Abstract
Background
Low-hemoglobin concentration and anemia are important risk factors for the health and development of women and children. The aim of this study was to investigate the correlation between blood indicators in early pregnancy among non-anemia women and anemia in the third trimester among pregnant women in China with uncomplicated pregnancies >36 weeks.
Methods
This was a multicenter, prospective cohort study. Pregnant women registered at the survey hospitals from May 2019 to December 2020 were included and followed up until delivery and discharge. The predictive value of serum ferritin (SF) and routine blood indexes (platelet count, red blood cell count, hemoglobin level, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration) were analyzed using a receiver operating characteristic (ROC) curve for the occurrence of anemia in the third trimester.
Results
The area under the ROC curve of the first trimester hemoglobin for predicting anemia during late pregnancy (cutoff value 128 g/L, sensitivity 82.3%, specificity 49.6%) and iron deficiency anemia (cutoff value 124 g/L, sensitivity 66.3%, specificity 66.4%) in the third trimester was larger than those of other blood variables.
Conclusions
Hemoglobin levels in the first trimester were significantly better predictors of anemia during the third trimester than the other indices. Our study contributes to the clinical practice of early intervention for anemia, thus taking effective measures to improve maternal and infant outcomes.
Background
Anemia affects approximately 2 billion people worldwide or 30% of the world’s population. According to statistics, the prevalence of anemia during pregnancy decreased from 41% in 2010 to 36% in 2019, with no significant improvement over the decade [Citation1]. In China, the reported prevalence varies from 10.5% to 23.5%, which cannot be ignored [Citation2]. The prevalence of iron deficiency (ID) in Chinese urban pregnant women was 48.16%, and that of iron deficiency anemia (IDA) was 13.87% [Citation3]. Causes of anemia in pregnancy include iron deficiency, folic acid deficiency, vitamin B12 deficiency, vitamin A deficiency, vitamin E deficiency, globin formation disorder, and aplastic anemia. Among them, ID is the primary cause of anemia during pregnancy. Up to 30% of individuals with ID may have IDA [Citation1].
IDA can cause substantial damage to pregnant women and infants. It increases the risk of hypertension during pregnancy, premature rupture of membranes, puerperal infections, postpartum depression, fetal growth restriction, fetal hypoxia, premature birth, neonatal asphyxia, and neonatal hypoxic-ischemic encephalopathy [Citation4–8]. Some experiments have shown that anemia during pregnancy affects the histomorphological changes of the placenta, leading to the adaptation of the placental blood vessels, and the placenta becomes hypoxic, which may lead to adverse fetal outcomes [Citation9].
A complete blood count in the first trimester is recommended for all pregnant women to screen for anemia [Citation10]. It would be helpful for pregnant women and doctors to know which blood variables in early pregnancy predict anemia in the third trimester. However, to the best of our knowledge, this issue has not been studied extensively in China. Therefore, this study was to investigate the correlation between blood indicators in early pregnancy and anemia in the third trimester among pregnant women in China.
Methods
Relevant diagnoses
At present, the diagnosis of ID depends on the serum ferritin (SF) content, which is the most effective indicator that is easy to obtain. Although WHO recommends an SF threshold of 15 μg/L, up to 50% of patients with true ID can be missed [Citation11]. Thus, we used an SF level <20 μg/L as the diagnostic criterion for ID according to the Chinese guideline [Citation12]. To identify the presence of anemia during pregnancy, we used the anemia criteria from the United States Centers for Disease Control and Prevention: <110 g/L in the first trimester, <105 g/L in the second trimester, and <110 g/dL in the third trimester [Citation13].
Study design and population
Our study was a multicenter prospective cohort study based on the West China Second Hospital Iron Deficiency Cohort in China (IRONWOMEN), which was registered in ClinicalTrials.gov (NCT 03961074). This study was approved by the Medical Ethics Committee of the West China Second Hospital of Sichuan University and was conducted in accordance with the Declaration of Helsinki.
A total of 6 tertiary hospitals (West China Second Hospital, Chongqing Maternal and Child Health Care Hospital, Hubei Provincial Maternal and Child Health Care Hospital, Yan’an University Affiliated Hospital, Xiamen City Maternal and Child Health Care Hospital, and Zhejiang University Medical College Affiliated Obstetrics and Gynecology Hospital) participated and were located in four regions in China (east, south, southwest, and northwest). The participating hospitals routinely detected blood parameters during pregnancy, and the detection methods met the corresponding standards. The indicators included the platelet (PLT) count, red blood cell (RBC) count, hemoglobin (Hb) level, hematocrit (Ht) level, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and SF level.
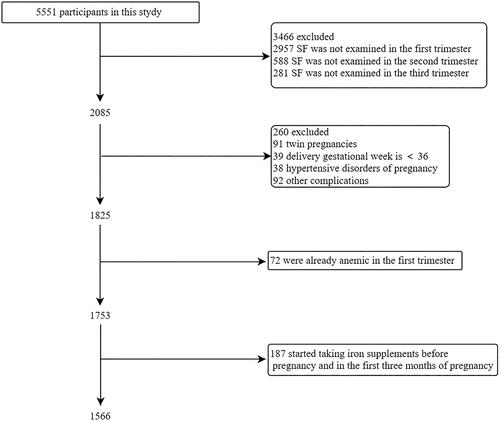
The study was conducted in May 2019 with the primary aim of investigating the diagnosis, treatment, and prognosis of ID and IDA in pregnant Chinese women. Pregnant women were included during their first prenatal visit to the participating hospital and were followed up until delivery and discharge. The following inclusion criteria were used: 1) establishment of a health record in the participating hospitals between May 2019 and December 2020, 2) planned to deliver at the survey sites (hospitals), and 3) signed informed consent. Women with preterm birth at gestational week <36, those with twin pregnancies, those with development of hypertensive disorders of pregnancy in the current pregnancy (including chronic hypertension, gestational hypertension, and preeclampsia), those diagnosed with medical complications at the time of the current pregnancy (including thyroid dysfunction, hemolysis, elevated liver enzymes and low platelets syndrome, liver diseases, kidney diseases, autoimmune diseases and hemoglobinopathies), those with anemia during pregnancy in the first trimester of the current pregnancy, and those who started taking iron supplementation before pregnancy and in the first 3 months were excluded from the present analysis. All women donated blood samples during the first, second, and third trimesters of pregnancy to determine 8 variables: PLT count, RBC count, Hb level, Ht level, MCV, MCH, MCHC, and SF level. Early pregnancy was defined as gestational weeks < 14, middle pregnancy as gestational weeks < 28, and late pregnancy as gestational weeks ≥28. Various iron supplements were used in different regions of China at the discretion of the attending physician based on the mother’s blood test results. Thus, there were no predefined protocols for prescribing iron preparations after the first 3 months of pregnancy.
Biochemical procedures
Blood PLT, RBC, Hb, Ht, MCV, MCH, MCHC, and SF values were measured at each hospital’s laboratory. The detection of hematological indices and SF levels in various medical institutions has reached scientific research requirements. The patients sat quietly and rested for 30 min, after which professional technicians collected their venous blood samples. The PLT count, RBC count, Hb level, Ht level, MCV, MCH, and MCHC were detected using the XE-2100 automatic blood cell analyzer (Sysmex) and its matching kit. The SF level was detected using immunoturbidimetry.
Statistical analysis
The Shapiro-Wilk method and histograms were used to test the data for normality. Data with a normal distribution or approximately normal distribution are represented as mean ± standard deviation. Data with a skewed distribution are expressed as median (25th percentile, 75th percentile) or median (interquartile range). Count data are described as percentages. The paired design t-test or paired design symbol rank sum test was used to compare the clinical data and blood data between the first and second trimesters, first and third trimester. The Wilcoxon–Mann–Whitney test was used to compare quantitative data between pregnant women with and without anemia in the third trimester, and the chi-square test or Fisher exact test was used to analyze qualitative data. ROC curves were constructed to determine the optimal cutoff of blood variables for the prediction of anemia during pregnancy occurring in the third trimester. All analyses were conducted using SPSS Statistics version 26.0 (IBM Corp.), and the statistical significance was set at p < .05.
Results
Demographic characteristics
A total of 1566 women without anemia in the first trimester were analyzed in this study (). The characteristics of the study population were shown in . Overall, 63 (4.0%) and 124 (7.9%) women developed anemia during the second and third trimesters, respectively. 47 (3.0%) pregnant women had anemia in both the second and third trimesters. The mean gestational age at delivery was 39.1 weeks, and 825 (52.7%) women delivered vaginally. The average blood collection times during the first, second, and third trimesters were 11.3, 23.4, and 33.0 weeks, respectively. In total, 1243 (79.4%) women received iron supplements after the first 3 months of pregnancy.
Table 1. Demographic characteristics.
Clinical data and blood parameters during pregnancy
PLT, RBC, Hb, Ht, MCHC, and SF values decreased from early to late pregnancy, whereas MCV and MCH levels increased. An Hb level <105 g/L in the second trimester was found in 63 (4.0%) of women, and an Hb level <110 g/L in the third trimester was found in 124 (7.9%) women. SF levels <20 ng/mL were found in 590 women (37.7%) in the second trimester and 741 women (47.3%) in the third trimester. In this study, 5.1% and 5.7% of pregnant women without anemia in early pregnancy developed IDA during the second and third trimesters, respectively ().
Table 2. Clinical data and blood parameters during pregnancy.
Anemia characteristics during the first trimester
The characteristics of 124 women in the first trimester who developed anemia in the third trimester were investigated (). According to chi-square tests, the basic demographic characteristics (ethnicity, household registration, annual income, nulliparous women, delivery mode, and duration of iron or folic acid supplementation) of pregnant women were not associated with anemia. Among the 124 patients with anemia in the third trimester, 47 (37.9%) had anemia in the second trimester. Compared with the non-anemia group, the anemia group showed low values of RBCs, Hb, Ht, and MCHC during the first trimester. In contrast, the PLT, MCH, MCV, and SF values did not differ between the two groups.
Table 3. Demographic characteristics and blood variables of women with and without anemia.
Blood variables in the first trimester as predictors of anemia in the third trimester
The areas under the curve (AUCs) of the ROC curves were 0.70 for Hb during the first trimester (), suggesting that Hb levels in the first trimester were better predictors of anemia in the third trimester than other blood variables. The optimal cutoff values determined by the ROC were 128 g/L for Hb in the first trimester (sensitivity, 82.3%; specificity, 49.6%).
Table 4. Screening characteristics of optimal cutoff values suggested by ROC curves for predicting anemia (third trimester).
Blood variables in the first trimester as predictors of IDA in the third trimester
The AUCs of the ROC curves were 0.71 for Hb during the first trimester (). Hb levels in the first trimester were better predictors of IDA in the third trimester than the SF level. The optimal cutoff values determined by ROC curves were 124 g/L for IDA in the first trimester (sensitivity, 66.3%; specificity, 66.4%).
Table 5. Screening characteristics of optimal cutoff values suggested by ROC curves for predicting IDA (third trimester).
Discussion
The present study’s results demonstrated that 8.9% (140/1566) of Chinese women without anemia during the early trimester developed anemia in pregnancy, 4.0% (63/1566) of Chinese healthy women developed anemia in the second trimester, and 7.9% (124/1566) developed anemia in the third trimester. 47 (3.0%) pregnant women have anemia in both the second and third trimesters. 5.1% (77/1503) of pregnant women without anemia in their first and second trimesters developed anemia in their third trimester. The Hb level in the first trimester exhibited a greater AUC of the ROC curve for predicting anemia (cutoff value 128 g/L, sensitivity 82.3%, specificity 49.6%) and IDA (cutoff value 124 g/L, sensitivity 66.3%, specificity 66.4%) in the third trimester than the other blood variables. Our results are like those of a Japanese study [Citation14], which suggested that the optimal cutoff value (126 g/L) of Hb yielded a sensitivity of 83% and a specificity of 59% [Citation14]. A prospective non-interventional single-center cohort study in France also identified first-trimester hemoglobin as the best predictor of anemia in the third trimester with a cutoff value of 120 g/L (specificity 87.5%, sensitivity 36.7%) [Citation15]. Compared to these two studies, the size of the present study is a strength.
Much information is known about the consequences of anemia during pregnancy, including the increased risk of low birth weight (<2500 g), preterm birth, perinatal mortality, and neonatal mortality [Citation16]. Maternal anemia also increases the risk of maternal death during and after childbirth, especially in less developed countries. An updated analysis showed that the odds ratio for maternal deaths was 0.71 (95% confidence interval: 0.60–0.85) for a 10 g/L greater mean Hb in late pregnancy [Citation17]. The Hb concentration in the first trimester is an accurate predictor of anemia occurring in the third trimester. If the Hb is less than 124 g/L in early pregnancy, an SF count should be performed to confirm IDA, but if the Hb value is between 110 and 124 g/L, iron supplementation could be provided.
Iron requirements for pregnant women increase gradually from 0.8 mg/day in the first trimester to 7.5 mg/day in the third trimester and can even reach 10 mg/day at 32–40 weeks of gestation. The total iron requirement throughout pregnancy is up to 1240 mg [Citation7]. In a large review of premenopausal women [Citation18], only 20% had presumed iron reserves >500 mg (defined as an SF concentration >70 µg/L) and would potentially be able to complete pregnancy without iron supplementation [Citation18]. In our study, the median values of SF in the first, second, and third trimesters of pregnancy were 63.3 μg/L, 23.3 μg/L, and 20.2 μg/L, respectively. Although 79.4% of healthy pregnant women already took iron supplements after the first 3 months of pregnancy, as many as 47.3% of healthy Chinese women developed ID in the third trimester, and 5.7% developed IDA in the third trimester. Our study contributes to the clinical practice of early intervention for anemia, thus taking effective measures to improve maternal and infant outcomes.
The Hb value in the first trimester was able to efficiently distinguish women at an elevated risk of anemia during the third trimester. Of 769 women with an Hb level <128 g/L during the first trimester, 96 (12.5%) developed anemia during the third trimester, whereas only 28 of 797 (3.5%) with an Hb level ≥128 g/L during the first trimester developed anemia during the third trimester. This result may provide validation for early initiation of iron supplementation even in women without anemia but with lower Hb levels in the first trimester, and it may lead to a decrease in the frequency of anemia in pregnancy.
In clinical practice, SF is used as a criterion to determine the need for iron supplementation. However, the cost implications of iron-related markers surpass the determination of a blood count. Moreover, SF acts as an acute-phase reactant protein during infections and is affected by alcohol excess, liver disease and malignancy [Citation19]. We suggest that Hb could be an important indicator to predict anemia in the third trimester of pregnancy in clinical practice. Early oral iron supplementation has been shown to improve hematological indices and reduce the incidence of anemia [Citation20]. Therefore, iron supplementation for women with an Hb level <128 g/L early in pregnancy may help prevent anemia during labor.
The WHO aims to reduce the prevalence of anemia in women of reproductive age by half between 2010 and 2025, but currently available interventions, such as iron therapy, do not appear to be working on the scale required to meet this aim [Citation6]. Clinically, there is still a lack of high-quality randomized controlled trials to prove that intervention for anemia in pregnancy, especially in the early stage of pregnancy, can reduce the incidence of adverse outcomes; however, actively monitoring and stabilizing the hemoglobin index in the early stage of pregnancy is indeed beneficial to prevent the occurrence of anemia during pregnancy.
Conclusions
This prospective study demonstrated that approximately 7.9% of women without anemia during the first trimester developed anemia during the third trimester in China, and 5.7% of women developed IDA. The Hb value in the first trimester most efficiently predicted anemia in the third trimester compared to other blood variables.
Author contributions
Yue Zeng participated in the study design, data collection, statistical analyses and was a major contributor in writing the manuscript. Guolin He conceived of the whole study, supervised the work overall, and carried out the study design and corrections of the manuscript. Both authors approved the final manuscript for submission.
Ethical statement
This study was approved by the Medical Ethics Committee of the West China Second Hospital of Sichuan University (project approval number: 2016-009). All participants provided written informed consent at enrollment.
Transparency declaration
The contributing authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported and no important aspects of the study have been omitted.
Funding
This study was supported by National Key Research and Development Program of China (2021YFC2701501). This work was not associated with any commercial entity.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available to protect patients’ privacy.
References
- Stevens GA, Paciorek CJ, Flores-Urrutia MC, et al. National, regional, and global estimates of anaemia by severity in women and children for 2000-19: a pooled analysis of population-representative data. Lancet Glob Health. 2022;10(5):1–7. doi: 10.1016/S2214-109X(22)00084-5.
- Si S, Peng Z, Cheng H, et al. Association of vitamin D in different trimester with hemoglobin during pregnancy. Nutrients. 2022;14(12):2455. doi: 10.3390/nu14122455.
- Tan J, He G, Qi Y, et al. Prevalence of anemia and iron deficiency anemia in chinese pregnant women (IRON WOMEN): a national cross-sectional survey. BMC Pregnancy Childbirth. 2020;20(1):670. doi: 10.1186/s12884-020-03359-z.
- Benson CS, Shah A, Stanworth SJ, et al. The effect of iron deficiency and anaemia on women’s health. Anaesthesia. 2021;76 Suppl 4(S4):84–95. doi: 10.1111/anae.15405.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138(4):663–674. doi: 10.1097/AOG.0000000000004559.
- Juul SE, Derman RJ, Auerbach M. Perinatal iron deficiency: implications for mothers and infants. Neonatology. 2019;115(3):269–274. doi: 10.1159/000495978.
- Georgieff MK. Iron deficiency in pregnancy. Am J Obstet Gynecol. 2020;223(4):516–524. doi: 10.1016/j.ajog.2020.03.006.
- Haider BA, Olofin I, Wang M, et al. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2013;346(jun21 3):f3443–f3443. doi: 10.1136/bmj.f3443.
- Stangret A, Skoda M, Wnuk A, et al. Mild anemia during pregnancy upregulates placental vascularity development. Med Hypotheses. 2017;102:37–40. doi: 10.1016/j.mehy.2017.03.007.
- Agarwal AM, Rets A. Laboratory approach to investigation of anemia in pregnancy. Int J Lab Hematol. 2021;43 Suppl 1(S1):65–70. doi: 10.1111/ijlh.13551.
- Tang C, King K, Ross B, et al. Iron deficiency in women: clearing the rust of silence. Lancet Haematol. 2022;9(4):e247–e248. doi: 10.1016/S2352-3026(22)00079-5.
- Society of Perinatal Medicine, Chinese Medical Association. Guideline for diagnosis and treatment of iron deficiency and iron deficiency anemia in pregnancy. Chin J Perinat Med. 2014;17:451–454.
- Recommendations to prevent and control iron deficiency in the United States. Centers for disease control and prevention. Mmwr Recomm Rep. 1998;47:1–29. PMID:9563847.
- Noshiro K, Umazume T, Hattori R, et al. Hemoglobin concentration during early pregnancy as an accurate predictor of anemia during late pregnancy. Nutrients. 2022;14(4):839. doi: 10.3390/nu14040839.
- Resseguier AS, Guiguet-Auclair C, Debost-Legrand A, et al. Prediction of iron deficiency anemia in third trimester of pregnancy based on data in the first trimester: a prospective cohort study in a High-Income country. Nutrients. 2022;14(19):4091. doi: 10.3390/nu14194091.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and Middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103(2):495–504. doi: 10.3945/ajcn.115.107896.
- Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and Middle-income countries. The Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X.
- Means RT. Iron deficiency and iron deficiency anemia: implications and impact in pregnancy, fetal development, and early childhood parameters. Nutrients. 2020;12(2):447. doi: 10.3390/nu12020447.
- Koperdanova M, Cullis JO. Interpreting raised serum ferritin levels. BMJ. 2015;351:h3692. doi: 10.1136/bmj.h3692.
- Reveiz L, Gyte GM, Cuervo LG, et al. Treatments for iron-deficiency anaemia in pregnancy. Cochrane Database Syst Rev. 2011;1813 (10):CD003094. doi: 10.1002/14651858.CD003094.pub3.