?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
Epidural-related maternal fever increases the incidence of Category II fetal tracings. To compare the effectiveness of low-flow oxygen inhalation and cooling treatment for parturients with Category II fetal tracings caused by epidural-related maternal fever.
Methods
We investigated 200 pregnant women who accepted epidural analgesia during labor and had body temperature exceeding 38 °C during labor. Among the patients, 99 and 101 were randomly allocated to receive cooling treatment group (control group) and oxygen inhalation (oxygen group), respectively. The primary outcome was the incidence of Category II fetal heart rate tracings.
Results
The incidence of Category II fetal heart rate tracings in the control group was significantly higher than that in the oxygen group. However, no significant differences were noted between the two groups in terms of the Apgar scores; amniotic fluid turbidity; or maternal outcomes, including cesarean section rate, forceps delivery rate, lateral resection rate, manual removal of placenta rate, the amount of intrapartum hemorrhage, and hemorrhage at postpartum 2 h. Oxygen inhalation therapy was more effective than cooling treatment in reducing the incidence of Category II tracings.
Conclusion
Low-flow and short-term oxygen inhalation for parturients with epidural-related maternal fever reduces the incidence of Category II fetal heart rate tracings, but had no significant influence on the mode of delivery or neonatal outcomes.
Background
Noninfectious fever induced by labor epidural analgesia (LEA) is defined as epidural-related maternal fever (ERMF), and more than 20% of the women receiving epidural analgesia develop ERMF [Citation1–3]. Studies have shown weak correlations between the duration of LEA plus dose of analgesics and the persistence of maternal fever [Citation4,Citation5].
Maternal fever during delivery has been linked to several transient neonatal complications [Citation6–9]. Elevated maternal temperature is associated with increased oxygen consumption by both the mother and fetus, which in turn leads to fetal hypoxia/intrapartum acidosis. Umbilical artery sampling performed immediately after delivery provides the most accurate diagnosis of fetal acidemia (pH < 7.15). However, fetal acidemia is known to affect the autonomic nervous system and thereby cause changes in the FHR. Therefore, FHR tracing may be regarded as an alternative to umbilical artery sampling [Citation10]. Continuous fetal electronic monitoring is one of the most common screening methods for fetal hypoxia/intrapartum acidosis [Citation11]. As defined by the National Institute of Child Health and Human Development (NICHD) [Citation12], category II fetal heart rate tracing lies in between Category I tracing, which reflects no hypertensive acidosis, and Category III fetal heart rate tracing, which is indicative of highly suspected fetal acidosis. Category II tracings, due to their uncertainty, require close monitoring because they carry the risk of progression to Category III tracings [Citation13], which are known to be associated with fetal hypoxia, acidosis, and encephalopathy [Citation14].
It would be worthwhile to minimize the incidence of Category II tracings associated with ERMF. Therefore, it is important to address the increase in temperature, which is associated with the development of Category II tracings; the most direct method would be cooling treatment for parturient. Randomized controlled trial had shown that cooling treatment both oral and intravenous acetaminophen can benefit parturients and fetuses with intrapartum maternal fever [Citation15]. On the other hand, Category II tracings cause changes in maternal oxygen consumption, and maternal oxygen (O2) administration is one of the most commonly used intrauterine resuscitation techniques during labor. Moore et al. have shown that maternal hyperoxia may have a positive impact on neonatal outcomes; in particular, in the presence of suspected fetal distress, hyperoxia has been shown to have a positive impact on fetal heart rate [Citation16,Citation17].
Objectives
This randomized controlled trial was aimed at comparing the effectiveness of low-flow oxygen inhalation and cooling treatment in the reduction of Category II fetal tracings caused by ERMF.
Material and methods
Study population
This investigation was designed as a randomized controlled trial. The study protocol was approved by the Clinical Committee of Guangzhou Women and Children’s Medical Center (protocol number: 201939000), and all subjects enrolled in this study provided written informed consent. After recruitment of the subjects, the trial was registered in China clinical trial registry (http://www.chictr.org.cn/editx.asp, Date of registration: 5/9/2019, Principal investigator: ChiCTR1900025653). The reporting of this study is in accordance with the CONSORT guidelines.
Pregnant women who were managed at our institution during daytime between 5 May 2019 and 30 August 2019 were considered eligible for this study. All the enrolled women had accepted epidural analgesia during labor. The criteria for inclusion in this study were as follows: (1) singleton pregnancy with the gestational age more than 37 weeks; (2) planned vaginal delivery at the hospital; (3) cephalic presentation; (4) no perinatal transmission disease; (5) no suspected fetal coagulation dysfunction; (6) pre-epidural temperature of <37 °C; and (7) body temperature above 38 °C during labor. The exclusion criteria for this study were as follows: (1) inability to sign the informed consent form; (2) bilateral availability of upper limb venous access (3) absence of upper extremities; (4) medical history of myocardial infarction, heart failure, maternal hepatitis, or HIV infection; (5) history of severe allergies, specifically a history of allergy to silicone or plastic; (6) preeclampsia; (7) intake of an antipyretic 6 h before enrollment in the study; (8) presentation other than vertex (i.e. breech or transverse); (9) gestational age of <37 weeks; or (10) suspected fetal coagulation dysfunction.
Only women who accepted epidural analgesia were enrolled, and the analgesic was administered according to the woman’s request. Detailed explanation and informed consent for participation in the study was obtained. The investigators responsible for data collection and analysis were blinded to the group assignment.
Implementation of epidural analgesia
Epidural analgesia was induced once uterine contractions were regular and cervical dilatation was ≥1 cm. Arrangements were made for the immediate availability of emergency medicine, atropine, and ephedrine. The epidural catheter was placed at the L3-L4 level. First, a test dose of 4 ml of 1% lidocaine was injected via the catheter, followed by a bolus dose of 10 ml of ropivacaine 0.0625% (lot number: H20140763, Astra Zeneca, Sweden) with 0.3 µg/mL sufentanil (batch number: H20054256, Yichang Renfu Pharmaceutical Co., Ltd.). Then, the epidural catheter was connected to the pulse pump with the following settings: loading dose, 10 ml; injection rate, 6 ml/h; patient-controlled analgesia dose, 8 ml; maximum dose, 40 ml/h; and lockout interval, 10 min. After 15 min, the depth of anesthesia was assessed using the skin scratch test. At any time during delivery, if the parturient had breakthrough pain and the pain score of the visual analog scale was >3, an additional 5 ml of 0.1% ropivacaine was administered.
Measurement of body temperature and treatment of fever
A wireless thermometer, iThermonitor (Rui Ren Medical, China), was connected to the central control platform to measure the axillary temperature throughout the delivery process (10 values per second). Body temperature sensors were installed according to product instructions and previous study [Citation13]. Fever was defined as an axillary temperature of ≥38 °C, as measured by the iThermonitor.
The enrolled women were randomly allocated to the cooling treatment group (control group; n = 99) or the oxygen inhalation group (oxygen group; n = 101) by random number table. The random allocation sequence was generated by one researcher and was not unsealed by the other researcher until women received epidural analgesia. In the cooling treatment group (control group), when the axillary temperature of women was more than 38 °C, oral paracetemol, pseudoephedrine, dextromethorphan and chlorpheniramine maleate suspension (Tylenol, Shanghai Johnson & Johnson Pharmaceutical Co., Ltd.) was administered. On the other hand, women allocated in the oxygen inhalation group (oxygen group) were administered low-flow oxygen inhalation with 100% oxygen at a rate of 2 L/min for 30 min when the axillary temperature is increased to >38 °C.
Obstetricians determined the stages of labor. The first stage of labor was defined as the period between the occurrence of regular contractions and full dilation of cervix; the second stage, as the time between full dilation of cervix and delivery of fetus; and the third stage, as the period between delivery of fetus and delivery of placenta.
Fetal heart rate tracing and abnormal fetal heart rate treatment
Fetal heart rate tracings were classified according to the categories defined by the United States National Institute of Child Health and Human Development (revised in 2008) [Citation10]. Category I tracings are considered normal, and no intervention is required. Category III tracings are considered abnormal and indicate the need for immediate intrauterine resuscitation and intervention. Category II tracings vary widely depending on the level of acidosis and need to be managed according to the following Assess-Begin-Clear-Determine (ABCD) processing protocol, which has been developed at our institution: (1) Assess: This step involves quick and systematic evaluation of oxygenation of intrauterine fetus and identification of possible reasons of fetal hypoxia. (2) Begin: Once comprehensive evaluation and identification of possible causes are completed, targeted interventions should be implemented as per the clinical situation. (3) Clear: If improvement in FHR is still not satisfactory after intrauterine resuscitation, it is necessary to prepare for rapid delivery, including assisted vaginal delivery or cesarean section. (4) Determine: The final delivery plan must be appropriately chosen.
Data collection
Data were collected from all the enrolled women for the following demographic and basic clinical characteristics: gestational age, height, weight, body mass index (BMI), and duration of labor. Additionally, data were collected on maternal clinical characteristics, including maternal temperature, delivery outcomes, amount of postpartum hemorrhage, and occurrence of puerperal infection. The following fetal parameters were assessed: primary outcome, Category II fetal heart tracings[Citation12]; secondary outcome, neonatal Apgar scores[Citation18,Citation19]; and amniotic fluid turbidity. Maternal blood pressure and heart rate were continuously monitored during labor.
Statistical analysis
The required sample size was calculated based on the incidence of Category II tracings obtained from previous trials. Assuming the rate in cooling-group was 71%, and rate in oxygen-group was 89%, with α = 0.05 and a power of 0.9, the minimum sample size required for each group, to achieve a ratio of 1:1, was 99.
All statistical analyses in this study were performed using SPSS software (ver. 21.0; SPSS Inc., USA). The measurement data were expressed in terms of mean and standard deviation (), and the intergroup differences were compared by t test. The enumeration data were expressed in terms of the number of cases and percentage [n (%)], and the intergroup differences in the two groups were determined by chi square test or rank sum test. p < 0.05 was considered to indicate statistical significance.
Results
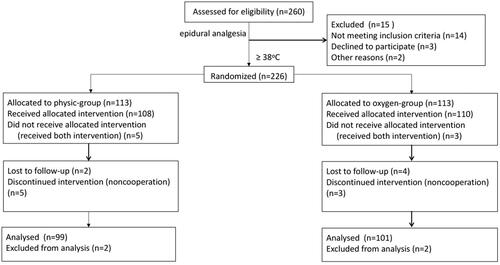
In this study, we investigated 200 parturients with ERMF; 99 were randomly assigned to the control group, and 101 were randomized to the oxygen group ().
No significant differences were noted between the two groups in terms of the general demographic variables (). Additionally, there were no significant inter-group differences for the maternal-related outcomes ().
Table 1. Maternal demographic and clinical characteristics.
Table 2. Maternal-related outcomes.
The incidence of Category II fetal heart tracings in the control group was significantly higher than that in the oxygen group (87.9% vs. 74.3%; p = 0.014). However, the two groups did not show any differences in the Apgar scores recorded from 1 min to 10 min and degree of amniotic fluid turbidity ().
Discussion
Two study groups in this randomized controlled trial were comparable in terms of baseline characteristics. Maternal-related outcomes between two groups were comparable. The main clinical findings were as follows: the incidence of Category II fetal heart tracings in the oxygen inhalation group was significantly lower than that in the control group; however, the neonatal Apgar scores from 1 to 10 min and the degree of amniotic fluid turbidity did not show any significant differences between the two groups.
Intrapartum maternal fever is associated with increased tissue oxygen consumption, which leads to hypoxia, thereby increasing the incidence of Category II fetal tracings. Continuous monitoring of FHR fluctuations, which is associated with maternal contractions, is a standard practice for the positive assessment of the fetal status during delivery [Citation10]. Firstly, fetal temperature is positively correlated to maternal temperature. Research has shown that maternal oral temperature is significantly correlated with fetal skin temperature, which is 0.75 °C less than the fetal core temperature [Citation20]. Maternal fever leads to pyrexia in the fetus, which may exacerbate the preexisting hypoxia due to increased tissue oxygen consumption and the resulting rightward shift of the fetal oxygen-dissociation curve. During the process of delivery, uterine contractions lead to changes in the intrauterine pressure. This, in turn, may cause intermittent interruptions in uterine and cord blood flow, which can jeopardize oxygen delivery to the fetus and reduce fetal oxygenation [Citation21]. Secondly, abnormal maternal temperature may also disrupt the acid-base balance in the placenta. Studies have shown a significant correlation between the heat flux measured immediately before scalp blood sampling and the pH of fetal scalp blood, thereby indicating that fetal scalp heat flux is associated with the metabolic status of the fetus [Citation22]. This is reflected as changes in FHR tracings. Moreover, the fetal brain regulates FHR by modulating sympathetic and parasympathetic pathways and changes in cerebral hypoxia are reflected as changes in FHR, which may be visualized through fetal heart tracings [Citation11].
In this study, we found that oxygen inhalation therapy was more effective than cooling treatment in reducing the incidence of Category II tracings. However, oxygen inhalation therapy did not result in any significant differences in the incidence of delivery or neonatal outcomes. Some Category II tracings may progress to Category III tracings. Category II tracings with moderate variability and/or accelerations may be considered favorable because their presence is indicative of the lack of fetal acidosis. However, Category II tracings that are not accompanied by spontaneous or provoked accelerations, minimal/absent variability, or deep decelerations (i.e. FHR drops to 70 bpm or less) show a tendency to progress to Category III. In such cases, immediate corrective measures are needed, such as intrauterine resuscitation or cesarean section, forceps delivery, lateral resection [Citation10]. Our study results indicated that oxygen inhalation did not have any impact on these delivery outcomes, which meant that oxygen treatment did not reduce the occurrence of these high-risk Category II tracings. From another point of view, the reason for the occurrence of such high-risk Category II tracings might not only be the increase of fetal oxygen consumption, but some other factors. Raghuraman et al. found that correction of maternal hypoxia during delivery did not lead to the resolution of the high-risk Category II fetal heart rate tracing or reduce its recurrence; intrauterine air resuscitation in parturients who did no have oxygen desaturation has been found to be comparably effective with oxygen inhalation in improving the lactic acid levels in the umbilical artery in parturients with Category II fetal tracing [Citation23,Citation24]. Moors et al. who have defined FHR pattern in terms of the Federation of Gynecology and Obstetrics (FIGO) classification, also reported similar conclusions to those in our study that supplemental oxygen did not reduce the adverse outcomes of those high-risk Category II tracings [Citation17]. Moreover, they found that the requirement of lateral resections on fetal indications was significantly lesser following maternal oxygen inhalation in the subgroup with abnormal FHR tracings; this result is different from ours. This discrepancy may be attributed to the differences between the experimental designs of their study and ours. However, another study suggested that maternal overoxygenation during intrauterine resuscitation may lead to an increase in oxygen free radicals, which in turn cause cellular damage [Citation25]. Therefore, we intended to compare the effectiveness of treatment with low-flow and short-term oxygen inhalation.
Limitations of the study
Our study has certain limitations. We had a relatively small sample size in this study. In addition, the investigation did not include a comparison of the blood samples of the umbilical cord artery, which is considered essential to accurately establish the diagnosis of fetal acidemia. Lastly, because of technical limitations, we did not monitor the intrauterine temperature during delivery and the fetal temperature. In future studies, we intend to conduct further investigations in population groups of more than 1,000, with the inclusion of additional indicators.
Conclusion
To summarize, the results of the present study indicate that low-flow and short-term oxygen inhalation for parturients with ERMF could decrease the incidence of Category II fetal heart rate tracings, without any significant effect on the mode of delivery or neonatal outcomes.
Ethics approval and consent to participate
The study protocol was approved by the Clinical Committee of Guangzhou Women’s and Children’s Medical Center (protocol number: 201939000). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All subjects enrolled in this study provided written informed consent.
Author contributions
BSZ performed data analysis; BL wrote the manuscript; QNW collected data; JXJ and XRS designed most of the investigation. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data sets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Sultan P, David AL, Fernando R, et al. Inflammation and epidural-related maternal fever: proposed mechanisms. Anesth Analg. 2016;122(5):1–7. doi:10.1213/ANE.0000000000001195.
- Sharpe EE, Arendt KW. Epidural labor analgesia and maternal fever. Clin Obstet Gynecol. 2017;60(2):365–374. doi:10.1097/GRF.0000000000000270.
- Zhao B, Li B, Wang Q, et al. The relationship between epidural analgesia and intrapartum maternal fever and the consequences for maternal and neonatal outcomes: a prospective observational study. J Matern Fetal Neonatal Med. 2021;35(25):5354–5362.
- Zhao BS, Li B, Wang QN, et al. Time- and dose-dependent correlations between patient-controlled epidural analgesia and intrapartum maternal fever. BMC Anesthesiol. 2021;21(1):31. doi:10.1186/s12871-021-01249-1.
- Yin H, Hu R. A cohort study of the impact of epidural analgesia on maternal and neonatal outcomes. J Obstet Gynaecol Res. 2019;45(8):1435–1441. doi:10.1111/jog.13988.
- Burgess APH, Katz JE, Moretti M, et al. Risk factors for intrapartum fever in term gestations and associated maternal and neonatal sequelae. Gynecol Obstet Invest. 2017;82(5):508–516. doi:10.1159/000453611.
- Törnell S, Ekéus C, Hultin M, et al. Low apgar score, neonatal encephalopathy and epidural analgesia during labour: a swedish registry-based study. Acta Anaesthesiol Scand. 2015;59(4):486–495. doi:10.1111/aas.12477.
- Greenwell EA, Wyshak G, Ringer SA, et al. Intrapartum temperature elevation, epidural use, and adverse outcome in term infants. Pediatrics. 2012;129(2):e447-454–e454. doi:10.1542/peds.2010-2301.
- Morton S, Kua J, Mullington CJ. Epidural analgesia, intrapartum hyperthermia, and neonatal brain injury: a systematic review and meta-analysis. Br J Anaesth. 2021;126(2):500–515. doi:10.1016/j.bja.2020.09.046.
- Arnold JJ, Gawrys BL. Intrapartum fetal monitoring. Am Fam Physician. 2020;102(3):158–167.
- Knupp RJ, Andrews WW, Tita ATN. The future of electronic fetal monitoring. Best Pract Res Clin Obstet Gynaecol. 2020;67:44–52. doi:10.1016/j.bpobgyn.2020.02.004.
- Macones GA, Hankins GD, Spong CY, et al. The 2008 national institute of child health and human development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666. doi:10.1097/AOG.0b013e3181841395.
- Alfirevic Z, Devane D, Gyte GM, et al. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2017;2(2):Cd006066. doi:10.1002/14651858.CD006066.pub3.
- American college of obstetricians and gynecologists; society for maternal-fetal medicine. Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123:693–711.
- Mehraban S, Nematian S, Mehraban SS, et al. Randomized control trial of intravenous acetaminophen for reduction of intrapartum maternal fever. Am J Obstet Gynecol MFM. 2021;3(1):100287. doi:10.1016/j.ajogmf.2020.100287.
- Moors S, Joshi R, Bullens LM, et al. A randomized controlled trial studying the effect of maternal hyperoxygenation on fetal heart rate in suspected fetal distress. Physiol Meas. 2020;41(11):115002. doi:10.1088/1361-6579/abc0b6.
- Moors S, Bullens LM, van Runnard Heimel PJ, et al. The effect of intrauterine resuscitation by maternal hyperoxygenation on perinatal and maternal outcome: a randomized controlled trial. Am J Obstet Gynecol MFM. 2020;2(2):100102. doi:10.1016/j.ajogmf.2020.100102.
- Cnattingius S, Johansson S, Razaz N. Apgar score and risk of neonatal death among preterm infants. N Engl J Med. 2020;383(1):49–57. doi:10.1056/NEJMoa1915075.
- Casey BM, McIntire DD, Leveno KJ. The continuing value of the apgar score for the assessment of newborn infants. N Engl J Med. 2001;344(7):467–471. doi:10.1056/NEJM200102153440701.
- Macaulay JH, Bond K, Steer PJ. Epidural analgesia in labor and fetal hyperthermia. Obstet Gynecol. 1992;80(4):665–669.
- Yli BM, Kjellmer I. Pathophysiology of foetal oxygenation and cell damage during labour. Best Pract Res Clin Obstet Gynaecol. 2016;30:9–21. doi:10.1016/j.bpobgyn.2015.05.004.
- Rudelstorfer R, Simbruner G, Sharma V, et al. Scalp heat flux and its relationship to scalp blood pH of the fetus. Am J Obstet Gynecol. 1987;157(2):372–377. doi:10.1016/s0002-9378(87)80175-8.
- Raghuraman N, López JD, Carter EB, et al. The effect of intrapartum oxygen supplementation on category II fetal monitoring. Am J Obstet Gynecol. 2020;223(6):905.e901–905.e907. doi:10.1016/j.ajog.2020.06.037.
- Raghuraman N, Wan L, Temming LA, et al. Effect of oxygen vs room air on intrauterine fetal resuscitation: a randomized noninferiority clinical trial. JAMA Pediatr. 2018;172(9):818–823. doi:10.1001/jamapediatrics.2018.1208.
- Torres-Cuevas I, Parra-Llorca A, Sánchez-Illana A, et al. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017;12:674–681. doi:10.1016/j.redox.2017.03.011.