Abstract
The study aimed to investigate whether serum IL-1β, FoxO1and Sesn2 concentrations differed between threatened preterm labor (TPL) and uncomplicated pregnancies. This study was conducted on 54 women with TPL pregnancies and 26 healthy pregnant women. The TPL group was further divided into two subgroups according to the gestational age at delivery. Patients who gave birth within 48–72 hours after the hospitalization were referred to as preterm delivery (PD) and those who gave birth at ≥37 weeks were referred to as term delivery (TD). Maternal levels of serum IL-1β, FoxO1 and Sesn2 were measured with the use of enzyme-linked immunosorbent assay kits. The mean maternal serum IL-1β and FoxO1 of PD were significantly higher than TD (p<.000*) and the control group (p < .000*). The mean maternal serum IL-1β, FoxO1 level of TD was significantly higher than the control group (p<.000*). The mean maternal serum Sesn2 levels of TD and the control group were significantly higher than the preterm group (p<.000*). The mean maternal serum Sesn2 level of the control group was significantly higher than the TD group (p <.000*). A negative correlation was found between serum concentration of serum IL-1β, and FoxO1 with the gestational week of delivery (r= −0.722, p< .000*for, IL-1β; r = −0.625, p < .000* for FoxO1). A positive correlation was found between the serum concentration of serum Sesn2 with the gestational week of delivery (r = 0.507, p<.000* for sesn2). High serum IL-1β, FoxO1 levels, and low Sesn2 levels may have the potential to be used as biomarkers for the differentiation of PD and TD.
Introduction
Preterm birth is the main cause of neonatal and maternal morbidity and mortality and affects approximately 10–12% of all pregnancies [Citation1]. 70% of newborn deaths occur because of the etiological basis of prematurity [Citation2–5]. It is proven that there is an inflammatory response or oxidative stress that triggers the accumulation of cytokines, such as IL-1, IL-6, IL-8, and metalloproteinases, in the membranes, umbilical cord, and placenta. The onset of preterm labor can begin with these chemokines, thereby increasing uterine contractions and cervical maturation [Citation6–9].
Interleukin-1β (IL-1β) is a potent pro-inflammatory cytokine that initiates and enhances a wide variety of effects associated with immune and host responses to microbial invasion and tissue damage. The production and release of IL-1β are stimulated by pathogen-associated molecular model molecules which involve several steps [Citation10]. IL-1β is first synthesized as biologically inactive pro-IL-1β, processed by caspase-1 as mature, biologically active IL-1β, and then released into the extracellular environment. Although recent publications have greatly increased our knowledge of the mechanisms involved in the production and processing of IL-1β, the mechanisms of IL-1β secretion and maturation remain unclear. Different models of the non-classical secretory pathway used by monocytes, macrophages, and dendritic cells to release IL-1β have been reported [Citation10]. A better understanding of IL-1β is of great therapeutic importance. This is thought to be especially important in understanding the mechanisms of normal birth and in the treatment of preterm births [Citation10, Citation11].
Sestrins (Sesns), metabolic proteins induced by stress, are known to protect cells against various harmful stimuli such as DNA damage, oxidative stress, starvation, endoplasmic reticulum (ER) stress, and hypoxia. Sesns are regulated mainly through the activation of the key energy sensor AMP-dependent protein kinase (AMPK) and inhibition of rapamycin complex 1 (mTORC1) [Citation12]. There is evidence that Sesns2, the transcriptional target of p53, triggers AMPK activation and reduces mTORC1 signaling, especially in mouse and human decidual cells [Citation12, Citation13]. Preclinical studies using mouse models and human decidual cells may provide a basis for therapeutics currently used in the treatment of preterm births [Citation12–14].
Forkhead box O1 (FoxO1) is a proinflammatory modulator. It was reported that its expression increased in myometrial biopsies taken from women who gave birth at term and preterm [Citation15]. In addition, deletion of FoxO1 in human myometrial cells attenuates the capacity of IL-1β to induce inflammatory gene expression [Citation13]. Specifically, FoxO1 knockdown was reported to significantly reduce the release of IL-6, IL-8, and prostaglandins (PGE2 and PGF2α) from IL-1β [Citation16, Citation17]. IL-1 β, FoxO1, and Sesn2 may be important biomarkers in predicting increased inflammatory response and preterm birth [Citation18, Citation19].
We hypothesized that IL-1β, and FoxO1, which are important in the inflammatory response, and the cellular adaptation molecule sestrin may be found in different compositions in healthy pregnant women and preterm birth groups. Therefore, this study aims to determine serum Foxo1, IL-1β, and Sesns2 levels in patients with threatened preterm labor. Thus, it is possible to distinguish between high-risk pregnancies and false preterm birth. To our knowledge, this is the first study to investigate maternal serum levels of FoxO1 and Sesn2 in pregnancies with preterm and term deliveries. We evaluated whether these parameters affected the course of preterm labor.
Materials and methods
This case-control study was carried out following the approval of our university’s Ethics Committee based on international Helsinki principles (date: December 09, 2017; number: 83045809-604.0102-A26). All participants were given detailed instructions and they signed informed consent forms before recruitment. A total number of 80 pregnant women were included who were between 28 ± 0 and 35 ± 6 weeks of gestation: 26 women with normal pregnancies, and 54 patients who had been diagnosed with "threatened preterm labor" (TPL) at the Cerrahpasa Medical Faculty Department of Obstetrics and Gynecology between March 2017 and April 2019. TPL was defined as the presence of at least four regular and painful uterine contractions in 20 min or six contractions in 1 h, lasting at least 30 s, which can be measured with an electronic cardiotocography device, or 20 mm cervical dilatation or 80% of effacement on examination.
The TPL group was further divided into two subgroups according to the gestational age at delivery including women in the "preterm delivery group" (PD, n = 27) and the ones who gave birth at ≥ 37 weeks "term delivery group" (TD, n = 27). All patients in the PD group gave birth within 48–72 hours after admission. The clinical and laboratory data of the TPL group, consisting of PD and TD groups, were compared with the control group consisting of women with healthy pregnancies (control group n = 26).
While determining the exclusion criteria, the need for urgent treatment was tried to be taken into account. Multiple pregnancies, uterine anomaly, premature rupture of membranes, placental abruption, fetal distress (electronic cardiotocography device was used for the diagnosis of fetal distress), placenta previa, preeclampsia/eclampsia, chronic hypertension, diabetes mellitus, gestational diabetes, hyperthyroidism, fetal growth restriction (FGR), heart disease, infection, polyhydramnios, oligohydramnios, cervical cerclage or surgery, anemia (hemoglobin <10 g/mL), and increased leukocyte count (white blood cell WBC > 15,000/mm3). Infection was excluded based on CRP (C-reactive protein), urine culture, body temperature, leukocyte count, and pelvic examination findings. We also excluded pregnant women who smoked and used drugs, such as salicylic acid, nonsteroidal anti-inflammatory drugs, antihypertensive drugs, or antibiotics. Gestational age was determined according to the following criteria: until the last menstrual period and/or crown-rump length (CRL) on first-trimester ultrasound [Citation20]. Patient age information of the cases, weight, body mass index (BMI kg/m2), parity, and Bishop’s score, were recorded. The cervical canal length was determined by ultrasonography by the same obstetrician (Toshiba Xario, Tokyo, Japan). Blood samples were collected at room temperature (24 °C) before drug administration from the TPL and control groups. Complete blood count (CBC) was performed using an automated hematology analyzer (Cell-Dyn) 4000 (Abbott Diagnostics, Santa Clara, CA USA). A chemistry Analyzer (Roche/Hitachi, Basel, Switzerland) was used in the biochemistry department of the hospital. They were initially treated with hydration and if persistent uterine contractions occurred, they were given a 20 mg oral loading dose of nifedipine followed by 10–20 mg three to four times daily, adjusted according to uterine activity for up to 48 h. Betamethasone was given as an antenatal corticosteroid therapy for fetal lung maturation [Citation21].
Although the FoxO1 protein has a nuclear transcription function, it can be detected in the bloodstream like many intracellular molecules. It can be analyzed by the ELISA method [Citation22, Citation23]. With our current knowledge, we could not find any publication containing blood circulation value ranges in pregnant women. However, measurements have been made previously for all three parameters in our study in units similar to our findings, and standardized pregnancy values have not yet been determined. Serum samples for the determination of biomarkers IL-1β, FoxO1, and Sesn 2, were obtained from venous blood samples by centrifugation of coagulated samples for 30 min. Separated serum samples were stored in several storage containers in small aliquots at −80 °C until testing. Enzyme-linked immunosorbent assay (ELISA) was used (twice) to determine serum levels (Sunred Biotechnology Co., Shanghai, China; catalog number: 201-12-0629 for FoxO1; 201-12-0144 for IL-1β, and 201-12-4926 for Sesn2). In our study, all measurements were performed according to the manufacturer’s instructions. In the last step of the procedure, serum concentrations were determined by reading microplates at 450-nanometer absorbance according to the standard curve. Serum levels for FoxO1 and Sesn2 were expressed as ng/mL, and IL-1β serum level was expressed as pg/mL. Measurement ranges, precision, intra and inter-assay precision of kits for FoxO1, 0.124 ng/mL, 0.15–40 ng/mL, <10%, <12%, for Sesn2, 0.0853 ng/mL, 0.1–30 ng/mL, <10%, <12%, for IL-1β 15,013 pg/mL, 20–8000 pg/mL, <10%, and < 12%.
Statistical analysis was performed using the Statistical Package for the Social Science (SPSS) software version 23 (Chicago, IL). The Kolmogorov-Smirnov test was used to evaluate the normality of the distribution of variables. All analyses were two-tailed and p-values below 0.05 were considered statistically significant. The data are presented as mean ± standard deviation. FoxO1, IL-1β, and Sesn2 levels were converted logarithmically to obtain normal distributions, and the significance of the differences between the variables and patient subgroups was determined using the Bonferroni test after ANOVA. Spearman correlation coefficients (r) were calculated for nonparametric distribution parameters.
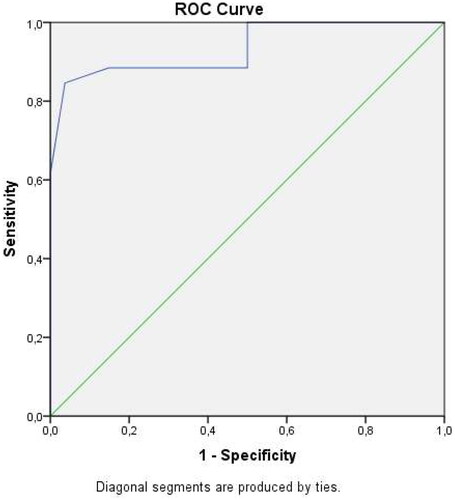
Boxplot diagrams were used for graphical visualization of endocan levels between subgroups. Boxes show the median (black line) and the 25th and the 75th centiles (top and bottom lines of the box). (ROC) analysis was used to determine the predictive threshold of maternal serum IL-1β, FoxO1, and Sesn 2 levels for predicting delivery within 48–72 h after admission.
Results
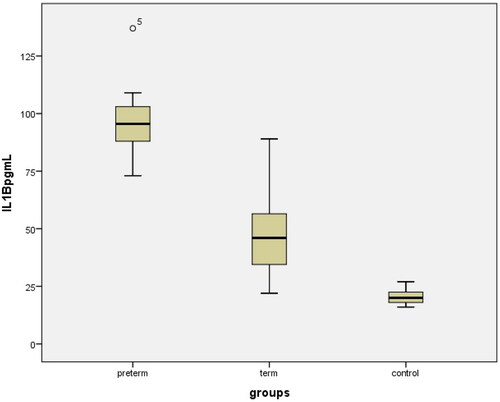
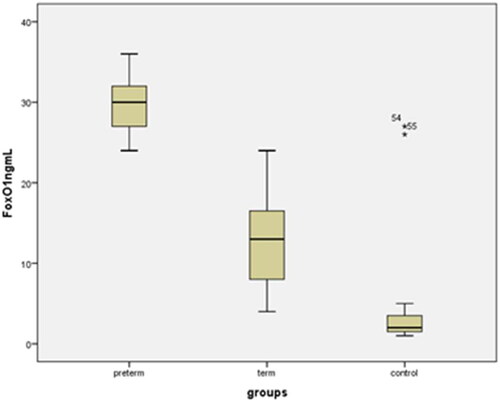
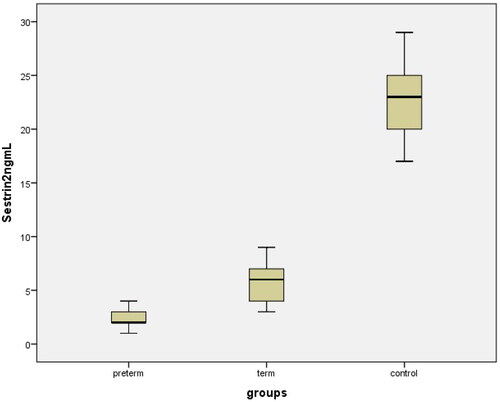
The clinical characteristics, laboratory parameters, and perinatal outcomes of the control and study groups are shown in . There were no statistically significant differences in mean maternal age, gravidity, parity, gestational week at blood sampling time, hemoglobin (Hb g/dL), WBC, CRP, platelets (x10 9/L) between the PD, TD, and control groups (p>.05). The mean gestational age at delivery, mean birth weight, and mean platelet volume (MPV) were significantly lower in PD patients than in TD and control subjects (p<.001). Body mass indices were significantly higher in the PD and TD groups than in the control group. The mean maternal serum Sesn2, FoxO1, and IL-1β concentrations in PD, TD, and uncomplicated pregnancies are shown in and (p1: PD-control; p2: TD-control; p3: PD-TD). Delivery within 48–72 h after admission occurred in 27 preterm deliveries (PD), and 53 cases delivered at ≥ 37 weeks with term delivery (TD) and control cases. A comparison of maternal and fetal characteristics of the study population is presented in .
Table 1. Maternal and fetal characteristics of the study population.
Table 2. Comparison of term group (term delivery + control) and preterm groups.
The mean maternal serum FoxO1 and IL-1β levels in the PD group were significantly higher than those in the TD and control groups (29.07 ± 6.233 ng/mL vs. 12.91 ± 5.45 ng/mL, 3.23 ± 2.95 ng/mL, p<.000); 95.64 ± 12.75 vs 47.36 ± 16.55 pg/mL, 20.24 ± 3.04 pg/mL, p<.000); The mean maternal serum Sesn2 level of the PD group was significantly lower than the TD and control groups (1.98 ± 0.7 ng/mL vs. 5.9 ± 1.51 ng/mL, 22.82 ± 3.1 ng/mLp<.000) (). FoxO1 and IL-1β levels were also significantly lower in the total TD group (TD + Control, n = 53) than in the PD group. The mean Sesn2 level was also significantly higher in the tTD group (TD + Control, n = 53) than in the PD group ().
Moderate negative correlations were found between serum concentrations of Foxo1 and IL-1β and gestational week of delivery, birth weight, MPV, cervical length, and body mass index at blood sampling (kg/m2) ().
Table 3. Correlation analyses between maternal serum IL-1β, Foxo1, and Sesn2 all other parameters in whole groups.
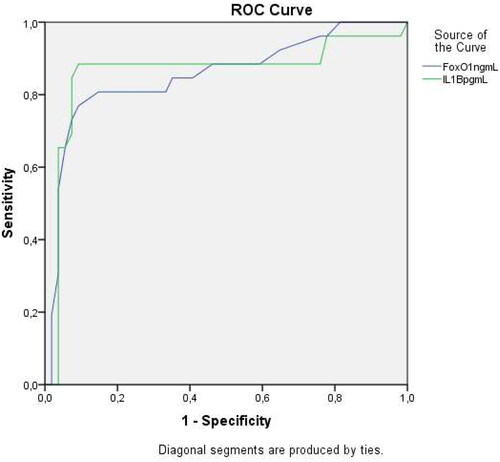
Receiver operating characteristic (ROC) curves were also evaluated. The threshold value for maternal serum FoxO1 in predicting delivery within 48–72 h was calculated as ≥ 27.23 ng/mL, and the area under the curve (AUC) was 0.855 (95% confidence interval (CI) 0.82–0.93, 0.853 p>.001 with 72.6% sensitivity and 76.5 specificity. The threshold value for maternal serum IL-1β in predicting delivery within 48–72 h was calculated as ≥82.23 pg/mL, and the area under the curve (AUC) was 0.861 (95% confidence interval (CI) 0.85–0.97, 0.853 p>.001 with 81.6% sensitivity and 79.5% specificity). () The threshold value for maternal serum Sesn2 in predicting delivery within 48–72 h was calculated as ≤1.67 ng/mL, and the area under the curve (AUC) was 0.905 (95% confidence interval (CI) 0.86–0.92, 0.883 p>.001 with 87.6% sensitivity and 77.6 specificity ().
Discussion
It is important to distinguish between true preterm birth cases and false preterm birth cases that give birth on time in pregnant women who are hospitalized with a diagnosis of risk of premature birth. A correct distinction shortens the hospital stay and protects pregnant women and newborns from the side effects of the treatment. In our study, maternal serum IL-1β levels were compared between TPL subgroups (PD and TD) and healthy control groups. It was also significantly higher in the term and control groups. In addition, maternal serum IL-1β concentration was negatively correlated with gestational age at delivery, birth weight, and cervical length. The results showed that serum FoxO1 and IL-1β levels were significantly higher in the PD group than in the term and control groups. In contrast, the Sesn2 levels were significantly lower in the PD group than in the term and control groups. Recent studies have shown that an increased maternal inflammatory response is associated with various pregnancy pathologies such as preeclampsia, preterm delivery, and fetal growth restriction. Park et al. showed that NLRP3 (NLRP3 sensor molecule, ASC adapter protein, and pro-caspase-1) inflammasome signaling induced by lipopolysaccharide (LPS) in human trophoblast cells caused Caspas1 activation, which in turn increases pro-IL-1β release. [Citation24]. The induced increase in mature IL-1β also showed that it elicits an excessive immune response. [Citation24]. Terzidou et al. reported that IL-1β is an early inflammatory precursor of the effective latent labor phase, which is prolonged in pregnant women who lack an effective inflammatory response, and that IL-1β may be effective in the induction of labor as a proinflammatory agent. [Citation25]. Mittal et al. revealed that while IL-1β initially upregulated the oxytocin receptor, prolonged exposure to IL-1β impairs the oxytocin response in myometrial cells and leads to a decrease in the amount of calcium in myocytes [Citation26]. It has been reported that uric acid and palmitic acid, which are molecules associated with metabolic disease, can stimulate inflammation in the placenta by activating the secretion of the NLRP3 inflammasome and IL-1β through the production of reactive oxygen species in trophoblasts. NLRP3 inflammation and increased IL-1β levels have been associated with pregnancy-related diseases such as recurrent pregnancy loss, hyperemesis, and pregnancy-related preeclampsia [Citation27]. Sterile intra-amniotic inflammation is an important etiological cause of spontaneous preterm births. Activation of the NLRP3 inflammasome has been reported to be one of the causes of sterile intraamniotic inflammation. Released caspase-1 causes increased IL-1 levels and premature birth. Lopez et al. performed ultrasound-guided NLRP3 inflame-inducing intra-amniotic S100B, resulting in a 50% preterm delivery and a high neonatal mortality rate of 59.7% [Citation28]. In cases where the specific NLRP3 inhibitor MCC950 was applied as a new treatment approach, the rate of preterm birth decreased to 35.7%, and the neonatal mortality rate was 26.7% [Citation18, Citation19]. IL-1 β levels were significantly higher than those in the control group, which was administered phosphate-buffered saline (PBS) instead of S100B [Citation25, Citation26]. We also found that IL-1β maternal levels were significantly higher in the preterm group than in the term group.
It has been reported that it can occur by increasing cAMP, progesterone, and IL-8 to induce decidualization in the endometrial tissue in preparation for human parturition. The increase in IL-1 β, IL-6, and IL-8 has also been reported to be due to FoxO1 upregulation [Citation29, Citation30]. FoxO1 plays a role in normal parturition mechanisms as a transcription factor related to both mitochondrial and endoplasmic reticulum functions. FoxO1 is also involved in mitochondrial stress and ER and is affected by placental senescence. When Lucy et al. examined post-term placental tissues, FoxO1 levels were found to be significantly lower than in placentas below 40 weeks of age [Citation31]. Since FoxO1 is at the junction of the oxidative stress and apoptotic pathways, it is reported to be a therapeutic target in the treatment of preterm pregnancy [Citation29, Citation30].
Recent studies suggest that Sesn2 may be a new potential therapeutic target molecule for trophoblast invasion, trophoblast functions, and prevention of pregnancy complications via the AMPK/mTORC1 pathway [Citation32]. Morbidly obese women are more likely to have pregnancy complications, such as preeclampsia, hypertension, preterm labor, and gestational diabetes. The relationship between maternal obesity, insufficient trophoblast invasion, and impaired trophoblast function is well known. Pregnancy and high metabolic activity of the placenta may be associated with inflammation that causes oxidative stress. In diabetes and preeclampsia, which impair placental function, inflammation of radical oxygen metabolites and oxidative stress levels increase in the placenta. Sesn2 is associated with the pathophysiology of ER stress, insulin resistance, type 2 diabetes, and cardiac diseases. Recently, hepatic Sesn2 was reported to be upregulated in response to ER stress during nonalcoholic fatty liver development [Citation33]. A growing body of research has shown that obese women have more pregnancies resulting in spontaneous preterm delivery than those with normal BMI. It has been reported to increase the possibility of preterm delivery due to sterile inflammation or metabolic mechanisms that are not yet clear, especially in pregnant women with BMI ≥ 30 (kg/m2) [Citation34, Citation35].
Sesn2 has been reported to suppress inflammatory pathways in the endothelium and macrophages. Knockdown of Sesn2 has been shown to increase palmitate-induced ER stress, inflammatory signaling, and apoptosis. Additionally, Sesn2 inhibits palmitate and attenuates palmitate-induced ER stress and inflammation-induced invasion of damaged trophoblasts via the AMPK/mTORC1 pathway. [Citation36–38] Lee et al. reported that sestrin 2 exerted a protective effect on the cell by reducing the radical oxygen space (ROS), possibly by inducing Nrf2, in mouse trophoblast cells that underwent palmitate-induced damage. Knockout of Sesn2 increases the levels of palmitate-induced phosphorylation and proinflammatory cytokine genes [Citation39]. This suggests that Sesn2 plays a protective role against blasts in trophoblasts. Little is known about the role of Sesn2 in trophoblast inflammatory response [Citation36–38]. In our study, the lowest Sesn2 levels were observed in the preterm delivery group. Further studies are needed to clarify whether the expression of Sesn2 in the human placenta and pregnant women with pathological structures such as preeclampsia and preterm birth is functionally related.
As a result, the predominant feature of preterm cases seems to be an inflammatory process compared to healthy pregnancies with high IL1-β and, FoxO1 levels, and low Sesn2 levels, which may be the result or cause of pathophysiological changes. TPL and PD may have similar symptoms, but different pathogenesis. This is the first study to focus on the changes in serum levels of IL-1β, FoxO1, and Sesn 2 in patients with TPL. Our findings demonstrated increased serum IL-1β, and FoxO1 levels in the TPL group, especially in the preterm delivery group. We speculate that increased maternal serum IL-1β and FoxO1 levels may be related to the inflammatory process which is the predominant feature of preterm delivery. Low sestrin levels indicate disruption of cellular homeostasis that would offset increased inflammatory performance. These findings would suggest that IL-1β, FoxO1, and Sesn 2 may be useful markers to define a high-risk group for preterm delivery in patients with threatened preterm labor and similar cervical length measures, which may benefit from increased surveillance and timely administration of corticosteroids for fetal lung maturity enhancement. Our study had several limitations. The main limitation of our study was the small sample size of the study groups. We had to keep our case group limited to avoid confusion caused by overlapping cases requiring different treatments. Further studies are required to confirm these findings.
Disclosure statement
The authors declare they have no conflict of interest.
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author at [email protected]
Additional information
Funding
References
- Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362(6):529–535. doi:10.1056/NEJMra0904308.
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70(2):1–51. 33814033
- Osterman M, Hamilton B, Martin JA, et al. Births: final data for 2020. Natl Vital Stat Rep. 2021; Feb70(17):1–50. PMID: 35157571.
- Singh S, Madhusudhan Dey M, Singh S. Biochemical markers as predictor of preterm labor - and their clinical relevance - the current. Gynecol Obstetr Reprod Med. 2022;3(28):12–15.
- Koç E, Bolat F, Kaymak Cihan M, et al. The effect of cord blood vitamin D level on bronchopulmonary dysplasia and other neonatal morbidities in preterm infants. Gynecol Obstetr Reprod Med. 2022;28(2):179–185.
- Gomez-Lopez N, Romero R, Xu Y, et al. Neutrophil extracellular traps in the amniotic cavity of women with intra-amniotic infection: a new mechanism of host defense. Reprod Sci. 2017;24(8):1139–1153. doi:10.1177/1933719116678690.
- Di Renzo GC, Tosto V, Giardina I. The biological basis and prevention of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:13–22. doi Epub 2018 Feb 16. PMID: 29703554. doi:10.1016/j.bpobgyn.2018.01.022.
- Daskalakis G, Arabin B, Antsaklis A, et al. Preterm labor: up to date. Biomed Res Int. 2019;2019:4870938–4870932. May 9 PMID: 31211139; PMCID: PMC6532298. doi:10.1155/2019/4870938.
- Chatur N, Castro M, Young Tai KF. Macular rash, thrombocytopenia, and hyperbilirubinemia in a preterm ınfant. Case Rep Pediatr. 2019; Apr 82019:4076740. PMID: 31093405; PMCID: PMC6476038. doi:10.1155/2019/4076740.
- Nadeau-Vallée M, Obari D, Quiniou C, et al. A critical role of interleukin-1 in preterm labor. Cytokine Growth Factor Rev. 2016;28:37–51. doi Epub 2015 Nov 30. PMID: 26684042. doi:10.1016/j.cytogfr.2015.11.001.
- Nadeau-Vallee M, Obari D, Beaudry-Richard A, et al. Preterm birth, and neonatal ınjuries: ımportance of ınterleukin-1 and potential of ınterleukin-1 receptor antagonists. Curr Pharm Des. 2017;23(40):6132–6141. PMID: 28847304. doi:10.2174/1381612823666170825145114.
- Deng W, Cha J, Yuan J, et al. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J Clin Invest. 2016;126(8):2941–2954. Aug 1 Epub 2016 Jul 25. doi:10.1172/JCI87715.
- Wolfson RL, Chantranupong L, Saxton RA, et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351(6268):43–48. doi:10.1126/science.aab2674.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27(1):2. doi:10.1186/s11658-021-00302-8.
- Paquette AG, MacDonald J, Bammler T, et al. Placental transcriptomic signatures of spontaneous preterm birth. Am J Obstet Gynecol. 2023;228(1):73.e1–73.e18. doi:10.1016/j.ajog.2022.07.015.
- Sakabe NJ, Aneas I, Knoblauch N, et al. Transcriptome and regulatory maps of decidua-derived stromal cells inform gene discovery in preterm birth. Sci Adv. 2020;6(49):86–96. doi:10.1126/sciadv.abc8696.
- Lappas M. Forkhead box O1 (FOXO1) in pregnant human myometrial cells: a role as a pro-inflammatory mediator in human parturition. J Reprod Immunol. 2013;99(1-2):24–32. ISSN 0165-0378. doi:10.1016/j.jri.2013.04.005.
- Graves DT, Milovanova TN. Mucosal ımmunity and the FOXO1 transcription factors. Front Immunol. 2019; Nov 2910:2530. PMID: 31849924; PMCID: PMC6896163. doi:10.3389/fimmu.2019.02530.
- Kim M, Kowalsky AH, Lee JH. Sestrins in physiological stress responses. Annu Rev Physiol. 2021;83(1):381–403. Feb 10 Epub 2020 Oct 28. PMID: 33113341; PMCID: PMC7878365. doi:10.1146/annurev-physiol-031620-092317.
- Hadlock FP, Harrist RB, Sharman RS, et al. Estimation of fetal weight with the use of head, body, and femur measurements – a prospective study. Am J Obstet Gynecol. 1985;151(3):333–337. doi:10.1016/0002-9378(85)90298-4.
- American college of obstetricians and gynecologists, committee on practice Bulletins-Obstetrics. Practice bulletin. Manage Preterm Labor Obstet Gynecol. 2016;1(159):e29.
- Kazemi Fard T, Ahmadi R, Akbari T, et al. FOXO1 and cytokines associations in patients with coronary artery disease. Cytokine. 2021;141:155443. doi:10.1016/j.cyto.2021.155443.V.
- Ibrahim HA, Zakaria SS, El-Batch MM, et al. The value of SIRT1/FOXO1 signaling pathway in early detection of cardiovascular risk in children with β-Thalassemia major. Biomedicines. 2022;10(10):2601. Oct 17 PMID: 36289866; PMCID: PMC9599077. doi:10.3390/biomedicines10102601.
- Park S, Shin J, Bae J, et al. SIRT1 alleviates LPS-ınduced IL-1β production by suppressing NLRP3 ınflammasome activation and ROS production in trophoblasts. Cells. 2020;9:728. doi:10.3390/cells9030728.
- Terzidou V, Blanks AM, Kim SH, et al. Labor, and ınflammation ıncrease the expression of oxytocin receptor in human amnion. Biol Reprod. 2011;84(3):546–552. doi:10.1095/biolreprod.110.086785.
- Mittal P, Romero R, Tarca AL, et al. A molecular signature of an arrest of descent in human parturition. Am J Obstet Gynecol. 2011;204(2):177.e15–33. PMID: 21284969; PMCID: PMC3053040. doi:10.1016/j.ajog.2010.09.025.
- Tamura K, Ishikawa G, Yoshie M, et al. Glibenclamide inhibits NLRP3 inflammasome-mediated IL-1β secretion in human trophoblasts. J Pharmacol Sci. 2017;135(2):89–95. ISSN 13478613. doi:10.1016/j.jphs.2017.09.032.
- Gomez-Lopez N, Romero R, Garcia-Flores V, et al. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomes. Biol Reprod. 2019;100(5):1306–1318. doi:10.1093/biolre/ioy264.
- Li JK, Lai PF, Tribe RM, et al. Transcription factors regulated by cAMP in the smooth muscle of the myometrium at human parturition. Biochem Soc Trans. 2021;49(2):997–1011. doi:10.1042/BST20201173.
- Fernando F, Veenboer GJ, Oudijk MA, et al. TBX2, a novel regulator of labor. Medicina. 2021;57(6):515. doi:10.3390/medicina57060515.
- Lucy AB, McKeating DR, Fisher JJ, et al. Post-term pregnancy is associated with increases in markers of senescence and. Mitochondrial Alterat Through Gestat Placent Pathol. 2021;100:104.
- Hussain T, Murtaza G, Metwally E, et al. The role of oxidative stress and antioxidant balance in pregnancy. Mediators Inflamm. 2021;2021:9962860–9962811. doi:10.1155/2021/9962860.
- Han D, Kim H, Kim S, et al. Sestrin2 protects against cholestatic liver injury by inhibiting endoplasmic reticulum stress and NLRP3 inflammasome-mediated pyroptosis. Exp Mol Med. 2022;54(3):239–251. doi:10.1038/s12276-022-00737-9.
- Liu B, Xu G, Sun Y, et al. Association between maternal pre-pregnancy obesity and preterm birth according to maternal age and race or ethnicity: a population-based study. Lancet Diabetes Endocrinol. 2019;7(9):707–714. ISSN 22138587. doi:10.1016/S2213-8587(19)30193-7.
- Tersigni C, Neri C, D’Ippolito S, et al. Impact of maternal obesity on the risk of preterm delivery: insights into pathogenic mechanisms. J Maternal-Fetal Neonatal Med. 2022;35(16):3216–3221. doi:10.1080/14767058.2020.1817370.
- Hwang HJ, Jung TW, Choi JH, et al. Of sestrin2 increases pro-inflammatory reactions and ER stress in the endothelium via an AMPK-dependent mechanism. Biochim Biophys Acta Mol Basis Dis. 2017;1863(6):1436–1444. doi:10.1016/j.bbadis.2017.02.018.
- Yang JH, Kim KM, Kim MG, et al. Role of sestrin2 in the regulation of proinflammatory signaling in macrophages. Free Radic Biol Med. 2015;78:156–167. doi:10.1016/j.freeradbiomed.2014.11.002.
- Yang K, Xu C, Zhang Y, et al. Sestrin2 suppresses classically activated macrophages-mediated inflammatory response in myocardial infarction through inhibition of mTORC1 signaling. Front Immunol. 2017;8:728. doi:10.3389/fimmu.2017.00728.
- Lee S, Shin J, Hong Y, et al. Sestrin2 alleviates palmitate-induced endoplasmic reticulum stress, apoptosis, and defective invasion of human trophoblast cells. Am J Reprod Immunol. 2020;83(4):e13222. Epub 2020 Feb 5. PMID: 31958198. doi:10.1111/aji.13222.