Abstract
Objective
This study aimed to investigate changes in the cervical strain rate (SR), cervical length (CL), and uterine artery blood flow parameters during early pregnancy in women with cervical insufficiency and evaluate the clinical efficacy of these markers for screening of cervical insufficiency in early pregnancy.
Methods
This retrospective study in 60 pregnant women with cervical insufficiency and 100 normal pregnant women was conducted between September 2021 and January 2023 and measured ultrasound parameters of the cervix during early pregnancy. The cervical SR, CL, and uterine artery resistance index (RI) were measured in both groups at 11–14 weeks of gestation. Strain elastography represented by the SR was used to assess the hardness of the internal and external cervical openings.
Results
During early pregnancy, the SR at the internal and external cervical openings were significantly higher in the cervical insufficiency group than those in the normal pregnancy group (SR I: 0.19 ± 0.018% vs. 0.16 ± 0.014%; SR E: 0.26 ± 0.028% vs. 0.24 ± 0.025%; p < .001). The CL was significantly shorter in the cervical insufficiency group than that measured in the normal pregnancy group (34.3 ± 2.9 mm vs. 35.2 ± 1.99 mm; p = .036), while cervical blood perfusion was also poorer in the cervical insufficiency group than that in the normal pregnancy group (uterine artery RI: 0.76 ± 0.07 vs. 0.74 ± 0.05; p = .048). Receiver operating characteristic (ROC) curve analysis showed that the optimal critical values for diagnosing cervical insufficiency were 0.17% for SR I, 0.25% for SR E, 33.8 mm for CL, and 0.78 for uterine artery RI. Of these parameters, the ROC curve for SR I had the largest area under the curve [AUC = 0.89 (p < .001)], with the highest sensitivity (78%) and specificity (82%). Multivariate logistic regression analysis demonstrated that the SR at the internal cervical opening (OR 17.47, 95% confidence interval (CI) 5.08–60.08; p < .001) and CL (OR 5.05, 95% CI 1.66–15.32; p = .004) still showed significant differences between the two groups.
Conclusion
Cervical elastography is an effective tool for screening early pregnancy cervical insufficiency. The SR at the internal cervical opening is a valuable indicator for screening cervical insufficiency and has superior clinical efficacy for screening for this condition compared to that of CL and the uterine artery blood flow index.
1. Introduction
According to statistics from The Lancet, the estimated number of premature births each year has reached 15 million and the global average premature birth rate is about 10%. As a consequence, premature birth is ranked as the top complication of pregnancy [Citation1]. Cervical insufficiency (CI) accounts for 8–9% of premature births and 8–25% of extremely preterm births and mid-to-late recurrent miscarriages [Citation2]. CI is characterized by structural or functional abnormalities of the cervix that lead to progressive, painless shortening and dilation of the cervix in the mid-to-late stages of pregnancy, resulting in an inability to maintain the pregnancy to full term [Citation3]. Preterm births caused by CI often occur before 28 weeks of gestation and are associated with higher rates of disability and mortality. Moreover, repeated mid-to-late recurrent miscarriages can lead to adverse outcomes such as intrauterine adhesions or fallopian tube blockages, thereby causing secondary female infertility [Citation4]. The latest guidelines issued in 2019 by the Society of Obstetricians and Gynecologists of Canada (SOGC) [Citation5] suggest that high-risk women with a history of CI should undergo vaginal ultrasound measurements of cervical length (CL) every two weeks between weeks 16–24 of pregnancy, with a CL ≤ 25 mm serving as the basis for diagnosing CI and performing cervical cerclage. However, research has found that using a critical value of 25 mm for CL to predict CI or preterm birth in low-risk women with no history of CI in early to mid-pregnancy has a sensitivity of only 37.3% [Citation6]. Therefore, relying solely on this criterion may overlook the fact that women with CI have a high rate of miscarriage or preterm birth of the fetus in the first pregnancy [Citation7]. Accordingly, it is essential to develop an effective method for predicting CI during early pregnancy.
Elastography is a noninvasive imaging technique used to measure the hardness of a region of interest [Citation8]. Currently, cervical elastography [Citation9,Citation10] mainly includes two methods: strain elastography and shear wave elastography. Strain elastography involves applying pressure to tissues using an ultrasound probe to cause tissue deformation which is quantified by the strain rate (SR). Under external pressure, softer tissues are more easily deformed, resulting in a higher SR [Citation11–13]. Cervical hardness is influenced by various pressures from the uterus, the growing fetus and the amniotic sac, as well as passive pressure from the uterine wall [Citation14]. Moreover, changes in cervical hardness during pregnancy occur earlier than changes in CL [Citation15]. Cervical elastography is considered a promising tool for predicting CI in early pregnancy. A recent study reported that cervical cone elastography is superior to CL for predicting CI, although the study only included a limited number of cases [Citation16]. In addition, only a small number of studies have investigated the relationship between cervical blood perfusion in early pregnancy and CI.
The aims of the current study were therefore to retrospectively analyze the cervical SR, CL, and uterine artery resistance index (RI) in pregnant women with and without early pregnancy CI and to identify independent predictive factors related to CI among these markers, compare their predictive efficacy, and enable early screening of high-risk groups for CI. This approach has the objectives of enhancing personalized prenatal management, determining the appropriate time for surgery, reducing the fetal abortion rate and preterm birth rate in first pregnancy in patients with CI.
2. Materials and methods
2.1. Subjects
The study was a retrospective ultrasound investigation conducted between September 2021 to January 2023 at Shanghai East Hospital, a teaching hospital affiliated to Tongji University. The study included pregnant women with early pregnancy CI (n = 60) as the case group and normal pregnant women (n = 100) as the control group. The inclusion criteria were: (a) singleton pregnancy, including assisted reproductive and natural conception; (b) aged 20–50 years and gestational age between 11 and 14 weeks, determined initially by measuring crown-rump length (CRL) in early pregnancy; (c) the case group consisted of pregnant women with a history of mid-to-late recurrent miscarriages or preterm births who were diagnosed with CI in the mid-to-late stages of pregnancy and underwent cervical cerclage (Shirodkar or MacDonald technique); (d) the control group consisted of pregnant women with no history of mid-to-late recurrent miscarriages or preterm births and who had a full-term delivery for the current pregnancy. The exclusion criteria were: (a) twin pregnancy; (b) vaginal bleeding in the first trimester; (c) uterine fibroids ≥ 5 cm, (d) Nabothian cysts of the cervix ≥1 cm; (e) spontaneous preterm birth with no evidence of CI history; (f) history of a cervical cone biopsy. The study was approved by the Shanghai East Hospital Clinical Research Ethics Committee under review number [2022] and Research Review No. (194), with all participants providing informed consent.
2.2. Instruments and methods
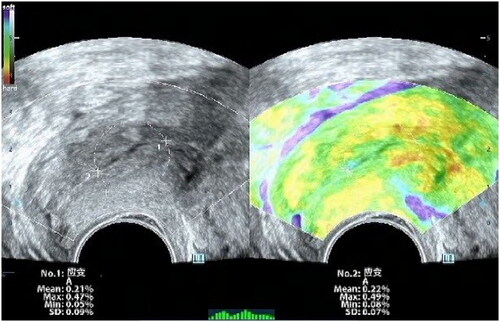
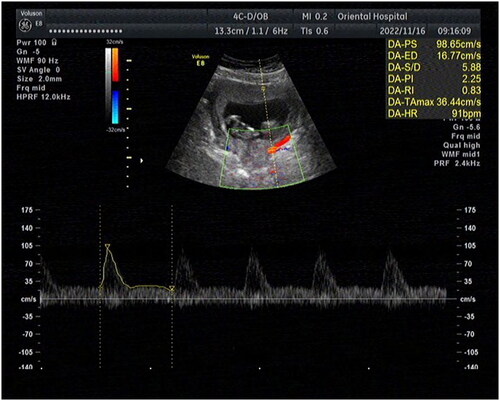
The study utilized a Voluson E8 color Doppler ultrasound system (GE Healthcare) with a RIC5-9-D endo-cavity probe (4-9 MHz) and a 4 C–D abdominal probe (3–10 MHz). Participants were required to empty their bladder before the examination. The endo-cavity probe was then placed at the vaginal fornix to obtain a mid-sagittal view of the cervix that showed clear images of the internal and external cervical openings and the anterior and posterior lips of the cervix. CL measurements were taken, followed by cervical strain elastography. The elastography area encompassed the entire cervix. Once a complete green quality bar was obtained through appropriate manual compression, the dual-mode (grayscale and elastography) was used to trace the contour of the cervix in the B-mode and determine the location and area of cervical elastography. As shown in , the region of interest was set as a circular area with a diameter of 1/2 CL, placed at both the internal and external cervical openings. The elastography software computed SR for both internal and external cervical openings as percentages. Finally, the abdominal probe was positioned horizontally on the lower abdomen of the pregnant woman. The probe was then tilted vertically toward the cervical external opening at a specific angle to obtain a clear coronal view of the cervix. The color Doppler mode was activated to display the smaller descending branches of the uterine artery on both sides of the cervix (), with the RI of the uterine arteries on both sides then measured and averaged. All ultrasound examinations were performed by the same experienced obstetric sonographer who had over 10 years of experience. In the first 20 patients, the elastography images of the internal and external cervical openings were measured twice to assess intra-rater reliability.
2.3. Elastography image analysis
Cervical elastography images represent the hardness of the cervix expressed as the SR. A larger SR indicated greater displacement of the cervix under pressure, with the image appearing red, indicating lower hardness (i.e. softness of the cervix). An intermediate SR image appeared green, while a smaller SR appeared blue, indicating higher cervical hardness.
2.4. Statistical methods
SPSS version 27.0 conducted statistical analysis. Intra-rater reliability was assessed via Spearman’s correlation. Continuous data were presented as mean ± standard deviation
(x¯±s) and median (minimum value, maximum value) [M (min, max)]. Group comparisons employed the t-test for normally distributed data and the Mann–Whitney U test for non-normally distributed data. Categorical variables were represented in terms of frequencies and percentages, and intergroup differences were determined using the χ2 test. ROC curve analysis determined optimal critical values for ultrasound parameters predicting CI. Single-factor and multiple-factor logistic regressions calculated odds ratios (OR) and 95% confidence intervals (CI). A significance level of α = 0.05 was adopted, with p < .05 indicating statistically significant predictors of CI presence.
3. Results
presents the general characteristics and neonatal outcomes of the two groups of pregnant women. The CI group had a higher parity than the control group (p < .001), along with a significantly earlier average gestational age at delivery (p < .001). Neonates in the CI group also exhibited significantly lower average birth weights when compared to those in the control group (p < .001). Moreover, the Apgar score at 1 min post-birth for CI group neonates was notably lower than that of the control group (p < .001) ().
Table 1. Maternal characteristics and neonatal outcomes of the study group.
presents the results of cervical SR, CL, and uterine artery RI between the two groups. The internal cervical opening (SR I 0.19 ± 0.018%) was significantly softer in the CI group than in the control group (SRI 0.16 ± 0.014%) (p < .001), while the external cervical opening (SRE 0.26 ± 0.028%) was significantly softer in the CI group than in the control group (SRE 0.24 ± 0.025%) (p < .002). The intraclass correlation coefficients for SRI and SRE were 0.84 and 0.88, respectively (p < .001). Furthermore, the CL in the CI group (34.3 ± 2.91 mm) was significantly shorter than that in the control group (35.2 ± 1.99 mm) (p = .036), while the uterine artery RI in the CI group (0.76 ± 0.07) was significantly higher than that in the control group (0.74 ± 0.05) (p = .048).
Table 2. Cervical elastography, cervical length and arteria uterina RI width of the study group.
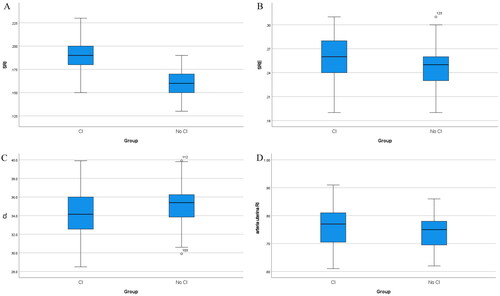
Box plots showed that the median values of SRs at the cervical internal and external openings, CL, and uterine artery RI were significantly different between the CI and control groups ().
Figure 3. Box-And-whisker plots of (A) the strain rate of the internal cervical opening, (B) the strain rate of the external cervical opening, (C) cervical length and (D) arteria uterina RI between the cervical insufficiency and no cervical insufficiency groups.
ROC curve analysis showed that the optimal critical values for diagnosing CI were 0.17% for the internal opening SR, 0.25% for the external opening SR, 33.8 mm for the CL, and 0.78 for the uterine artery RI. The sensitivity and specificity for diagnosing CI using the internal opening SR were 0.78 and 0.82, respectively, which were better than those for the external opening SR (0.62 and 0.68), CL (0.48 and 0.75), and uterine artery RI (0.45 and 0.80). The areas under the ROC curves for the internal opening SR, external opening SR, CL, and uterine artery RI, and the combination of these four parameters were 0.89 (95% CI, 0.83–0.94), 0.66 (95% CI, 0.57–0.75), 0.59 (95% CI, 0.50–0.69), 0.59 (95% CI, 0.50–0.70), and 0.92 (95% CI, 0.88–0.97), respectively (, ).
Figure 4. Subject work characteristics (ROC) curves and area under the curve (AUC) for the strain rate of the internal cervical opening, the strain rate of the external cervical opening, cervical length, uterine artery RI and the combination of these five parameters predicting cervical insufficiency of 0.89, 0.66, 0.59, 0.59, 0.59 and 0.92, respectively.
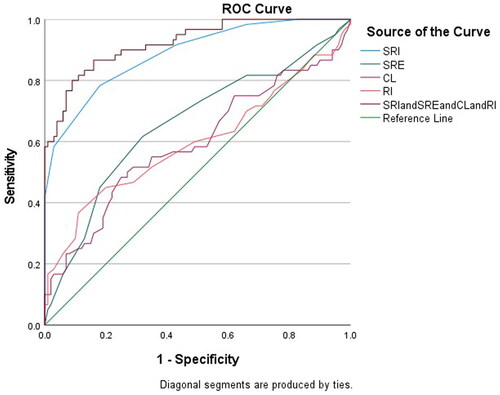
Table 3. ROC curve analyses of cervical elastography, cervical length, and arteria uterina RI width for the diagnosis of cervical insufficiency.
The results of the univariate logistic regression analysis using CI as the dependent variable and individual parameters with significant differences between the two groups as independent variables showed that parity (OR = 3.257; 95% CI, 2.146–4.943; p < .001), SR I > 0.17% (OR = 16.47; 95% CI, 7.412–36.597; p < .001), SR E > 0.25% (OR = 3.418; 95% CI, 1.751–6.673; p < .001), CL < 33.8 mm (OR = 2.806; 95% CI, 1.423–5.535; p = .003), and uterine artery RI > 0.78 (OR = 3.273; 95% CI, 1.615–6.632; p = .001) were all independent predictors of early pregnancy CI. Of these parameters, SR I had the highest odds ratio and the best predictive performance. After adjusting for individual parameters in the multivariate logistic regression analysis, SR I (adjusted OR 17.474; 95% CI, 5.082–60.085; p < .001), CL (adjusted OR 5.054; 95% CI, 1.667–15.326; p < .001), and parity (adjusted OR 3.976; 95% CI, 2.183–7.241; p = .004) still showed significant differences between the two groups ().
Table 4. Univariate and multivariate logistic regression analyses of individual parameter related to cervical insufficiency.
4. Discussion
CI is one of the main causes of mid-to-late pregnancy miscarriages and preterm births and results in relatively high rates of preterm disability and death [Citation4]. Currently, the clinical approach to CI mainly involves prophylactic cervical cerclage. However, the efficacy and prognosis of mid-pregnancy prophylactic cervical cerclage are not as good as those carried out in early pregnancy [Citation17]. Therefore, it is necessary to identify the relevant risks of CI in early pregnancy. Changes in cervical hardness during pregnancy occur earlier than changes in CL, making the analysis and evaluation of cervical hardness in early pregnancy a key focus in perinatal medicine. The potential value of cervical elastography for evaluating cervical softness as a predictor of preterm birth has been demonstrated previously [Citation18–20]. However, most of these earlier studies were conducted in the mid-term or late-term of pregnancy [Citation21], with some studies including only a small number of cases [Citation16]. More research is therefore required to provide objective and quantitative clinical indicators for effective early pregnancy screening of CI and to reduce the rate of preterm birth. This study investigated the critical values of cervical SR, CL, and uterine artery RI for diagnosing early pregnancy CI, along with their sensitivity and specificity, and also determined the clinical diagnostic efficacy of these markers. It was found that the SR at the internal opening of the cervix was a reliable indicator for screening early pregnancy CI, with a sensitivity of 0.78 and a specificity of 0.82, and that a threshold of SR I > 0.17%, yielded the highest predictive performance (adjusted OR 17.474).
The cervix is composed of collagen fibers, proteoglycans, elastic proteins, smooth muscles, fibroblasts, epithelial cells, and blood vessels. Of these, collagen fibers determine the hardness of the cervix, while proteoglycans influence the arrangement and thickness of collagen fibers. Under normal circumstances, the distribution of the collagen fibers is more uniform at the internal opening of the cervix compared to that at the external opening [Citation22]. The “cervical remodeling” process during pregnancy [Citation23] involves cervical softening, shortening, and dilatation, with abnormal cervical remodeling leading to CI. Physiological and biochemical studies of “cervical remodeling” during pregnancy in mice [Citation24] suggest that a deficiency of Type I and II small leucine-rich proteoglycans (SLRPs) and decreased expression of lysyl oxidase and lysyl hydroxylase result in premature collagen degradation, laxity, rupture, and that early softening and maturation of the cervix are associated with CI. These mechanisms support our findings that the cervix of patients with early pregnancy CI has a relatively high SR. Furthermore, the internal opening of the cervix bears direct pressure from the contents of the uterine cavity, making it prone to high strain in early pregnancy, with SRI being a good predictor of early pregnancy CI. Our study also demonstrated that SRI was lower than SRE, indicating that the internal opening of the cervix is harder than the external opening. This finding is consistent with the results of an in vitro study by Rosado-Mendez et al. [Citation25] that reported a significant increase in cervical hardness from the distal (vaginal) end to the proximal (uterine) end.
The uterine artery originates from the internal iliac artery and is divided into ascending and descending branches. The blood circulation of the cervix is supplied mainly by the descending branch of the uterine artery [Citation26]. Using ultrasound to measure the RI of the descending branch of the uterine artery reflects cervical blood perfusion and evaluates cervical function. The results of our study showed that the uterine artery RI in the CI group was significantly higher (p < .05) than that in the control group, with an optimal critical value of 0.78 for the uterine artery RI providing a diagnostic marker for CI. Microbial studies [Citation27] have suggested that proteases from Porphyromonas vaginalis can degrade clotting factors and disrupt physiological coagulation in the cervical vaginal canal, thereby adversely altering cervical blood perfusion. This finding is consistent with our study that cervical blood perfusion in CI patients was worse than that in normal pregnant women.
Ultrasound elastography for assessing cervical hardness mainly includes strain elastography and shear wave elastography. Strain elastography software has the advantages of ease of use and availability [Citation28]. These advantages coupled with the limited safety data of the transmission beam of shear wave elastography being focused so close to the fetus [Citation10], led us to use strain elastography in this study. This involved three different ultrasound parameters of the cervix (cervical SR, CL, and uterine artery RI) being analyzed in early pregnancy CI and non-CI pregnant women, with the optimal critical values of each ultrasound parameter obtained for diagnosing early pregnancy CI. This study also compared the diagnostic efficacy of cervical SR, CL, and uterine artery RI for diagnosing early pregnancy CI. However, the study had several limitations. First, in order to eliminate confounding factors, we excluded CI patients with twin pregnancies. Second, multiple studies have shown good inter-rater reliability for cervical SR [Citation16,Citation18,Citation21], although in our study, the ultrasound measurements were performed by a single sonographer, and therefore inter-rater reliability analysis was not conducted. Finally, due to the small sample size, the study did not further examine a predictive model for screening CI by combining multiple ultrasound indicators with CI risk factors in early pregnancy. This model could be pursued using an expanded case sample, including cases of twin pregnancies and a more comprehensive and in-depth study.
5. Conclusions
In summary, cervical elastography is an effective tool for screening early pregnancy CI, with the SR at the internal opening of the cervix shown to be a reliable indicator for screening CI. The efficacy of SR to predict CI is superior to that of CL and uterine artery blood flow parameters.
Authors contributions
Hua Jiang performed the literature review, carried out echocardiography measurements, selected participants, collected the clinical data and conducted the data analysis. Bo Zhang checked the validity of the data, provided financial support for the experiments and reviewed the paper. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- The Lancet. The unfinished agenda of preterm births. Lancet. 2016;388(10058):1. doi: 10.1016/S0140-6736(16)32170-5.
- Thain S, Yeo GSH, Kwek K, et al. Spontaneous preterm birth and cervical length in a pregnant Asian population. PLOS One. 2020;15(4):e0230125. doi: 10.1371/journal.pone.0230125.
- Liu Y, Yang D, Jiang Y, et al. Quantification of cervical stiffness changes in single and twin pregnancies using the E-cervix technique. Am J Obstet Gynecol MFM. 2023;5(2):100804. doi: 10.1016/j.ajogmf.2022.100804.
- Nazzaro G, Saccone G, Miranda M, et al. Cervical elastography using E-cervix for prediction of preterm birth in singleton pregnancies with threatened preterm labor. J Mater-Fetal Neonatal Med. 2022;35(2):330–8. doi: 10.1080/14767058.2020.1716721.
- Brown R, Gagnon R, Delisle M-F. No. 373-cervical insufficiency and cervical cerclage. J Obstet Gynaecol Can. 2019;41(2):233–247. doi: 10.1016/j.jogc.2018.08.009.
- Romero R, Yeo L, Miranda J, et al. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41(1):27–44. doi: 10.1515/jpm-2012-0272.
- McIntosh J, Feltovich H, Berghella V, et al. The role of routine cervical length screening in selected high-and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2–B7. doi: 10.1016/j.ajog.2016.04.027.
- Thomas A. Imaging of the cervix using sonoelastography. Ultrasound Obstet Gynecol. 2006;28(3):356–357. doi: 10.1002/uog.3813.
- Öcal FD, Çekmez Y, Erdoğdu E, et al. The utility of cervical elastosonography in prediction of cervical insufficiency: cervical elastosonography and cervical insufficiency. J Mater-Fetal Neonatal Med. 2015;28(7):812–818. doi: 10.3109/14767058.2014.933801.
- O'Hara S, Zelesco M, Sun Z. Can shear wave elastography of the cervix be of use in predicting imminent cervical insufficiency and preterm birth?-preliminary results. Ultrasound Med Biol. 2019;45:S111–S112. doi: 10.1016/j.ultrasmedbio.2019.07.368.
- Suthasmalee S, Moungmaithong S. Cervical shear wave elastography as a predictor of preterm delivery during 18–24 weeks of pregnancy. J Obstet Gynaecol Res. 2019;45(11):2158–2168. doi: 10.1111/jog.14094.
- Ono T, Katsura D, Yamada K, et al. Use of ultrasound shear-wave elastography to evaluate change in cervical stiffness during pregnancy. J Obstet Gynaecol Res. 2017;43(9):1405–1410. doi: 10.1111/jog.13379.
- Stout MJ, Zhou Y, Wylie KM, et al. Early pregnancy vaginal microbiome trends and preterm birth. Am J Obstet Gynecol. 2017;217(3):356. e351–356. e318.
- Moghaddam AO, Lin Z, Sivaguru M, et al. Heterogeneous microstructural changes of the cervix influence cervical funneling. Acta Biomater. 2022;140:434–445. doi: 10.1016/j.actbio.2021.12.025.
- Son M, Miller ES. Predicting preterm birth: cervical length and fetal fibronectin. Semin Perinatol. 2017;41(8):445–451. doi: 10.1053/j.semperi.2017.08.002.
- Chen CY, Chen CP, Sun FJ. Assessment of the cervix in pregnant women with a history of cervical insufficiency during the first trimester using elastography. Acta Obstet Gynecol Scand. 2020;99(11):1497–1503. doi: 10.1111/aogs.13942.
- Lim K, Butt K, Crane JM. Ultrasonographic cervical length assessment in predicting preterm birth in singleton pregnancies. J Obstet Gynaecol Can. 2011;33(5):486–499. doi: 10.1016/S1701-2163(16)34884-8.
- Dymanowska-Dyjak I, Stupak A, Kondracka A, et al. Elastography and metalloproteinases in patients at high risk of preterm labor. J Clin Med. 2021;10(17):3886. doi: 10.3390/jcm10173886.
- Köbbing K, Fruscalzo A, Hammer K, et al. Quantitative elastography of the uterine cervix as a predictor of preterm delivery. J Perinatol. 2014;34(10):774–780. doi: 10.1038/jp.2014.87.
- Patberg ET, Wells M, Vahanian SA, et al. Use of cervical elastography at 18–22 weeks’ gestation in the prediction of spontaneous preterm birth. Am J Obstet Gynecol. 2021;225(5):525. e521–525. e529. doi: 10.1016/j.ajog.2021.05.017.
- Jiang L, Peng L, Rong M, et al. Nomogram incorporating multimodal transvaginal ultrasound assessment at 20 to 24 weeks’ gestation for predicting spontaneous preterm delivery in low-risk women. Int J Womens Health. 2022;14:323–331. doi: 10.2147/IJWH.S356167.
- Yan Y, Basij M, Garg A, et al. Spectroscopic photoacoustic imaging of cervical tissue composition in excised human samples. PLOS One. 2021;16(3):e0247385. doi: 10.1371/journal.pone.0247385.
- Zhou L, Jiang R, Meng J, et al. Three-dimensional remodeling of collagen fibers within cervical tissues in pregnancy. J Innov Opt Health Sci. 2023;16(04):2243005. doi: 10.1142/S1793545822430052.
- Colon-Caraballo M, Lee N, Nallasamy S, et al. Novel regulatory roles of small leucine-rich proteoglycans in remodeling of the uterine cervix in pregnancy. Matrix Biol. 2022;105:53–71. doi: 10.1016/j.matbio.2021.11.004.
- Rosado-Mendez IM, Palmeri ML, Drehfal LC, et al. Assessment of structural heterogeneity and viscosity in the cervix using shear wave elasticity imaging: initial results from a rhesus macaque model. Ultrasound Med Biol. 2017;43(4):790–803. doi: 10.1016/j.ultrasmedbio.2016.12.006.
- Jaraquemada JMP, Mónaco RG, Barbosa NE, et al. Lower uterine blood supply: extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstet Gynecol Scand. 2007;86(2):228–234. doi: 10.1080/00016340601089875.
- Lithgow KV, Buchholz VC, Ku E, et al. Protease activities of vaginal porphyromonas species disrupt coagulation and extracellular matrix in the cervicovaginal niche. NPJ Biofilms Microbiomes. 2022;8(1):8. doi: 10.1038/s41522-022-00270-7.
- Helmi H, Siddiqui A, Yan Y, et al. The role of noninvasive diagnostic imaging in monitoring pregnancy and detecting patients at risk for preterm birth: a review of quantitative approaches. J Mater-Fetal Neonatal Med. 2022;35(3):568–591. doi: 10.1080/14767058.2020.1722099.