Abstract
Objective
Pelvic artery embolization (PAE) is a uterus-saving treatment for postpartum hemorrhage (PPH); however, subfertility or abnormal placentation for subsequent pregnancy has been a concern in several previous reports. This study aimed to investigate the impact of PAE on subsequent pregnancies in women with a history of PPH.
Methods
A retrospective cohort study was conducted on women transferred to the tertiary center for PPH and delivered for the next pregnancy at the same center later. The study group was divided into two groups based on PAE application to treat previous PPH.
Results
Of the 62 women included, 66% (41/62) had received PAE for the previous PPH, while 21 had not. Pregnancy outcomes for subsequent pregnancies were compared between the PAE and non-PAE groups. The PAE group had a higher estimated blood loss volume for the present delivery than the non-PAE group (600 vs. 300 mL, p = 0.008). The PAE group also demonstrated a higher incidence of placenta previa (4.8% vs. 24.4%, p = 0.080) and placenta accreta (0% vs. 14.6%, p = 0.082) than the non-PAE group, although the difference was not statistically significant.
Conclusion
These findings suggest that the use of PAE to treat PPH may increase the risk of bleeding, placenta previa, and placenta accreta spectrum in subsequent pregnancies.
Introduction
Postpartum hemorrhage (PPH) remains a leading cause of maternal mortality and sometimes results in serious morbidities. Traditionally, estimated blood loss in excess of 500 ml after vaginal birth or 1,000 ml after cesarean delivery was used to diagnose PPH; however, the American College of Obstetricians and Gynecologists (ACOG) stated a new classification for PPH: cumulative blood loss ≥ 1,000 ml or blood loss with signs of hypovolemia within 24 h after the birth regardless of delivery mode [ACOG 2017] [Citation1]. Among the causes of peripartum maternal deaths worldwide, PPH accounts for approximately 10–20% [Citation2]. Appropriate timing and amount of transfusion are always the most important intervention for PPH; nevertheless, a hysterectomy should be followed when bleeding does not stop, and the patient’s condition deteriorates despite conservative treatments such as transfusion and administration of uterotonics [Citation3].
In the recent two decades, pelvic artery embolization (PAE) using angiography has greatly lowered the hysterectomy rate. Therefore, the uterus could be saved for the next conception, and complications from the emergent operation (usually subtotal hysterectomy) could be avoided [Citation4,Citation5]. Although PAE is an innovative modality for PPH to preserve fertility, several studies suggested the possibility of association with subfertility afterward and a higher risk for recurrence of PPH with abnormal placentation, including the placenta accreta spectrum [Citation6–9]. Since randomized controlled trials or other large prospective studies are not very easy for PPH management, the evidence of PAE’s long-term effects is limited. Therefore, this study aimed to evaluate obstetric outcomes and complications in the subsequent pregnancy among women who had experienced PPH and had been treated by PAE for the previous pregnancy.
Methods
A retrospective cohort study was conducted at Seoul National University Bundang Hospital, a single tertiary center, and included women who had been transferred from local clinics or other primary maternity hospitals to manage PPH between January 2010 and December 2020. Most primary maternity hospitals were conveniently located within a 15-km radius of the tertiary center. Although the administration of several uterotonics and insertion of intrauterine balloon tamponades were available in some of these facilities, the patient was transferred if massive transfusions or other further interventions were needed, or the unstable hemodynamic status persisted or worsened. The management protocol at our center for referred patients with PPH has been previously described [Citation10]. Additionally, CT angiography was applied to selected patients to localize the active bleeding point and determine whether PAE was needed [Citation11]. The study protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital (Approval No. B-1906/544-102), and it was conducted according to the principles expressed in the Declaration of Helsinki.
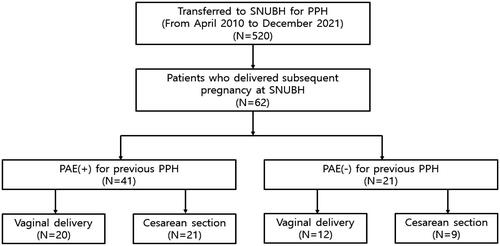
During the study period, 520 patients met the initial inclusion criteria. Those who gave birth for the subsequent pregnancy at the same center were also checked (). The baseline maternal characteristics were reviewed, including age, parity, gestational age at delivery, delivery mode, PPH treatment modalities applied, and the amount of transfusion for both the previous and subsequent pregnancies. For the subsequent pregnancy, the antenatal hemoglobin and hematocrit levels one month before delivery and other underlying obstetric complications were additionally reviewed from electronic medical records. Neonatal outcomes were also checked, including birthweight, Apgar scores, and neonatal intensive care unit (NICU) admission. The obstetric outcomes for the next pregnancy to evaluate were duration of hospital stay, intensive care unit (ICU) admission, estimated blood loss, vital signs, the presence of placenta previa, uterine rupture, placenta accreta spectrum, the levels of hemoglobin and hematocrit immediately after delivery (in 24 h), the amount of transfusion, intrauterine balloon tamponade insertion, use of PAE, and peripartum hysterectomy. The study population was divided into two groups according to the application of PAE for the previous pregnancy with PPH: those who had undergone PAE during their previous PPH management (PAE group) and those who had not (non-PAE group). The outcomes mentioned above were compared between two groups using the Mann–Whitney U-test for continuous variables and Fisher’s exact test for categorical variables. Statistical significance was set at p < 0.05. Data analysis was performed using SPSS 26.0 (IBM, Armonk, NY, USA).
Results
There were 62 women who gave birth to the subsequent pregnancy after the PPH event at the same center. Among them, 66% (41/62) had received PAE for the previous pregnancy with PPH ().
Among the 41 patients who underwent PAE, 22.0% (9/41) not only received uterine artery embolization but also had embolization of the unilateral ovarian artery. When comparing the baseline characteristics for the previous pregnancy with PPH, the median value of maternal age was significantly higher in the PAE group than in the non-PAE group (32 vs. 30 years, p = 0.013) (). The proportion of nulliparity, the median value of gestational age at delivery, and the Cesarean section rate were comparable between the two groups. The proportion of ICU admission and massive transfusion defined as packed red blood cells (pRBC) was higher in the PAE group than in the non-PAE group; however, the difference did not reach statistical significance (73% vs. 48%, p = 0.056; 85% vs. 62%, p = 0.054, respectively).
Table 1. Baseline characteristics of the study population according to the exposure to pelvic artery embolization at the previous pregnancy complicated with postpartum hemorrhage.
also demonstrates the baseline maternal characteristics for the subsequent or current pregnancy. For the subsequent pregnancy, maternal age was significantly higher in the PAE group than in the non-PAE group (35 vs. 33 years, p = 0.008). Gestational age at delivery, the rate of Cesarean section, initial antenatal hemoglobin and hematocrit, and other obstetric underlying complications such as pregnancy-induced hypertension and diabetes mellitus were not different between the two groups.
Detailed obstetric and neonatal outcomes are shown in . The duration of hospital stay and the rate of ICU admission were comparable between the two groups. The PAE group revealed a higher median value of estimated blood loss than the non-PAE group (600 vs. 300 ml, p = 0.008). The proportion of placenta previa was 24.4% for the PAE group and 4.8% for the non-PAE group; however, the difference did not reach statistical significance (p = 0.080). Placenta accreta spectrum was found only in the PAE group (15%), while none in the non-PAE group (p = 0.088).
Table 2. Obstetric and neonatal outcomes compared between the group with pelvic embolization for the previous pregnancy and the group without.
The median value of immediate postpartum hemoglobin level was significantly lower in the PAE group than in the non-PAE group (10.4 vs. 11.8 mg/dL, p = 0.010). Hematocrit showed the same results (32% for the PAE group vs. 34% for the non-PAE group, p = 0.010). The rates of pRBC transfusion were 34% for the PAE group and 10% for the non-PAE group; however, the difference was not statistically significant (p = 0.063). About 12% of patients received PAE again for the subsequent pregnancy in the PAE group. Approximately 15% (6/41) of the PAE group underwent peripartum hysterectomy, while the non-PAE group had no case of hysterectomy (p = 0.088). Most of peripartum hysterectomy was performed due to persistent uterine atony despite of uterotonic agents or failure to remove placenta caused by placenta accreta spectrum syndrome (Supplementary table S1).
The sub-analysis for the PAE group according to the delivery mode for the subsequent pregnancy was performed in . About half of the PAE group underwent a Cesarean section (21/41) (). About 38% (8/21) underwent Cesarean section due to previous Cesarean delivery. Other indications were antenatally diagnosed placenta previa (4), maternal request (4), suspected placenta accreta spectrum (3) and fetal breech presentation (2). The median value of gestational age at delivery was significantly shorter in the Cesarean section group than in the vaginal delivery group (37 vs. 39 weeks, p = 0.003). The rate of placenta previa diagnosed in the current pregnancy was significantly higher in the Cesarean section group than in the vaginal delivery group (43% vs. 5%, p = 0.009). The duration of hospital stay, the rates of ICU admission, postpartum tachycardia, and placenta previa were significantly worse in the Cesarean section group than in the vaginal delivery group (p < 0.05 for all). The Cesarean section group received more transfusions than the vaginal delivery group (52% vs. 15%, p = 0.020). The rates of uterine balloon tamponade insertion and PAE applied for the current pregnancy were comparable between the two groups divided according to the delivery mode; however, 29% of the Cesarean section group underwent peripartum hysterectomy while there was no hysterectomy in the vaginal delivery group (p = 0.021).
Table 3. Obstetric outcomes compared between the group with cesarean section and the group with vaginal delivery for this pregnancy among those who had undergone pelvic artery embolization.
Discussion
The principal implications of this study were (i) PAE seems to increase the blood loss for the next delivery; (ii) the median value of immediate postpartum hemoglobin and hematocrit levels were significantly lower in the PAE group than in the non-PAE group; (iii) the rates of peripartum hysterectomy and placenta accreta spectrum might be higher for the subsequent pregnancy for those treated with PAE, although the difference did not reach the statistical significance; (iv) approximately 12% of patients had to undergo PAE repeatedly for the subsequent pregnancy in the PAE group; (v) about 15% of the PAE group ended up peripartum hysterectomy while none received hysterectomy in the non-PAE group; and (vi) Cesarean section tends to increase the risk of more bleeding and other adverse outcomes for the subsequent pregnancy in the cases who had undergone PAE for the previous delivery.
Recent studies have reported conflicting findings regarding the potential adverse effects of PAE on subsequent pregnancies. Poggi et al. found that pregnancies following PAE for PPH had an increased risk of placenta accreta spectrum than those without PAE [Citation9]. Additionally, Imafuku et al. suggested that subsequent pregnancies after PAE for severe PPH carry a high risk of severe PPH recurrence [Citation12]. In two recent meta-analyses, PAE was associated with higher rates of placenta accreta spectrum and PPH recurrence in subsequent pregnancies. However, the severity or condition of PPH might not be consistent in all those reviewed articles [Citation13,Citation14]. Lee et al. found less effectiveness of PAE for the subsequent pregnancy with recurrent PPH to achieve hemostasis in patients with the experience of PPH for the previous pregnancy [Citation15]. Regarding the impact of these emboli on the uterus, Colgan et al. reported on the pathologic features of the uterus following PAE, and in their observations, no cases identified polyvinyl alcohol particles (PVA) emboli within the endometrium. They characterized this phenomenon by noting that the PVA material, coupled with a pronounced chronic inflammatory reaction caused by foreign body giant cells, effectively occluded the uterine vessel lumen. They speculated that myometrial vessels probably intercept and trap smaller PVA particles before these can reach the endometrium [Citation16]. According to animal studies, damage to the myometrium can lead to impaired myometrium functioning, acting as a primary cause for the invasion of extravillous trophoblasts, resulting in the formation of placenta accreta spectrum [Citation17]. Consequently, it’s thought that damage to the myometrium caused by PAE could be associated with increased placenta accreta spectrum. In our data, of the 21 mothers who had a cesarean section this time and had received PAE during their previous pregnancy, 13 underwent primary cesarean section. Excluding two with breech presentation and one with suspected placenta abruption, ten had either placenta previa or placenta accreta spectrum.
Cho et al. showed that PAE at the first delivery increased the rate of PAE at the second delivery, and this result partly coincides with our finding [Citation18]. Women who had undergone PAE during their previous delivery seem to experience more obstetric complications and more severe conditions of PPH. Additionally, Pyeon et al. found postpartum PAE as a risk factor for primary ovarian failure following delivery [Citation19]. Therefore, obstetricians should be vigilant for the pregnancy and delivery of patients who have experienced PPH for the previous pregnancy, particularly those planning Cesarean delivery, as they require additional care during delivery and postpartum. Thorough evaluation of placentation via ultrasonography in the second and third trimesters is also recommended to prepare for placenta accreta spectrum [Citation20,Citation21]. Ultrasonographic findings such as presence of interrupted retroplacental space and placental lacunae are known as signs of placenta accreta spectrum disorders. However, there was a large multicenter prospective study reporting the rate and outcomes of emergency Cesarean section in women diagnosed as placenta previa and/or placenta accreta spectrum disorders and this study suggested that prenatal ultrasound for abnormal placentation cannot entirely predict the risk of emergency delivery [Citation22].
This study has some limitations because of the retrospective nature of the design and the small sample size. Moreover, as shown in , cases with far more severe PPH were likely to undergo PAE rather than other conservative management; therefore, selection bias for the PPH risk factors developed for the inclusion of the initial study population. Including ethical issues, there are a few critical difficulties in performing randomized controlled trials or prospective studies for treatment methods and outcomes of PPH. The diagnosis of PPH and the management protocol for PPH vary according to the facility and experience of the centers. In addition, long-term follow-ups for the patients transferred to the tertiary center for PPH are hardly feasible. Although selection bias is likely to be the weak point of this study, the result could be said to be meaningful because the data were collected from only those who had been diagnosed with PPH not controlled by common conservative treatments from primary clinics and had received the same PPH protocol management at our center. In fact, the information for the next pregnancy at the same tertiary center as the transfer for the previous delivery complicated with PPH could be followed up in detail to check the effect of the previous PAE. Future research with a large sample size population or national data is essential to achieve safe delivery for those with a history of PPH from the previous pregnancy and decrease maternal mortality caused by PPH worldwide.
Supplemental Material
Download MS Word (21.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, JYP. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Committee on Practice Bulletins. Obstetrics practice bulletin no. 183. Practice bulletin no. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130(4):e168–e186.
- Bienstock JL, Eke AC, Hueppchen NA. Postpartum hemorrhage. N Engl J Med. 2021;384(17):1635–1645. doi:10.1056/NEJMra1513247.
- Lambrecht S, Van De Velde M. Interventional radiology for the obstetric patient. Curr Opin Anaesthesiol. 2020;33(4):566–570. doi:10.1097/ACO.0000000000000884.
- Deux JF, Bazot M, Le Blanche AF, et al. Is selective embolization of uterine arteries a safe alternative to hysterectomy in patients with postpartum hemorrhage? AJR Am J Roentgenol. 2001;177(1):145–149. doi:10.2214/ajr.177.1.1770145.
- Hunter LA. Exploring the role of uterine artery embolization in the management of postpartum hemorrhage. J Perinat Neonatal Nurs. 2010;24(3):207–214. doi:10.1097/JPN.0b013e3181e8c994.
- Chauleur C, Fanget C, Tourne G, et al. Serious primary post-partum hemorrhage, arterial embolization and future fertility: a retrospective study of 46 cases. Hum Reprod. 2008;23(7):1553–1559. doi:10.1093/humrep/den122.
- Delotte J, Novellas S, Koh C, et al. Obstetrical prognosis and pregnancy outcome following pelvic arterial embolisation for post-partum hemorrhage. Eur J Obstet Gynecol Reprod Biol. 2009;145(2):129–132. doi:10.1016/j.ejogrb.2009.03.013.
- Oberg AS, Hernandez-Diaz S, Palmsten K, et al. Patterns of recurrence of postpartum hemorrhage in a large population-based cohort. Am J Obstet Gynecol. 2014;210(3):229.e1-8–229.e8. doi:10.1016/j.ajog.2013.10.872.
- Poggi SH, Yaeger A, Wahdan Y, et al. Outcome of pregnancies after pelvic artery embolization for postpartum hemorrhage: retrospective cohort study. Am J Obstet Gynecol. 2015;213(4):576.e1-5–576.e5. doi:10.1016/j.ajog.2015.06.063.
- Oh KJ, Hong JS, Youm J, et al. Can coagulopathy in post-partum hemorrhage predict maternal morbidity? J Obstet Gynaecol Res. 2016;42(11):1509–1518. doi:10.1111/jog.13098.
- Jung YM, Kim HJ, Choi WS, et al. CT angiography for the management of postpartum hemorrhage refractory to conservative treatment. J Matern Fetal Neonatal Med. 2022;35(21):4081–4088. doi:10.1080/14767058.2020.1846708.
- Imafuku H, Yamada H, Morizane M, et al. Recurrence of post-partum hemorrhage in women with a history of uterine artery embolization. J Obstet Gynaecol Res. 2020;46(1):119–123. doi:10.1111/jog.14129.
- Zhang XQ, Chen XT, Zhang YT, et al. The emergent pelvic artery embolization in the management of postpartum hemorrhage: a systematic review and meta-analysis. Obstet Gynecol Surv. 2021;76(4):234–244. doi:10.1097/OGX.0000000000000887.
- Matsuzaki S, Lee M, Nagase Y, et al. A systematic review and meta-analysis of obstetric and maternal outcomes after prior uterine artery embolization. Sci Rep. 2021;11(1):16914. doi:10.1038/s41598-021-96273-z.
- Lee CH, Yoon CJ, Lee JH, et al. Recurrent postpartum hemorrhage at subsequent pregnancy in patients with prior uterine artery embolization: angiographic findings and outcomes of repeat embolization. Br J Radiol. 2022;95(1136):20211355. doi:10.1259/bjr.20211355.
- Colgan TJ, Pron G, Mocarski EJ, et al. Pathologic features of uteri and leiomyomas following uterine artery embolization for leiomyomas. Am J Surg Pathol. 2003;27(2):167–177. doi:10.1097/00000478-200302000-00004.
- Ma Y, Hu Y, Ma J. Animal models of the placenta accreta spectrum: current status and further perspectives. Front Endocrinol. 2023;14:1118168. doi:10.3389/fendo.2023.1118168.
- Cho GJ, Shim JY, Ouh YT, et al. Previous uterine artery embolization increases the rate of repeat embolization in a subsequent pregnancy. PLoS One. 2017;12(9):e0185467. doi:10.1371/journal.pone.0185467.
- Pyeon SY, Noh E, Cho GJ. Long-term effect on ovarian function after uterine artery embolization during the postpartum period: a nationwide population-based study. Reprod Sci. 2023;30(10):2990–2995. doi:10.1007/s43032-023-01257-1.
- Einerson BD, Gilner JB, Zuckerwise LC. Placenta accreta spectrum. Obstet Gynecol. 2023;142(1):31–50. doi:10.1097/AOG.0000000000005229.
- Einerson BD, Shamshirsaz AA, Stephenson ML, et al. The need for presurgical evaluation for placenta accreta spectrum. Am J Perinatol. 2023;40(9):996–1001. doi:10.1055/s-0043-1761639.
- Lucidi A, Fratelli N, Maggi C, et al. Determinants of emergency delivery in pregnancies complicated by placenta previa or placenta accreta spectrum disorders: analysis of ADoPAD cohort. Ultrasound Obstet Gynecol. [cited 2023 Sep 12]. doi:10.1002/uog.27465.