Abstract
Objective
To assess pre-term birth, low birth-weight and growth restriction according to maternal thyroid screening results and subsequent treatment.
Methods
This is a nonintervention nested case-control study derived from 10,052 asymptomatic women previously screened during the first trimester marker with anti-thyroid peroxidase antibodies, serum thyroid stimulating hormone, and free thyroxine. Screening results had been classified as positive with one or more markers outside the normal range and referred to an endocrinologist. Cases were 512 women with positive results and information on recommended treatment: 204 thyroxine, propylthiouracil or surgery, and 308 no treatment or only iodine. Controls were a sequential sample of 1292 women with negative results. All cases and controls had information on gestation at delivery or birth-weight. Outcome measures were pre-term birth (<37 weeks), low birth-weight (<2.5 kg) and, for singletons, small for gestational age (SGA; <10th percentile).
Results
Among singleton pregnancies, there was a higher prevalence of both pre-term birth (risk ratio (RR) 1.69, 95% confidence interval (CI) 1.21–2.36, p < .002) and low birth-weight (RR 1.72, 95% CI 1.13–2.62, p < .02) in cases compared with controls. An increase in low birth-weight was also present in term pregnancies, but not significant (RR 1.80, 95% CI 0.78–4.14, p = .16), and there was no difference in SGA prevalence (1.24, 95% CI 0.93–1.65, p = .14). Among cases there was no significant difference in these rates according to treatment even after logistic regression, allowing for the individual screening marker levels and maternal weight.
Conclusions
Women with positive thyroid screening results are at increased risk of pre-term birth regardless of thyroid dysfunction or subsequent treatment. An association with low birth-weight is probably secondary to early delivery.
Introduction
Routine screening in early pregnancy using thyroid antibodies and thyroid hormones can identify those at high risk of for subclinical thyroid dysfunction. We have previously shown that in the Czech Republic, a single relatively large hospital can readily incorporate maternal screening for thyroid disease into a routine first trimester Down’s syndrome screening program [Citation1]. In our program, about 12% of women had positive screening test results and were referred to an endocrinologist, who recommended treatment for hypothyroidism in 25% and hyperthyroidism in 1% [Citation2].
Thyroid dysfunction in pregnancy is associated with pre-term birth. In a 2015 systematic review of 15 studies carried out at different stages of pregnancy, meta-analysis found that the prevalence of pre-term birth was higher in overt hypothyroidism compared with a reference group (odds ratio (OR) 1.19, 95% confidence interval (CI) 1.12–1.26, p < .00001) [Citation3]. There was an increase of the same magnitude for hyperthyroidism (OR 1.24, 95% CI 1.17–1.31, p < .00001) but differences were not statistically significant for subclinical hypothyroidism (OR 1.11, 95% CI 0.90–1.37, p = .32) and isolated hypothyroxinemia (OR 1.22, 95% CI 0.88–1.70, p = .24). A recent systematic review included 11 studies of specifically on subclinical hyperthyroidism did not find an association with pre-term birth (OR = 0.96, 95% CI 0.74–1.25) [Citation4].
The reference group in each study comprised women with thyroid antibodies or hormones in the normal range. Hence, this type of study does not exclude the possibility that all women with positive thyroid screening results are at high risk of pre-term birth, regardless of clinical outcome. Those with thyroid dysfunction identified by antenatal screening are offered treatment and this might reduce their risk of pre-term birth, although there is not strong evidence for this. In a 2015 Cochrane Review of antenatal screening randomized trials [Citation5], only two were identified. Neither found a statistically significant difference between the screening and control arms with risk ratio (RR) 0.99 (95% CI 0.80–1.24, p = .55) [Citation6] and 0.71 (95% CI 0.42–1.20, p = .20) [Citation7] although the first included case-finding in the control arm, which may have reduced any difference. Moreover, the first also reported on the prevalence of low birth-weight, which also did not differ (RR 0.97, 95% CI 0.74–1.27). In a 2018 systematic review of thyroxine randomized trials in women with subclinical hypothyroidism, only two were identified and a meta-analysis yielded an RR of 0.84, which was not statistically significant (95% CI 0.59–1.21, p = .35) [Citation8]. In a subsequent review, which included seven randomized trials and six observational studies, the RR was 0.77 with 95% CI 0.47–1.25 [Citation9].
We therefore report the rate of pre-term birth as well as low birth-weight and growth restriction among women who participated in the Czech screening study. In a nested case-control study, these outcomes were compared between those with positive and negative results and within the positive group, according to clinical diagnosis and treatment.
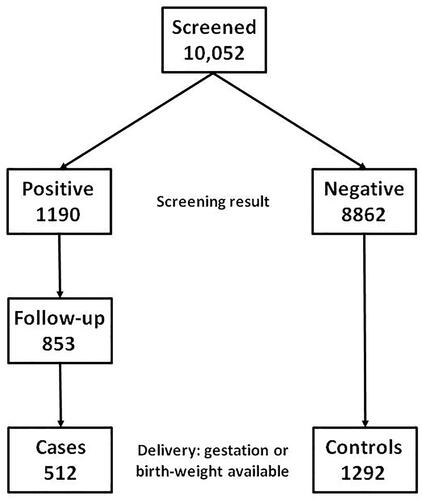
Methods
shows the derivation of cases and controls. The total screened population comprised 10,052 asymptomatic women tested between November 2009 and September 2015 using TPO, TSH, and fT4; from March 2013 until September 2015 only TPO and TSH were used, as previously described [Citation1]. The decision not to continue using fT4 was made because of financial rather than scientific reasons. The test was carried out at the same time as the 11–13 weeks Combined test for Down syndrome [Citation10]. At the time of testing, the normal range for each marker in the Czech Republic was: TPO less than 5.6 kU/l; TSH 0.35–4.94 mU/l; and FT4 9.0–19.1 pmol/l; the same ranges were used for singleton and twin pregnancies. A result was regarded as positive if at least one of these markers was out of range. Such women were referred to an endocrinologist.
There were 1190 with positive screening results and in a previous follow-up study, hospital records were examined to determine the recommendation of the endocrinologist and the consequent sequence of events [Citation2]. Specifically, each record was categorized according to whether the endocrinologist recommended no further action, repeat testing, treatment, and if so the type. The study reported on 818 where this information was available and for the current study a further 35 were added.
Cases comprised those with information on either the gestational age at delivery or birth-weight, for twins in at least one fetus. Controls comprised a sequential series of 1292 women with negative screening results and information on either gestational age or birth-weight. Maternal age and crown-rump length (CRL) and the number of fetuses were recorded at the time of screening and maternal weight was collected for cases.
The prevalence of pre-term birth (<37 weeks) and low birth-weight (<2.5 kg) was computed and fetal growth restriction was determined in singleton pregnancies by computing the prevalence of small for gestational age (SGA) using the 10th percentile cutoff in published weight charts from a large series in neonates in London [Citation11]. The relative risk (RR) was calculated from the prevalence ratio and statistical significance evaluated using a Chi-square test; comparison was made between the rates in those with positive and negative results as well as among positives, between those who were treated and untreated. When comparisons were made between a small subgroup, such as treatment for hypothyroidism and hyperthyroidism, the Fisher’s exact test was used.
Singleton and twin pregnancies were analyzed separately. Logistic regression analysis was performed to determine if allowing for co-variables altered the prevalence comparisons. Regression parameters were derived by maximum likelihood estimation and the model assumption of little or no multi-collinearity between continuous variables was tested by assessment of tolerance and variance inflation statistics. The potential variables considered were maternal age and CRL, and among cases, the antibody and hormone levels, and maternal weight; only variables with a statistically significant difference between treated and untreated women were included, based on the Wilcoxon rank sum test (two-tail).
All tests were regarded as statistically significant if p < .05.
Results
The 512 cases comprised: 204 women recommended for treatment with thyroxine, propylthiouracil, or surgery, including six with twin pregnancies and 308 who were untreated or only received iodine, of whom 18 had twins. The 1292 controls included 13 with twins. Within each of these case groups and allowing for twin status, there were no statistically significant differences in TPO, TSH, or FT4 according to whether or not information on delivery or birth-weight was available.
shows the basic characteristics of cases and controls. In singletons, there was a highly statistically significant greater median maternal age in cases, while in twins there was no such difference and median CRL did not differ in singletons or twins.
Table 1. Basic characteristics of cases and controls.
shows that, among singleton pregnancies, there was a 1.69-fold higher prevalence of both pre-term birth and low birth-weight in women with positive thyroid screening results compared with those with negative results (11.0% versus 6.5% and 7.9% versus 4.6%, respectively). The increase in pre-term births was highly statistically significant (p < .002) and for low birth-weight the 1.72-fold increase was significant but less so (p < .02). Among term pregnancies, the prevalence of low birth-weight was 1.80-fold higher in those with positive results compared with negative although this was not significant (p = .16). The prevalence of SGA was similar according to the screening result. In twin pregnancies, the RR of pre-term birth and low birth-weight prevalence according to positive or negative was close to 1.
Table 2. Prevalence of pre-term birth, low birth-weight, and small for gestational age according to the thyroid screening test result.
Logistic regression in singleton pregnancies was used to calculate ORs comparing prevalence in cases and controls after adjusting for maternal age. For pre-term birth, the adjusted odds were 1.77 (95% CI 1.22–2.56); for low birth-weight 1.77 (1.12–2.78); and for SGA 1.28 (0.92–1.78).
compares the prevalence of pre-term birth, low birth-weight, and SGA in those recommended for treatment with thyroxine, propylthiouracil, or surgery with those having no treatment or iodine alone. For singletons, the pre-term prevalence was similar among those treated or not (11.7% versus 10.4%; p = .65). Although there was a 1.6-fold higher prevalence of low birth-weight (10.0% versus 6.4%) overall and 2.3-fold in term pregnancies, neither reached statistical significance (p = .15 and p = .19, respectively). The prevalence of SGA was also similar among those treated or not (16.0% versus 13.3%; p = .40). In twins, the RRs for pre-term birth and low birth-weight were close to unity.
Table 3. Women with a positive thyroid screening test result: preterm birth and low birth-weight prevalence according to type of treatment.
The treated group comprised 199 with hypothyroidism, including six twins and five with hyperthyroidism, none with twins. Among singletons, the pre-term prevalence was 11.5% (22/191) in hypothyroid compared with 20.0% (1/5) in hyperthyroid cases (Fisher’s exact test, p = .47); the low birth-weight prevalences were 9.8% (18/184) and 20.0% (1/5), respectively (p = .41); and for SGA 14.8% (27/182) and 60.0% (3/5), which were statistically significant (p < .05).
The treatment recommendation was made by the endocrinologist on the basis of clinical examination, including the specific antibody and hormone profile. shows the difference in profile between the treatment groups as well as other potential co-variables. As expected, those recommended thyroxine, propylthiouracil or surgery had substantially higher median TPO and TSH levels as well as somewhat lower fT4, all highly statistically significant differences. This was so in both singleton and twin pregnancies. Of the biophysical factors, median maternal weight differed in singletons (65 kg versus 63 kg), a significant difference (p < .02) and in twins (72 kg versus 68 kg), although not significantly (p = .82); the other two factors did not differ. Among the treated group those with hypothyroidism had higher TPO, TSH, and lower fT4 levels than those with hyperthyroidism, as well as higher weight, all statistically significant differences.
Table 4. Women with a positive thyroid screening test result: median antibody and hormone levels, and other factors, according to type of treatment.
shows the results of logistic regression in singleton pregnancies with positive screening results. The regression analysis was used to calculate ORs adjusted for the levels of TPO, TSH, fT4 (if available), and maternal weight. For none of the outcomes, pre-term birth, low birth-weight, or SGA was the OR statistically significant. Despite moderate correlations between the independent variables, the logistic model assumption of little or no multi-collinearity was demonstrated.
Table 5. Singleton cases: odds ratio, thyroxine, propylthiouracil or surgery compared with no treatment or iodine, adjusted for TPO, TSH, fT4 (when tested), and maternal weight.
Discussion
This study shows that women with positive thyroid screening results are at increased risk of pre-term birth regardless of thyroid dysfunction or subsequent thyroid treatment. The association between low birth-weight and positive screening results is probably secondary to early delivery.
The study is based on a large consecutive series of women screened for subclinical thyroid dysfunction and those with abnormal results were referred to an endocrinologist who recommended treatment. There was sufficient statistical power to compare the adverse outcomes of pregnancy according to screening results and subsequent treatment.
Nevertheless, it is possible that among women with positive screening results, the comparison between those recommended to have thyroxine, propylthiouracil, or surgery and those recommended no treatment or iodine is biased. This would occur if the former had a higher a priori chance of pre-term birth or low birth-weight. If so, the treatment in the former may have reduced the prevalence to that in the latter group. It would also occur if the two groups had the same a priori chance but treatment was iatrogenic, for example, increasing hypertensive disorders or placental abruption, although this has not been demonstrated [Citation9,Citation12, Citation13]. While the randomized trials of thyroxine in women with subclinical hypothyroidism did not individually show statistically significant reductions in pre-term birth, they were small and statistically under-powered. However, even when entered into a meta-analysis together with observational studies, statistical significance was not achieved (RR = 0.77, 95% CI 0.47–1.25) [Citation9].
Another limitation is that there is no information on compliance with treatment. A randomized trial in mildly iodine-deficient pregnant women in Thailand found that 88% complied with daily iodine supplementation or placebo [Citation14]. But thyroxine or propylthiouracil is likely to have stronger side effects than iodine supplementation and compliance may be lower.
Pre-term birth is a major cause of perinatal morbidity and mortality. Women with a previous pre-term birth have a more than double a priori risk as recurrence [Citation15]; other risk factors include short inter-pregnancy interval [Citation16] and Afro-Caribbean ancestry [Citation17]. Women at high risk are recommended to have serial second trimester cervical length measurements and progesterone supplementation [Citation18]. The current study suggests that women with singleton pregnancies and positive thyroid screening tests should also be considered at high risk. Further studies will be needed on the efficacy of progesterone treatment in this group of women.
The generalizability of our results is limited by the specific thyroid normal ranges adopted in the Czech Republic and the clinical interpretation of positive test results by local endocrinologists. The normal range for TSH, 0.35–4.94 mU/l, is higher than the range of 0.1–2.5 mIU/L specified by the Endocrine Society, which has recommended thyroxine treatment in above case (2.5 mIU/L) [Citation19]. If a lower range for TSH had been used, it is likely that more women would have received thyroxine. Moreover, some women with TSH between 2.5 mU/l and 4.94 mU/l, who might elsewhere have been diagnosed as having sub-clinical hypothyroidism, will have been included in the controls. However, this is not a large number of women − 129 (10%) among the 1292 controls – and will not substantially alter the comparison with cases.
In conclusion, women with positive thyroid screening results are at increased risk of pre-term birth regardless of thyroid dysfunction or subsequent thyroid treatment. However, the current study was not powerful enough to reliably consider hypothyroidism and hyperthyroidism separately, and to compare different treatments. More focused studies will be needed to determine if treatment could be improved so as to reduce the risk of pre-term birth and the consequent risk of low birth weight.
Ethics statement
Committee approval not required as this is a nonintervention study.
Acknowledgements
We thank the nursing staff of the Fetal Medicine Center and the Department of Endocrinology for their help in gathering outcome information. We also thank the gynecologists who referred patients to our center.
Disclosure statement
HC is a consultant to PerkinElmer Inc. Other authors have no financial interests.
Data availability statement
The data that support the findings of this study are available from the corresponding author, ID, upon reasonable request.
Additional information
Funding
References
- Dhaifalah I, Salek T, Langova D, et al. Routine first trimester screening for maternal thyroid disease. J Fetal Med. 2017;4(1):1–6. doi:10.1007/s40556-017-0112-8.
- Dhaifalah I, Havalova J, Langova D, et al. Women with positive first trimester thyroid disease screening results. J Fetal Med. 2019;6(3):123–126. doi:10.1007/s40556-019-00218-6.
- Sheehan PM, Nankervis A, Araujo Júnior E, et al. Maternal thyroid disease and preterm birth: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(11):4325–4331. doi:10.1210/jc.2015-3074.
- Nazarpour S, Amiri M, Bidhendi Yarandi R, et al. Maternal subclinical hyperthyroidism and adverse pregnancy outcomes: a systematic review and meta-analysis of observational studies. Int J Endocrinol Metab. 2022;20(3):e120949. doi:10.5812/ijem-120949.
- Spencer L, Bubner T, Bain E, et al. Screening and subsequent management for thyroid dysfunction pre-pregnancy and during pregnancy for improving maternal and infant health. Cochrane Database Syst Rev. 2015;2015(9):CD011263. (doi:10.1002/14651858.CD011263.pub2.
- Negro R, Schwartz A, Gismondi R, et al. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. 2010;95(4):1699–1707. doi:10.1210/jc.2009-2009.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(6):493–501. doi:10.1056/NEJMoa1106104.
- Yamamoto JM, Benham JL, Nerenberg KA, et al. Impact of levothyroxine therapy on obstetric, neonatal and childhood outcomes in women with subclinical hypothyroidism diagnosed in pregnancy: a systematic review and meta-analysis of randomized controlled trials. BMJ Open. 2018;8(9):e022837. doi:10.1136/bmjopen-2018-022837.
- Bein M, Yu OHY, Grandi SM, et al. Levothyroxine and the risk of adverse pregnancy outcomes in women with subclinical hypothyroidism: a systematic review and meta-analysis. BMC Endocr Disord. 2021;21(1):34. doi:10.1186/s12902-021-00699-5.
- Dhaifalah I, Salek T, Langova D, et al. Incorporating thyroid markers in Down’s syndrome screening protocols. Prenat Diagn. 2017;37(5):510–514. doi:10.1002/pd.5047.
- Nicolaides KH, Wright D, Syngelaki A, et al. Fetal medicine foundation fetal and neonatal population weight charts. Ultrasound Obstet Gynecol. 2018;52(1):44–51. doi:10.1002/uog.19073.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376(9):815–825. doi:10.1056/NEJMoa1606205.
- van Dijk MM, Vissenberg R, Fliers E, et al. Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(5):322–329. doi:10.1016/S2213-8587(22)00045-6.
- Verhagen NJE, Gowachirapant S, Winichagoon P, et al. Iodine supplementation in mildly iodine-deficient pregnant women does not improve maternal thyroid function or child development: a secondary analysis of a randomized controlled trial. Front Endocrinol. 2020;11:572984. doi:10.3389/fendo.2020.572984.
- Mercer BM, Goldenberg RL, Moawad AH, et al. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1999;181(5 Pt 1):1216–1221. doi:10.1016/s0002-9378(99)70111-0.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a metaanalysis. JAMA. 2006;295(15):1809–1823. doi:10.1001/jama.295.15.1809.
- Arndt J, Vess M, Cox CR, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Med Decis Making. 2009;29(2):175–181. doi:10.1016/s0002-9378(96)70048-0.
- Glover AV, Manuck TA. Screening for spontaneous preterm birth and resultant therapies to reduce neonatal morbidity and mortality: a review. Semin Fetal Neonatal Med. 2018;23(2):126–132. doi:10.1016/j.siny.2017.11.007.
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8):2543–2565. Erratum in: J Clin Endocrinol Metab. 2021;106(6):e2461. doi:10.1210/jc.2011-2803.