Abstract
Background
Maternal high blood pressure (BP) was associated with adverse pregnancy outcomes. This study aimed to synthesize evidence on the association between high BP prior to or in early pregnancy with maternal and fetal complications.
Methods
We searched the cohort studies assessing the effect of high BP in the Medline, Embase, Web of Science and China National Knowledge Internet databases. A random-effects model was used to estimate the pooled odds ratios (ORs) with 95% confidence intervals (CIs). The protocol was registered in PROSPERRO (CRD 42023414945).
Results
23 eligible studies were identified. High BP prior to or in early pregnancy was associated with higher odds of hypertensive disorders of pregnancy (OR 2.90, 95% CI 1.91–3.89), gestational hypertension (2.56, 2.01–3.12), preeclampsia (3.20, 2.66–3.74), gestational diabetes mellitus (1.71, 1.36–2.06), preterm birth (1.66, 1.39–1.93), stillbirth (2.01, 1.45–2.58) and neonatal intensive care unit admission (1.22, 1.08–1.37). Subgroup analyses indicated that pre-hypertension could significantly increase the odds of these outcomes except for stillbirth, though the odds were lower than hypertension.
Conclusions
High BP prior to or in early pregnancy was associated with adverse pregnancy outcomes and this association increased with hypertension severity. The findings emphasized an urgent need for heightened surveillance for maternal BP, especially pre-hypertensive status.
Introduction
Chronic hypertension complicates 1.5%–2.2% of pregnancy, and the rate increases by an average of 7.3% per year [Citation1]. Additionally, pre-hypertension, as a crucial stage for hypertension progression, affects a larger population than hypertension in national epidemiology studies and contributes to a prevalence of high blood pressure (BP) being 53.01% (95% confidence interval [CI], 51.13%–54.88%) [Citation2–4]. The 2017 guidelines of the American College of Cardiology/American Heart Association (ACC/AHA) bring up the term of pre-hypertension and highlight the importance due to its high prevalence among different age groups. For reproductive-age women, several clinical and national cohort studies have shown that the incidence rate of pre-hypertension is approximately 1–3 times that of hypertension [Citation5–7]. Therefore, high BP is an important public health concern in the pregnant population, which should attract more intensive monitoring to prevent its related complications.
The positive association of maternal high BP and adverse pregnancy outcomes has been well established by numerous epidemiological studies. According to a systematic review including 55 studies and 795,221 pregnancies, women with chronic hypertension have a high incidence of maternal and perinatal complications, such as preeclampsia (26%), cesarean delivery (41%), preterm delivery (28%) and neonatal intensive care unit (NICU) admission (21%) [Citation8]. Emerging evidence suggests that women who have pre-hypertension prior to or in early pregnancy, compared to normotensive women, have an increased risk of pregnancy complications, especially gestational hypertension and preeclampsia. We find that a few studies investigate the influence of both pre-hypertension and hypertension using the same population; their reported outcomes are limited in hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), cesarean delivery, preterm delivery, small for gestational age (SGA) and NICU admission [Citation5–7,Citation9–13]. These individual reports are usually from single center studies that provide valuable data for a given population, but they are of limited use for wider extrapolation. Nevertheless, collectively, they may enable accurate risk assessment of adverse pregnancy outcomes in affected women.
Nowadays, estimating adverse pregnancy outcomes presents a challenge due to the absence of strong evidence on systemic risk assessment in women who have high BP. In the interest of providing referable data for clinical guidance, we carried out a systematic meta-analysis of cohort studies to clarify the impact of high BP prior to or in early pregnancy on subsequent maternal and fetal complications.
Methods
Search strategy
We did a comprehensive literature review across Medline, Embase, Web of Science and China National Knowledge Internet (CNKI) from September 22, 1982 to September 22, 2022. The keywords and Mesh terms were searched separately and then combined in each database (Table S1). No language restrictions were applied. We followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The protocol has been registered in PROSPERRO (CRD 42023414945).
Study selection criteria
We included prospective, retrospective and ambispective cohort studies that reported the association between maternal high BP prior to or in early pregnancy (before 20 weeks’ gestation) and pregnancy outcomes. We excluded studies reporting less than 20 women with high BP prior to or in early pregnancy that were identified to be non-representative [Citation8], as well as studies that did not describe exposure definition and relevant outcome data.
Data extraction
After removing duplicate literatures, XJL and XWL independently screened studies that potentially met the inclusion criteria by reviewing titles and abstracts, followed by a full-text assessment. The discrepancies were reviewed by MJ. We included studies that reported different outcomes using the same cohort database. If studies reported a duplicate outcome, we selected the study with the largest number of women or relevant outcomes.
The following information of each study were extracted: first author’ name, publication date, study period, data source, country, sample size, demographic characteristics, exposure definition and, outcomes. High BP was categorized as pre-hypertension (“pre-hypertension”, “prehypertension”, “elevated BP” and “high-normal BP”) and hypertension (overall “hypertension”, “stage 1 hypertension” and “stage 2 hypertension”). In the included studies, pre-hypertension and hypertension were defined by the guidelines of 2017 ACC/AHA, 2019 Japanese Society of Hypertension (JSH), International Classification of Diseases Ninth Revision (ICD-9), ICD-10, the World Health Organization/International Society of Hypertension (WHO/ISH), and medical records. Pregnancy outcomes included maternal complications (e.g. HDP, gestational hypertension, preeclampsia, GDM, cesarean delivery, and placental abruption) and fetal complications (e.g. preterm delivery, SGA, stillbirth, and NICU admission). We carefully examined and recorded the definitions of pre-/hypertension, diagnosis period, sample size for comparisons, adverse pregnancy outcomes, and measures of association. Each study was scored according to the adapted Newcastle-Ottawa Scale (NOS) for cohort study with a total score ranging from 0 to 9 (low quality, score ≤ 3; moderate quality, 3 < score ≤ 6; high quality, 7 < score ≤ 9) (Table S3).
Statistical analysis
Quantitative meta-analysis was conducted for an outcome when more than one study presented relevant data. A random-effects estimate of the pooled odds ratio (OR) and 95% confidence intervals (CIs) for each outcome was generated with the use of the Mantel-Haenszel method. A subgroup meta-analysis was performed to compare the effect sizes and heterogeneity for pre-hypertension and hypertension. Heterogeneity was assessed using the I2 statistic, with substantial heterogeneity defined as an I2 value greater than 75% [Citation14]. Meta-regression was conducted for the outcome with substantial heterogeneity to investigate the effect of potential modifications, including region (North America, Asia, or others), study period (before 1998, 1998–2013, or after 2013), and sample size (<1000, 1000–10,000 or >10,000). Positive regression coefficients indicated an increase in the effect size whereas negative coefficients show a decrease. Sensitivity analysis was performed for each meta-analysis to explore the effect of the result of a single study on the pooled effect size by removing one study at a time. Publication bias was investigated by Egger’s test and shown in the funnel plots. All statistical analyses were performed using Stata version 16, and all tests and confidence intervals were two-sided with a significance level of 0.05.
Results
Study selection and characteristics
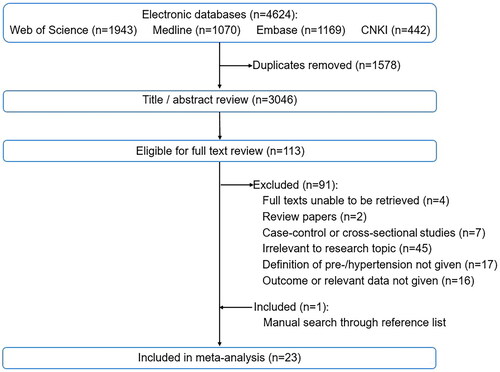
Of the 3046 titles and abstracts, 113 were relevant for full-text review and 22 met the inclusion criteria for systematic review. Excluded studies with detailed reasons were listed in Table S2. One additional article was incorporated after searching through the reference lists of identified articles using the snowball method. Finally, a total of 23 cohort studies were included in the meta-analysis (). showed the basic information of studies, demographic characteristics, definitions of exposure, extracted pregnancy outcomes and NOS grading scores.
Table 1. Overview of the characteristics of the 23 included cohort studies.
The included studies reported the effects of high BP prior to or in early pregnancy on maternal complications (HDP, n = 4; gestational hypertension, n = 6; preeclampsia, n = 9; GDM, n = 3; cesarean delivery, n = 2; placental abruption, n = 6; HELLP, n = 3), as well as fetal complications (preterm birth, n = 9; SGA, n = 3; stillbirth, n = 3; NICU admission, n = 2). The studies published between 2008 and 2022 were carried out in the United States (n = 7) [Citation5,Citation6,Citation11,Citation12,Citation15,Citation21,Citation29], China (n = 5) [Citation7,Citation9,Citation10,Citation23,Citation24], Canada (n = 2) [Citation16,Citation27], Japan (n = 2) [Citation17,Citation18], Netherlands [Citation20], Uruguay [Citation25], Colombia [Citation26], Korea [Citation30], Tanzania [Citation31], Norway [Citation32] and Latvia [Citation33] (n = 1 each). There were 4 studies including multiple gestations with sample sizes ranging from 3380 to 878,680, and the remaining 17 studies only included singleton births ranging from 293 to 1,078,323.
Quality assessment
The Newcastle-Ottawa grading scores ranged from 5 to 8. There were 21 literatures were of high quality, and no studies were excluded following the NOS scores (Table S4). The weaknesses were mainly from the non-representative pregnant population, inconsistent definitions of exposure, inadequate comparability, and a rough description of follow up rate.
High BP prior to or in early pregnancy and maternal outcomes
There were 16 studies that reported associations between high BP prior to or in early pregnancy and maternal complications. The pooled effect sizes of their associations were shown in the forest plot using subgroup meta-analysis for pre-hypertension and hypertension ().
Figure 2. Summary forest plot for maternal complications in women with high blood pressure prior to or in early pregnancy. Meta-analysis for each maternal outcomes was calculated by a subgroup analysis for pre-/hypertension using Random-effects Mantel-Haenszel method. NA: not applicable, 95% CIs: 95% confidence intervals.
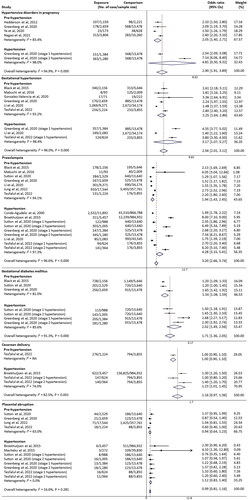
Involving 2122 HDP incidents in 38,207 women/births, four studies could be pooled in the meta-analysis for HDP [Citation6,Citation15,Citation30,Citation33]. Women with high BP prior to or in early pregnancy were more likely to have HDP than normotensive women with a pooled OR being 2.90 (95% CI 1.91–3.89, I2 = 94.9%). There was a significant association for pre-hypertension (OR 2.05, 95% CI 1.40–2.71, I2 = 83.4%), but not for hypertension (OR 4.81, 95% CI 0.30–9.32, I2 = 98.0%).
There were six studies reporting data of the association between maternal BP and gestational hypertension (6815 incidents in 72,891 women/births) [Citation6,Citation7,Citation12,Citation21,Citation26,Citation31]. All studies could be pooled in the meta-analysis for pre-hypertension (15,574 women), and three studies for hypertension (4091 women) [Citation6,Citation7,Citation12]. Compared with normotensive controls (59,384 women), there was a significantly increased odds of gestational hypertension for women who had high BP (OR 2.56, 95% CI 2.01–3.12, I2 = 96.0%), and there was a greater effect size for hypertension (OR 3.17, 95% CI 1.07–5.27, I2 = 93.2%) than pre-hypertension (OR 2.25, 95% CI 1.64–2.86, I2 = 98.4%).
Nine studies were pooled in the meta-analysis for preeclampsia involving 67,471 incidents in 2,338,277 births [Citation5–7,Citation12,Citation17,Citation20,Citation21,Citation25,Citation30], and four studies reported associations of preeclampsia with pre-hypertension and hypertension [Citation5–7,Citation12]. There were significantly increased odds in women who had high BP (OR 3.20, 95% CI 2.66–3.74), pre-hypertension (OR 1.94, 95% CI 1.43–2.45), as well as hypertension (OR 4.28, 95% CI 3.15–5.41), but substantial heterogeneity was noted (I2 = 96.6% for overall, 94.1% for pre-hypertension and 97.3% for hypertension).
There were three studies that could be pooled in the hypertension-stratified meta-analysis for GDM (47,422 women) [Citation5,Citation6,Citation21], and two of which were available with both pre-hypertension and hypertension data (36,963 women) [Citation5,Citation6]; one with only pre-hypertension (10,459 women) [Citation21]. The forest plot showed significantly increased odds of GDM among women with high BP prior to or in early pregnancy (OR 1.71, 95% CI 1.36–2.05, I2 = 91.3%). Meanwhile, we found women who had hypertension (OR 2.02, 95% CI 1.49–2.56, I2 = 85.6%) were associated with a higher odds of GDM than those who had pre-hypertension (OR 1.34, 95% CI 1.08–1.59, I2 = 81.0%).
There were two studies (994,456 women/births) with available data of cesarean delivery [Citation12,Citation20]. The data on pre-hypertension was derived from one study, which showed no significant association (OR 1.00, 95% CI 0.90–1.10) [Citation12]. However, women who had hypertension could increase the odds of cesarean delivery accompanying with no substantial heterogeneity (OR 1.23, 95% CI 1.01–1.45, I2 = 74.0%).
There were six studies (1,446,717 women/births) reporting associations of maternal BP and placental abruption (2685 incidents) [Citation5,Citation6,Citation12,Citation20,Citation30,Citation31], but most of the odds failed to reach statistical significance. Both pre-hypertension and hypertension were not associated with placental abruption. The odds of placental abruption did not change significantly in women who had pre-hypertension (OR 0.94, 95% CI 0.64–1.23) or hypertension (OR 1.12, 95% CI 0.83–1.40) with low levels of heterogeneity at 63.0% and 0.0%.
In addition, three studies (1,516,021 women) reported data on HELLP syndrome [Citation6,Citation16,Citation32], one of which only provided related frequencies rather than odds due to a low number of outcome [Citation6]. The pooled results of the remaining two studies showed maternal BP was not associated with HELLP syndrome (OR 3.03, 95% CI 0.30–5.77, I2 = 86.5%) (data not shown).
High BP prior to or in early pregnancy and fetal outcomes
There were twelve studies reporting associations between high BP prior to or in early pregnancy with fetal complications. By subgroups meta-analysis, the pooled odds for each fetal outcomes stratified by hypertension were shown in the forest plot ().
Figure 3. Summary forest plot for fetal complications in women with high blood pressure prior to or in early pregnancy. Meta-analysis for each fetal outcomes was calculated by a subgroup analysis for pre-/hypertension using Random-effects Mantel-Haenszel method. NA: not applicable; 95% CIs: 95% confidence intervals; NICU: neonatal intensive care unit.
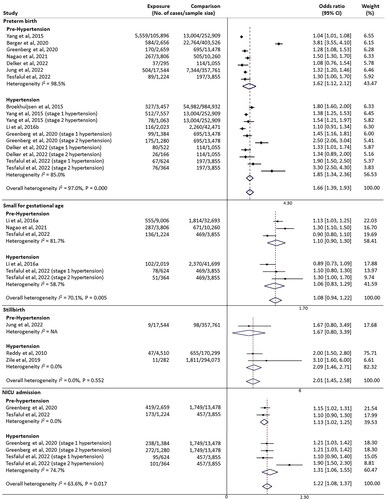
There were nine studies that could be pooled in the meta-analysis for preterm birth [Citation6,Citation10–12,Citation18,Citation20,Citation23,Citation27,Citation30]. A total of 110,631 of preterm birth among 2,323,068 births were included. There was significantly increased odds of preterm birth associated with high BP prior to or in early pregnancy (OR 1.66, 95% CI 1.39–1.93, I2 = 97.0%). Subgroup analysis showed women with hypertension (OR 1.85, 95% CI 1.34–2.36, I2 = 85.0%) were more likely to suffer from preterm birth than women with pre-hypertension (OR 1.62, 95% CI 1.12–2.12, I2 = 98.5%).
Three studies (63,851 women/births) were available with data for SGA [Citation9,Citation12,Citation18]. Both pre-hypertension (OR 1.10, 95% CI 0.90–1.30, I2 = 81.7%) and hypertension (OR 1.06, 95% CI 0.83–1.29, I2 = 58.7%) were not significantly associated with the odds of SGA. The overall pooled odds was also not significant (OR 1.08, 95% CI 0.94–1.22, I2 = 70.1%).
For the three studies focusing on stillbirth (841,469 births) [Citation15,Citation30,Citation33], one study showed no significant association of pre-hypertension and the odds of stillbirth (OR 1.67, 95% CI 0.80–3.49) [Citation30]; the other two studies were able to be pooled in the meta-analysis for hypertension subgroup and showed a significantly increased odds (OR 2.09, 95% CI 1.72–2.45, I2 = 0.0%). The overall pooled odds of stillbirth also significantly increased in women with high BP (OR 2.01, 95% CI 1.45–2.58, I2 = 0.0%, p = .552).
There were two studies with available data on NICU admission (24,868 women/births) [Citation6,Citation12]. The overall pooled odds of NICU admission was significantly higher among women with high BP (OR 1.22, 95% CI 1.08–1.37, I2 = 63.6%, p = .017). In the subgroup analysis, women with hypertension (OR 1.31, 95% CI 1.06–1.55) had a stronger association with the odds of NICU admission than those with pre-hypertension (OR 1.13, 95% CI 1.02–1.25), and no sustainable heterogeneity was noted (I2 = 0.0% for pre-hypertension, I2 = 74.7% for hypertension).
Meta-regression analysis, sensitivity analysis and publication bias
Overall heterogeneity was substantial for multiple meta-analyses; some of this was partially explained by subgroup analysis, which reduced heterogeneity to a lower level. Meta-regression analysis was carried out to explore the remaining heterogeneity for HDP, gestational hypertension, preeclampsia, and preterm birth (Table S5), but not for GDM whose results were from a small number of studies. We observed the residual heterogeneity was reduced significantly for gestational hypertension where the study period explained almost all of the heterogeneity (I2 = 4%, p = .004), followed by the geographical region of study (I2 = 24.23%, p = .006). The covariates in the meta-regression analysis also contributed to the heterogeneity of other outcomes to some extent, however, the residual heterogeneity still remained for preeclampsia and preterm birth.
The results of sensitivity analysis by removing one study at a time suggested that meta-analysis results were robust for most outcomes including HDP, preeclampsia, placental abruption, and preterm birth (Tables S6a, S6b, S6f, and S7a). There were limited impacts on the effect size or significance of pooled associations between maternal hypertension with gestational hypertension and NICU admission, as well as associations between maternal pre-hypertension with GDM, cesarean delivery, preterm birth, and SGA, respectively (Tables S6b, S6d, S6e, S7a, S7b, and S7c).
According to the funnel plots and Egger’s test results (Figure S1 and S2), there were no obvious evidence of publication bias for HDP (p = .642), gestational hypertension (p = .238), preeclampsia (p = .950), GDM (p = .303), a cesarean delivery (p = .782), placental abruption (p = .266), preterm birth (p = .224), SGA (p = .841), stillbirth (p = .807) and NICU admission (p = .411).
Discussion
In our systematic review and meta-analysis, 23 studies with retrospective or prospective cohort study design were included. Women with high BP or hypertension (including overall hypertension, stage 1 or stage 2 hypertension) prior to or in early pregnancy were associated with significantly increased odds of HDP, gestational hypertension, preeclampsia, GDM, preterm birth, stillbirth, and NICU admission. The odds of these outcomes, except for HDP and stillbirth, were also significantly increased in women with pre-hypertension, but the pooled odds were lower than those in women with hypertension. There were no evidence of the association between high BP with HELLP, cesarean delivery, placental abruption, and SGA.
A previous systematic review including 55 randomized controlled trials, cohort, and population studies has concluded that women with chronic hypertension have higher pooled incidences of adverse pregnancy outcomes compared with the US general pregnant population [Citation8]. Recently, another systematic review including 81 observational studies also provide evidence for an increased risk of adverse pregnancy outcomes associated with chronic hypertension [Citation34]. In contrast to the two previous reviews, we extracted retrospective and prospective cohort studies to investigate a relatively actual causality relationship between them. We additionally focused on the pre-hypertensive population as increasing evidence has emphasized the strict management of pre-hypertension due to its global health burdens [Citation35]. Our study supported previous conclusions that women with high BP should be assessed prior to or in early pregnancy and monitored closely for the potential development of pregnancy complications. Furthermore, we highlighted the importance of high-risk women incorporated with pre-hypertensive status for earlier and more effective surveillance.
We found high BP before or during early pregnancy was associated with the risk of gestational hypertension, preeclampsia, and preterm birth, however, significant heterogeneity existed between those studies. Besides a small portion of heterogeneity explained by hypertension-stratified subgroup analysis, the residual heterogeneity could be mostly explained by study time and region, but not by sample size for gestational hypertension. However, it was not helpful to reduce the substantial heterogeneity between the studies reporting preeclampsia or preterm birth via controlling these covariates. During the peri-conceptional period, maternal age, body mass index, ethnicity, educational level, parity, medical status, smoking, diet habit, socioeconomic status, and physical activity are the main risk factors for both hypertension and pregnancy outcomes [Citation36–39]. Meta-regression is not able to identify any other underlying causes of heterogeneity due to a lack of exploration or consistency of those factors mentioned above and a limited number of eligible studies. In addition, differences in controlling confounding factors and defining hypertension and outcomes, which are important to the credibility of results, may also be reasons for the heterogeneity.
Existing evidence has suggested several potential mechanisms likely accounting for the associations between high BP and adverse pregnancy outcomes. Immunity-inflammation perturbations play a critical role in the development of hypertension [Citation40]; increased inflammation response prior to pregnancy (high sensitivity C-reactive protein, excess reactive oxidative species, and pro-inflammation cytokines) in hypertensive women is believed to contribute to various pregnancy complications [Citation41]. Moreover, high BP is associated with the strongest evidence for the causation of cardiovascular disease, which is usually accompanied with vascular endothelial dysfunction [Citation42]. Maternal vascular dysfunction during periconception can lead to defective placentation, inadequate remodeling of spiral arteries and poor crosstalk of the maternal-fetal interface, then finally causes a complicated pregnancy [Citation43,Citation44]. The underlying pathological mechanisms overlapped by high BP and adverse pregnancy outcomes may be a widely recognized explanation for the biological link between them. Those mechanisms need further theoretical research to prove.
Our systematic review and meta-analysis has some strengths. The rigorous process about the search strategy, article selection, data extraction, and quality assessment was carried out by two independent investigators. The unique study design of cohort study could guarantee the evidence quality, and the included studies were mostly of high quality (21 studies) using population-based data from multiple-center research institutions (19 studies). Moreover, the included studies had a relatively large number of women/births (18 studies with a sample size >10,000), which were sufficient to be used to analyze and compare the impacts of different stages of hypertension on a variety of pregnant outcomes in the same population.
However, there are also some limitations. As there were few studies met the selection criteria for the outcomes of HELLP, stillbirth, and cesarean delivery, it was difficult to investigate the pooled effect sizes in the subgroups of hypertension. Then, most studies did not report the lifestyle characteristics of the targeted population (e.g. smoking, alcohol consumption or physical exercise), though baseline demographics information was presented. The demographic variables (e.g. maternal age, income, and education level) limited the assessments of heterogeneity source owing to their discontinuity. Moreover, the measurement of BP and definitions of outcomes were variable across studies, and meta-regression was not conducted to evaluate the impact of these differences due to limited relevant data. Finally, this review concentrated on the studies that investigated short-term outcomes including maternal complications during pregnancy and birth outcomes. The potential impact on subsequent long-term health of high BP prior to or in early pregnancy might be an area for further research.
Conclusions
In conclusion, high BP prior to or in early pregnancy significantly increased the odds of adverse maternal and fetal outcomes, and the odds was higher in hypertensive women compared with pre-hypertensive status. Given the heterogeneity and limited number of included studies, our findings should be interpreted cautiously. The findings of this meta-analysis support the importance of increased antenatal surveillance for women with abnormal blood pressures before or during early pregnancy to enable early identification of maternal and fetal risk. In the future, there is an urgent need for a deep understanding of the pathophysiology of abnormal blood pressure, particularly pre-hypertension, to inform the development of predictive and diagnostic tools and enhance therapeutic interventions of hypertension-associated pregnancy complications.
Authors contributions
Conceptualization, XJL and NL; Methodology, XJL, MJ, and XWL; Software, MJ; Validation, YLZ, LZ, and ZWL; Data Curation, XJL and YXW; Writing-Original Draft Preparation, MJ; Writing-Review & Editing, NL; Supervision, RWY and ZWL; Funding Acquisition, XJL and NL.
Supplemental Material
Download MS Word (225.6 KB)Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- McLaren RA, Al-Kouatly HB, Minkoff H. Change in prevalence of chronic hypertension in pregnancy after the updated ACC/AHA hypertension guidelines. Pregnancy Hypertens. 2022;29:61–63. doi: 10.1016/j.preghy.2022.06.004.
- Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China: results from the China hypertension survey, 2012-2015. Circulation. 2018;137(22):2344–2356. doi: 10.1161/circulationaha.117.032380.
- Balouchi A, Rafsanjani M, Al-Mutawaa K, et al. Hypertension and pre-hypertension in Middle east and North Africa (MENA): a meta-analysis of prevalence, awareness, treatment, and control. Curr Probl Cardiol. 2022;47(7):101069. doi: 10.1016/j.cpcardiol.2021.101069.
- Xiong P, Liu Z, Xiong M, et al. Prevalence of high blood pressure under 2017 ACC/AHA guidelines: a systematic review and meta-analysis. J Hum Hypertens. 2021;35(3):193–206. doi: 10.1038/s41371-020-00454-8.
- Sutton EF, Rogan SC, Lopa S, et al. Early pregnancy blood pressure elevations and risk for maternal and neonatal morbidity. Obstet Gynecol. 2020;136(1):129–139. doi: 10.1097/aog.0000000000003885.
- Greenberg VR, Silasi M, Lundsberg LS, et al. Perinatal outcomes in women with elevated blood pressure and stage 1 hypertension. Am J Obstet Gynecol. 2021;224(5):521.e1–521.e11. doi: 10.1016/j.ajog.2020.10.049.
- Li N, An H, Li Z, et al. Preconception blood pressure and risk of gestational hypertension and preeclampsia: a large cohort study in China. Hypertens Res. 2020;43(9):956–962. doi: 10.1038/s41440-020-0438-9.
- Bramham K, Parnell B, Nelson-Piercy C, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348(7):g2301–g2301. doi: 10.1136/bmj.g2301.
- Li N, Li Z, Ye R, et al. Preconception blood pressure and risk of low birth weight and small for gestational age: a large cohort study in China. Hypertension. 2016;68(4):873–879. doi: 10.1161/HYPERTENSIONAHA.116.07838.
- Yang Y, He Y, Li Q, et al. Preconception blood pressure and risk of preterm birth: a large historical cohort study in a Chinese rural population. Fertil Steril. 2015;104(1):124–130. doi: 10.1016/j.fertnstert.2015.03.024.
- Delker E, Bandoli G, LaCoursiere Y, et al. Chronic hypertension and risk of preterm delivery: national longitudinal study of adolescents to adult health. Paediatr Perinat Epidemiol. 2022;36(3):370–379. doi: 10.1111/ppe.12858.
- Tesfalul MA, Sperling JD, Blat C, et al. Perinatal outcomes and 2017 ACC/AHA blood pressure categories. Pregnancy Hypertens. 2022;28:134–138. doi: 10.1016/j.preghy.2022.03.004.
- Bateman BT, Bansil P, Hernandez-Diaz S, et al. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206(2):134.e131–134.e8. doi: 10.1016/j.ajog.2011.10.878.
- Ali M, van Os HJA, van der Weerd N, et al. Sex differences in presentation of stroke: a systematic review and meta-analysis. Stroke. 2022;53(2):345–354. doi: 10.1161/strokeaha.120.034040.
- Reddy UM, Laughon SK, Sun L, et al. Prepregnancy risk factors for antepartum stillbirth in the United States. Obstet Gynecol. 2010;116(5):1119–1126. doi: 10.1097/AOG.0b013e3181f903f8.
- Lisonkova S, Razaz N, Sabr Y, et al. Maternal risk factors and adverse birth outcomes associated with HELLP syndrome: a population-based study. BJOG. 2020;127(10):1189–1198. doi: 10.1111/1471-0528.16225.
- Mabuchi A, Yamamoto R, Ishii K, et al. Significance of high-normal blood pressure during early second trimester for predicting the onset of hypertensive disorders in pregnancy. Hypertens Pregnancy. 2016;35(2):234–241. doi: 10.3109/10641955.2016.1139719.
- Nagao T, Saito K, Yamanaka M. Prehypertension in early pregnancy is a risk factor for hypertensive disorders during pregnancy: a historical cohort study in Japan. Hypertens Pregnancy. 2021;40(1):51–55. doi: 10.1080/10641955.2020.1864637.
- Song P, Zhang Y, Yu J, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 2019;173(12):1154–1163. doi: 10.1001/jamapediatrics.2019.3310.
- Broekhuijsen K, Ravelli AC, Langenveld J, et al. Maternal and neonatal outcomes of pregnancy in women with chronic hypertension: a retrospective analysis of a national register. Acta Obstet Gynecol Scand. 2015;94(12):1337–1345. doi: 10.1111/aogs.12757.
- Black MH, Zhou H, Sacks DA, et al. Prehypertension prior to or during early pregnancy is associated with increased risk for hypertensive disorders in pregnancy and gestational diabetes. J Hypertens. 2015;33(9):1860–1867; discussion 1867. doi: 10.1097/hjh.0000000000000646.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/hyp.0000000000000066.
- Li N, Li Z, Ye R, et al. Preconception blood pressure and risk of preterm birth: a large cohort study in China. J Hypertens. 2016;34(11):2243–2247. doi: 10.1097/hjh.0000000000001069.
- Ye SC, Yang N, Wei MT, et al. Prehypertension prior to pregnancy is associated with hypertensive disorders of pregnancy and postpartum metabolic syndrome in chinese women. Hypertens Pregnancy. 2020;39(2):152–158. doi: 10.1080/10641955.2020.1748645.
- Conde-Agudelo A, Belizán JM. Risk factors for pre-eclampsia in a large cohort of latin American and Caribbean women. BJOG. 2000;107(1):75–83. doi: 10.1111/j.1471-0528.2000.tb11582.x.
- González-Valencia DP, Valero-Rubio SY, Fernando Grillo-Ardila C. Prehypertension as a risk factor for the development of perinatal complications: retrospective cohort study. Pregnancy Hypertens. 2020;21:203–207. doi: 10.1016/j.preghy.2020.04.016.
- Berger H, Melamed N, Davis BM, et al. Impact of diabetes, obesity and hypertension on preterm birth: population-based study. PLOS One. 2020;15(3):e0228743. doi: 10.1371/journal.pone.0228743.
- Okati-Aliabad H, Ansari-Moghaddam A, Kargar S, et al. Prevalence of hypertension and pre-hypertension in the middle east region: a systematic review & meta-analysis. J Hum Hypertens. 2022;36(9):794–804. doi: 10.1038/s41371-021-00647-9.
- Hedderson MM, Darbinian JA, Sridhar SB, et al. Prepregnancy cardiometabolic and inflammatory risk factors and subsequent risk of hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2012;207(1):68.e1–68.e9. doi: 10.1016/j.ajog.2012.05.017.
- Jung YM, Oh GC, Noh E, et al. Pre-pregnancy blood pressure and pregnancy outcomes: a nationwide population-based study. BMC Pregnancy Childbirth. 2022;22(1):226. doi: 10.1186/s12884-022-04573-7.
- Macheku GS, Philemon RN, Oneko O, et al. Frequency, risk factors and feto-maternal outcomes of abruptio placentae in Northern Tanzania: a registry-based retrospective cohort study. BMC Pregnancy Childbirth. 2015;15(1):242. doi: 10.1186/s12884-015-0678-x.
- Malmström O, Morken NH. HELLP syndrome, risk factors in first and second pregnancy: a population-based cohort study. Acta Obstet Gynecol Scand. 2018;97(6):709–716. doi: 10.1111/aogs.13322.
- Zile I, Ebela I, Rumba-Rozenfelde I. Maternal risk factors for stillbirth: a registry-based study. Medicina. 2019;55(7):326. doi: 10.3390/medicina55070326.
- Al Khalaf SY, O'Reilly É,J, Barrett PDB, et al. Impact of chronic hypertension and antihypertensive treatment on adverse perinatal outcomes: systematic review and meta-analysis. J Am Heart Assoc. 2021;10(9):e018494. doi: 10.1161/jaha.120.018494.
- Han M, Li Q, Liu L, et al. Prehypertension and risk of cardiovascular diseases: a meta-analysis of 47 cohort studies. J Hypertens. 2019;37(12):2325–2332. doi: 10.1097/hjh.0000000000002191.
- Ribeiro MM, Andrade A, Nunes I. Physical exercise in pregnancy: benefits, risks and prescription. J Perinat Med. 2022;50(1):4–17. doi: 10.1515/jpm-2021-0315.
- Li R, Lodge J, Flatley C, et al. The burden of adverse obstetric and perinatal outcomes from maternal smoking in an Australian cohort. Aust N Z J Obstet Gynaecol. 2019;59(3):356–361. doi: 10.1111/ajo.12849.
- Allehdan S, Basha A, Hyassat D, et al. Effectiveness of carbohydrate counting and dietary approach to stop hypertension dietary intervention on managing gestational diabetes mellitus among pregnant women who used metformin: a randomized controlled clinical trial. Clin Nutr. 2022;41(2):384–395. doi: 10.1016/j.clnu.2021.11.039.
- Laine K, Murzakanova G, Sole KB, et al. Prevalence and risk of pre-eclampsia and gestational hypertension in twin pregnancies: a population-based register study. BMJ Open. 2019;9(7):e029908. doi: 10.1136/bmjopen-2019-029908.
- Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. 2020;36(5):635–647. doi: 10.1016/j.cjca.2020.01.013.
- Deshmukh H, Way SS. Immunological basis for recurrent fetal loss and pregnancy complications. Annu Rev Pathol. 2019;14(1):185–210. doi: 10.1146/annurev-pathmechdis-012418-012743.
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75(2):285–292. doi: 10.1161/hypertensionaha.119.14240.
- Qu H, Khalil RA. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am J Physiol Heart Circ Physiol. 2020;319(3):H661–H681. doi: 10.1152/ajpheart.00202.2020.
- Jasper R, Skelding K. Cardiovascular disease risk unmasked by pregnancy complications. Eur J Intern Med. 2018;57:1–6. doi: 10.1016/j.ejim.2018.07.020.