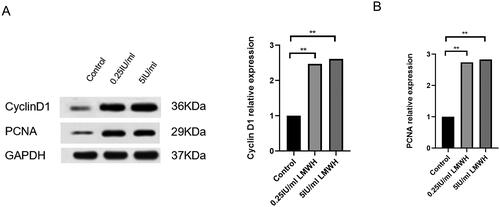
To further verify whether LMWH can promote the proliferation of decidual stromal cells, we detected the expression levels of proliferation-related proteins cyclin D1 and PCNA by Western blot. Compared with the control group, the protein expression levels of cyclin D1 and PCNA in the LMWH group were significantly increased, with a statistical difference (p < 0.05); however, the expression levels of cyclin D1 and PCNA in the 0.25-IU/ml LMWH and 5-IU/ml LMWH treatment groups showed no statistical difference (p > 0.05) ().
Figure 6. Effects of LMWH on the expression levels of cyclin D1 and PCNA in decidual stromal cells. A: Expression levels of cyclin D1 and PCNA proteins in the control group (0-IU/ml LMWH), 0.25-IU/ml LMWH and 5-IU/ml LMWH after cell co-culture. B: Cyclin D1 and PCNA protein expression. (The protein expression of MMP-2 and MMP-7 was quantified using GAPDH as a control). All experiments were conducted independently in triplicate, and the data were presented as mean ± SEM (n = 4) (*p < 0.05, **p < 0.01, ***p < 0.001, #p > 0.05, not significant).
The above results indicated that LMWH promoted the proliferation of human decidual stromal cells.
Low-molecular-weight heparin promotes the migration and invasion of human decidual stromal cells
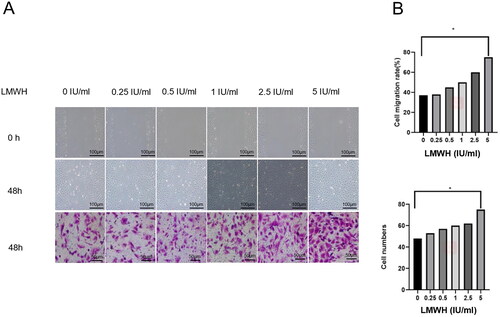
Different concentrations (0, 0.25, 0.50, 1, 2.50, and 5 IU/ml) of LMWH were co-cultured with early pregnancy normal human decidual stromal cells, and their migration and invasion were detected by scratch and Transwell assays. Compared with the control group, the migration and invasion of the decidual stromal cells in the LMWH group increased significantly after 48 h, and the difference between the two groups was statistically significant (p < 0.05) ().
Figure 7. Scratch and Transwell assays for exploring the effects of LMWH on the migration and invasion of human decidual stromal cells. A: Scratch assay: The effect of LMWH on the migration of human decidual stromal cells at 0 and 48 h. Transwell assay: The effects of LMWH on the invasion of human decidual stromal cells. B: Quantification of scratch and Transwell results. All experiments were conducted independently in triplicate, and the data were presented as mean ± SEM (n = 3) (*p < 0.05, **p < 0.01, ***p < 0.001, #p > 0.05, not significant).
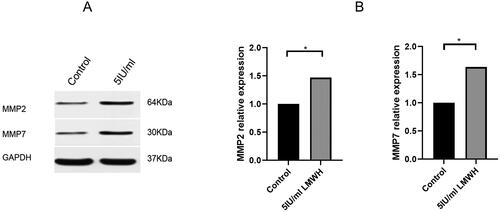
To further verify whether LMWH can promote the migration and invasion of decidual stromal cells, we detected the expression levels of migration-related proteins MMP-2 and MMP-7 by Western blot. Compared with the control group, after adding 5-IU/ml LMWH, the protein expressions of MMP-2 and MMP-7 in the cells were significantly increased, with a statistical difference (p < 0.05) ().
Figure 8. Effects of LMWH on the expression levels of MMP-2 and MMP-7 in decidual stromal cells. A: Expression levels of MMP-2 and MMP-7 proteins in the control group (0-UI/ml LMWH) and the experimental group (0.5-UI/ml LMWH) after co-culture. B: The protein expression of MMP-2 and MMP-7 was quantified using GAPDH as a control. All experiments were conducted independently in triplicate, and the data were presented as mean ± SEM (n = 6) (*p < 0.05, **p < 0.01, ***p < 0.001, #p > 0.05, not significant).
The above results indicated that LMWH promoted the migration and invasion of human decidual stromal cells.
Discussion
Recurrent spontaneous abortion is a major problem in the clinical diagnosis and treatment of common gynecological diseases, and it can seriously threaten patients’ physical and mental health. In recent years, LMWH has been widely used to treat RSA during pregnancy [Citation12, Citation31]. The anticoagulation action of LMWH was considered the key to controlling RSA. However, further research revealed that LMWH also effectively prevents URSA, indicating that LMWH may perform other functions in embryonic protection besides being an anticoagulant and antithrombotic agent [Citation27,Citation28]. The present study demonstrated that LMWH improved pregnancy outcomes by promoting the proliferation and migration of stromal cells in early pregnancy and enhancing the decidualization of stromal cells.
The maternal–fetal interface is a key site for mother–embryo nutrient exchange and cell signal transmission and comprises the decidua, placenta, myometrium, and the invasive placenta. These structures contain decidual cells, trophoblasts, and immune cells, and any factors that can change these structures and their functions will cause miscarriage [Citation32,Citation33]. The establishment of pregnancy represents the successful combination of two independent processes: endometrial decidualization and trophoblast invasion. Decidualization is still required after pregnancy due to incomplete endometrial decidualization. Decidualized stromal cells will continue to proliferate and interact with placental trophoblastic cells via migration to regulate the invasion of fetal trophoblasts, resist immune and oxidative stress responses during pregnancy, and regulate immune cell functions [Citation12,Citation13]. If the proliferation and migration abilities of decidual stromal cells are enhanced, the cells’ decidualization ability is also enhanced, and their interaction with placental trophoblast cells is improved. This outcome facilitates the invasion of trophoblasts, the enhancement of immune cell activity, and the implantation and development of embryos [Citation34].
The administration of LMWH can regulate trophoblast growth and division, inhibit the apoptosis of trophoblasts, and induce the invasion of trophoblasts [Citation35,Citation36]. Low-molecular-weight heparin participates in the adhesion and invasion of the blastocyst to uterine decidua, promoting both the proliferation of decidual cells and the formation of the placenta. Shih et al. [Citation37] found that the downregulated expression of E-cadherin and reduced adhesion ability in patients with abortion could affect the invasion of trophoblasts, thereby inducing abortion. The animal experiments conducted by Erden et al. [Citation19] confirmed that LMWH could improve the invasion of trophoblasts by regulating the expression of E-cadherin to prevent abortion. Studies have shown [Citation38] that low molecular heparin not only enhances the proliferation and invasive ability of trophoblasts, but is also able to promote endometrial angiogenesis through the release of vascular endothelial growth factor, which may be one of the mechanisms of action of LMWH in reducing pregnancy loss.
However, few studies exist on LMWH and stromal cell decidualization. By constructing an RSA mouse model, we found that the model’s embryo loss rate was significantly reduced after the injection of LMWH; the expression levels of decidualization-related factors IGFBP1 and PRL in the LMWH group of the RSA mice were significantly higher than those in the RSA group, indicating that in the LMWH group, LMWH enhanced the decidualization of stromal cells, which had the effect of protecting the fetus.
The decidualization of stromal cells is an important step in a successful pregnancy and plays a key role in the process of embryo implantation. Both IGFBP1 and PRL are used as marker molecules to measure the degree of decidualization [Citation39–41]. Endometrial stromal cell decidualization disorder can cause recurrent miscarriage. Studies have shown that in the process of stromal cell decidualization, the cell cycle factors cyclin D1 and PCNA are highly expressed in stromal cells [Citation42]. Cosmi et al. [Citation43] believed that PCNA participates in the process of endometrial proliferation. As demonstrated in well-known studies [Citation22], LMWH enhances the proliferation and invasion ability of trophoblasts.
Both MMP-2 and MMP-7, which are members of the extracellular MMP family, can degrade the extracellular matrix and enhance cell invasion. Studies have shown [Citation44] that decidual stromal cells can promote trophoblast migration by expressing MMP-2 and MMP-7. However, research on the decidualization of stromal cells by LMWH is rarely reported.
In our study, Western blot was used to detect changes in the expression levels of proliferation-related proteins cyclin D1 and PCNA and migration-related proteins MMP-2 and MMP-7 in decidual stromal cells treated with LMWH. The results showed that compared with the control group, the expression levels of cyclin D1, PCNA, MMP-2, and MMP-7 increased in the LMWH group. At different concentrations, LMWH could stimulate a significant increase in the proliferation and invasion of normal stromal cells in early pregnancy, indicating that LMWH could enhance the decidualization of stromal cells by promoting their proliferation in early pregnancy. Similar to the findings of X. Lv et al. [Citation15], the decidual stromal cells of patients with RSA had a lower degree of decidualization than that of normal pregnant women. It is speculated that the reduced degree of endometrial decidualization may cause an abnormal embryonic window period, thereby damaging the embryo or causing abnormalities. However, not all studies agree on the therapeutic role of LMWH in preventing recurrent pregnancy loss, and a randomized controlled trial showed [Citation45] that LMWH did not prevent RSA. The results of a randomized controlled trial study by Schleussner et al. that included a total of 449 patients with URSA from multiple centers [Citation28] showed that LMWH did not improve the rate of amnesties versus pregnancies in patients. A meta-analysis by Lv et al. showed [Citation46] that no single treatment regimen was significantly superior to controls for URSA, but treatment with LMWH alone had potential advantages.
This study has several limitations. The results would be more convincing if we could examine specific gene expression differences and potential metabolic pathways in uterine endometrial samples from patients with RSA and RSA + LMWH. However, due to limitations imposed by clinical treatment and ethical requirements, we were unable to obtain endometrial samples from RSA + LMWH patients. Further research at the animal level, investigating the effects of LMWH on pregnancy outcomes and decidualization in mice, would provide a deeper understanding of the specific regulatory mechanisms by which LMWH promotes early-stage endometrial decidualization. This could involve transcriptomic sequencing and metabolomics analysis to explore potential biological mechanisms and lay the foundation for studying the pathogenesis of pregnancy-related disorders such as placental dysfunction. Additionally, it may provide potential targets for improving treatment for patients with RSA in future clinical settings.
Conclusion
The pathogenic factors of RSA are complex, and LMWH may improve the pregnancy rate in patients with RSA via multiple pathways and multi-directional processes. Therefore, it is of great clinical significance to further investigate the effect of LMWH on the decidualization of stromal cells in early pregnancy and clarify its mechanism.
Ethical approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki(as was revised in 2013). The study was approved by Ethics Committee of the Yunnan First People’s Hospital (approval number: KHLL2019-KY015). Written informed consent was obtained from all participants.
Acknowledgements
We are particularly grateful to all the people who have given us help on our article.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Additional information
Funding
References
- Youssef A, Vermeulen N, Lashley EELO, et al. Comparison and appraisal of (inter) national recurrent pregnancy loss guidelines . Reprod Biomed Online. 2019;39(3):497–503. doi: 10.1016/j.rbmo.2019.04.008.
- El Hachem H, Crepaux V, May-Panloup P, et al. Recurrent pregnancy loss: current perspectives. Int J Womens Health. 2017;9:331–345. doi: 10.2147/IJWH.S100817.
- Pang L, Wei Z, Li O, et al. An increase in vascular endothelial growth factor (VEGF)and VEGF soluble receptor-1(sFlt-1)are associated with early recurrent spontaneous abortion . PLOS One. 2013;8(9):e75759. doi: 10.1371/journal.pone.0075759.
- Kolte AM, Olsen LR, Mikkelsen EM, et al. Depression and emotional stress is highly prevalent among women with recurrent pregnancy loss. Hum Reprod. 2015;30(4):777–782. doi: 10.1093/humrep/dev014.
- Arias-Sosa LA, Acosta ID, Lucena-Quevedo E, et al. Genetic and epigenetic variations associated with idiopathic recurrent pregnancy loss. J Assist Reprod Genet. 2018;35(3):355–366. doi: 10.1007/s10815-017-1108-y.
- Huang N, Gao Y, Zhang M, et al. METTL3-Mediated mA RNA methylation of ZBTB4 interferes with trophoblast invasion and maybe involved in RSA. Front Cell Dev Biol. 2022;10:894810. doi: 10.3389/fcell.2022.894810.
- Caruso A, De Carolis S, Di Simone N. Antiphospholipid antibodies in obstetrics: new complexities and sites of action. Hum Reprod Update. 1999;5(3):267–276. doi: 10.1093/humupd/5.3.267.
- Veglia M, D'Ippolito S, Marana R, et al. Human IgG antinuclear antibodies induce pregnancy loss in mice by increasing immune complex deposition in placental tissue: in vivo study. Am J Reprod Immunol. 2015;74(6):542–552. doi: 10.1111/aji.12429.
- Di Simone N, Castellani R, Caliandro D, et al. Monoclonal anti-annexin V antibody inhibits trophoblast gonadotropin secretion and induces syncytiotrophoblast apoptosis. Biol Reprod. 2001;65(6):1766–1770. doi: 10.1095/biolreprod65.6.1766.
- Ochoa-Bernal MA, Fazleabas AT. Physiologic events of embryo implantation and decidualization in human and non-human primates. Int J Mol Sci. 2020;21(6):1973. doi: 10.3390/ijms21061973.
- Cho HJ, Baek MO, Khaliq SA, et al. Microgravity inhibits decidualization via decreasing akt activity and FOXO3a expression in human endometrial stromal cells. Sci Rep. 2019;9(1):12094. doi: 10.1038/s41598-019-48580-9.
- Yang F, Zheng Q, Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the Maternal-Fetal interface. Front Immunol. 2019;10:2317. doi: 10.3389/fimmu.2019.02317.
- Lucas ES, Vrljicak P, Muter J, et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun Biol. 2020;3(1):37. doi: 10.1038/s42003-020-0763-1.
- Cottrell HN, Wu J, Rimawi BH, et al. Human endometrial stromal cell plasticity: reversible sFlt1 expression negatively coincides with decidualization. Hypertens Pregnancy. 2017;36(2):204–211.
- Lv X, Cai Z, Li S. Increased apoptosis rate of human decidual cells and cytotrophoblasts in patients with recurrent spontaneous abortion as a result of abnormal expression of CDKN1A and bax. Exp Ther Med. 2016;12(5):2865–2868. doi: 10.3892/etm.2016.3692.
- Larsen EC, Christiansen OB, Kolte AM, et al. New insights into mechanisms behind miscarriage. BMC Med. 2013;11(1):154. doi: 10.1186/1741-7015-11-154.
- Kaya Okur HS, Das A, Taylor RN, et al. Roles of estrogen receptor-α and the coactivator MED1 during human endometrial decidualization. Mol Endocrinol. 2016;30(3):302–313. doi: 10.1210/me.2015-1274.
- Chen P, Zhou L, Chen J, et al. The immune atlas of human deciduas with unexplained recurrent pregnancy loss. Front Immunol. 2021;12:689019. Published 2021 Jun 7 doi: 10.3389/fimmu.2021.689019.
- Mani SK, Julian J, Lampelo S, et al. Initiation and maintenance of in vitro decidualization are independent of hormonal sensitization in vivo. Biol Reprod. 1992;47(5):785–799. doi: 10.1095/biolreprod47.5.785.
- Castro-Leyva V, Zaga-Clavellina V, Espejel-Nunez A, et al. Decidualization mediated by steroid hormones modulates the innate immunity in response to group B streptococcal infection in vitro. Gynecol Obstet Invest. 2017;82(6):592–600. doi: 10.1159/000454770.
- Ahn JI, Yoo JY, Kim TH, et al. cAMP-response element-binding 3-like protein 1 (CREB3L1) is required for decidualization and its expression is decreased in women with endometriosis. Curr Mol Med. 2016;16(3):276–287. doi: 10.2174/1566524016666160225153659.
- Lazrak HH, René É, Elftouh N, et al. Safety of low-molecular-weight heparin compared to unfractionated heparin in hemodialysis: a systematic review and meta-analysis. BMC Nephrol. 2017;18(1):187. Published 2017. doi: 10.1186/s12882-017-0596-4.
- Zee AA, van Lieshout K, van der Heide M, et al. Low molecular weight heparin for prevention of venous thromboembolism in patients with lower-limb immobilization. Cochrane Database Syst Rev. 2017;8(8):CD006681. doi: 10.1002/14651858.CD006681.pub4.
- Bai W, Zhang X, Sun S, et al. Effect of low-molecular-weight heparins on anti-Xa peak levels and adverse reactions in Chinese patients with recurrent spontaneous abortion: a single-center, observational study. BMC Pregnancy Childbirth. 2021;21(1):683. doi: 10.1186/s12884-021-04161-1.
- Stief T. LMWH - action-monitoring for all patients. Acta Paediatr. 2012;101:e314.
- Yoneda M, Brosnan JF, Norris LA, et al. The effect of LMWH (tinzaparin) on coagulation and fibrinolytic activation in pregnant women at risk of thrombosis. Thromb Res. 2006;117(3):283–290. doi: 10.1016/j.thromres.2005.03.016.
- Jiang F, Hu X, Jiang K, et al. The role of low molecular weight heparin on recurrent pregnancy loss: a systematic review and meta-analysis. Taiwan J Obstet Gynecol. 2021;60(1):1–8. doi: 10.1016/j.tjog.2020.11.001.
- Schleussner E, Kamin G, Seliger G, et al. Low-molecular-weight heparin for women with unexplained recurrent pregnancy loss: a multicenter trial with a minimization randomization scheme. Ann Intern Med. 2015;162(9):601–609. doi: 10.7326/M14-2062.
- Luo J, Wang Y, Qi Q, et al. Sinomenine improves embryo survival by regulating Th1/Th2 balance in a mouse model of recurrent spontaneous abortion. Med Sci Monit. 2021;27:e927709. doi: 10.12659/MSM.927709.
- Wang S, Guo S, Hou X. MicroRNA-24 inhibits CDX1 expression in decidual tissues of recurrent spontaneous abortion mice to reduce the abortion risk. Adv Clin Exp Med. 2020;29(8):929–936. doi: 10.17219/acem/122173.
- de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;2014(7):CD004734. Published 2014 Jul 4
- PrabhuDas M, Bonney E, Caron K, et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16(4):328–334. doi: 10.1038/ni.3131.
- Xu L, Li Y, Sang Y, et al. Crosstalk between trophoblasts and decidual immune cells: the cornerstone of Maternal-Fetal immunotolerance. Front Immunol. 2021;12:642392. doi: 10.3389/fimmu.2021.642392.
- Hu WT, Huang LL, Li MQ, et al. Decidual stromal cell-derived IL-33 contributes to Th2 bias and inhibits decidual NK cell cytotoxicity through NF-kappaB signaling in human early pregnancy. J Reprod Immunol. 2015;109:52–65. doi: 10.1016/j.jri.2015.01.004.
- Bolnick AD, Bolnick JM, Kohan-Ghadr HR, et al. Enhancement of trophoblast differentiation and survival by low molecular weight heparin requires heparin-binding EGF-like growth factor. Hum Reprod. 2017;32(6):1218–1229. doi: 10.1093/humrep/dex069.
- Tersigni C, Marana R, Santamarìa A, et al. In vitro evidences of heparin’s effects on embryo implantation and trophoblast development[J]. Reprod Sci. 2012;19(5):454–462. doi: 10.1177/1933719111430994.
- Mori M, Barnard GF, Mimori K, et al. Overexpression of matrix metalloproteinase-7 mRNA in human Colon carcinomas. Cancer (Phi1a). 1995;75(S6):1516–1519. doi: 10.1002/1097-0142(19950315)75:6+<1516::AID-CNCR2820751522>3.0.CO;2-7.
- D'Ippolito S, Marana R, Di Nicuolo F, et al. Effect of low molecular weight heparins (LMWHs) on antiphospholipid antibodies (aPL)-mediated inhibition of endometrial angiogenesis. PLOS One. 2012;7(1):e29660. doi: 10.1371/journal.pone.0029660.
- Valatkaitė E, Baušytė R, Vitkevičienė A, et al. Decidualization potency and epigenetic changes in human endometrial origin stem cells during propagation. Front Cell Dev Biol. 2021;9:765265. doi: 10.3389/fcell.2021.765265.
- Peng Y, Jin Z, Liu H, et al. Impaired decidualization of human endometrial stromal cells from women with adenomyosis†. Biol Reprod. 2021;104(5):1034–1044. doi: 10.1093/biolre/ioab017.
- Gibson DA, Simitsidellis I, Kelepouri O, et al. Dehydroepiandrosterone enhances decidualization in women of advanced reproductive age. Fertil Steril. 2018;109(4):728–734.e2. doi: 10.1016/j.fertnstert.2017.12.024.
- Ma J, Li J, Yang S, et al. P57 and cyclin G1 express differentially in proliferative phase endometrium and early pregnancy decidua. Int J Clin Exp Med. 2015;8:5144–5149.
- Cosmi E, Saccardi C, Litta P, et al. Transvaginal ultrasound and sonohysterography for assessment of postpartum residual trophoblastic tissue[J]. Int J Gynaecol Obstet. 2010;110(3):262–∼264. doi: 10.1016/j.ijgo.2010.03.036.
- Xie J, Zhou X, Wang R, et al. Identification of potential diagnostic biomarkers in MMPs for pancreatic carcinoma. Medicine (Baltimore). 2021;100(23):e26135.)doi: 10.1097/MD.0000000000026135.
- Quenby S, Booth K, Hiller L, et al. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): an international open-label, randomised controlled trial. Lancet. 2023; 402(10395):54–61. doi: 10.1016/S0140-6736(23)00693-1.
- Lv S, Yu J, Xu X. A comparison of effectiveness among frequent treatments of recurrent spontaneous abortion: a bayesian network meta-analysis. Am J Reprod Immunol. 2018; 80(1):e12856.