Abstract
Objective
To investigate whether prenatal fibrinogen (FIB) or other related factors could be utilized to evaluate the risk of postpartum hemorrhage (PPH).
Methods
A retrospective study was conducted in a database from January 2015 to December 2019. A total of 128 patients were enrolled and evaluated with FIB, in which 55 patients were assigned to low FIB and 73 in normal FIB.
Results
According to the volume of blood loss, the mean of the low FIB group (<4 g/L) was markedly higher than that of the normal FIB group (≥4 g/L). Prenatal FIB was negatively correlated with PPH volume. The receiver operating characteristic (ROC) curve results indicated that the value of prenatal FIB was 0.701 to predict refractory PPH.
Conclusions
Prenatal FIB was significantly related to thrombin time (TT), which may be an independent factor to predict the coagulation state of prenatal pregnancy.
Introduction
Postpartum hemorrhage (PPH) is one of the most common delivery complications. It has been estimated by World Health Organization that PPH is responsible for the majority of maternal deaths, and more than 78,000 deaths have been reported annually [Citation1,Citation2]. It can also result in hemorrhagic shock, diffuse intravascular coagulation, and other serious complications, endangering the maternal life [Citation3].
Unfortunately, primary hospitals and basic delivery units are unable to detect and treat severe PPH. One possible reason may be that the screening and treatment equipment in these institutions are not effective enough [Citation4–6]. Therefore, early detection and effective treatment should be taken to solve this problem. Postpartum fibrinogen (FIB) used in PPH evaluation has been documented in a great number of studies, and elevated FIB levels for rectifying coagulation disorder in PPH are also under investigation [Citation7–10]. However, the potential association between prenatal FIB and PPH remains unclear.
Notably, accurate evaluation of bleeding volume is beneficial to the treatment of PPH and prevention from developing into severe PPH, maternal death, and additional adverse events [Citation11]. The present approaches mainly use the value of prenatal FIB and relevant antenatal examination factors to predict severe PPH. In addition, the correlation between PPH bleeding volume and the concentration of prenatal FIB is unclear. This paper was expected to provide a diagnostic marker for the risk detection of severe PPH, and its evaluation and rectification in clinical practice.
Materials and methods
We conducted a retrospective cohort study in Army Characteristic Medical Center (ACMC, formerly known as “Third Affiliated Hospital of Third Military Medical University”). We included cases diagnosed as “postpartum hemorrhage”, “severe postpartum hemorrhage”, and “refractory postpartum hemorrhage” from ACMC from January 2015 to December 2019. The main indicators were maternal age, gestational age, bleeding volume, laboratory tests, and fetal complications. This study was approved by ACMC Research Ethics Committee. All patients were hospitalized to give birth in ACMC.
The levels of FIB (Clauss assay) were measured by ACL-TOP700 automatic hemagglutination analyzer (Werfen, Bedford, MA). Prenatal FIB was measured closest to maternal delivery, and the measurement time was limited to three days before delivery. Bleeding volume postpartum was estimated by weighing surgical sponges and pads and by measuring collected blood. According to the concentration of prenatal FIB, all samples were divided into two groups: low FIB (<4 g/L) and normal FIB (≥4 g/L)). Additionally, all samples were classified into four groups based on the volume of blood loss: no PPH (less than 500 mL), PPH (500–1000 mL), severe PPH (over 1000 mL) and refractory PPH (). The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZOG) guideline defines PPH as >500 mL during puerperium and classifies severe PPH as blood loss of >1000 mL. Prenatal FIB was measured closest to maternal delivery, and the measurement time was limited to two days before delivery. 77.34% of the prenatal FIB was measured within 4 h before delivery. Refractory PPH is defined as severe PPH that cannot be stopped by conservative measures such as uterotonics, continuous uterine massage or compression, and requires surgery, uterine artery embolization (interventional treatment) or even uterine removal.
Statistical analysis
The data were analyzed using the SPSS 22.0 (IBM, Armonk, NY) software. Quantitative data were expressed as mean ± standard deviation and qualitative data as the number of cases (%). The measurement data of normal distribution were determined using t-tests; those of non-normal distribution were tested by Mann–Whitney’s U tests, and the count data were tested by χ2. The analysis of variance was used to compare the mean value of prenatal FIB in each degree of PPH and correct the possible baseline differences. Pearson’s correlation was also adopted to analyze the FIB and the relationship between thrombin time (TT). A linear regression equation was employed to analyze the mean value of PPH and six major factors for PPH. Receiver operating characteristic (ROC) curves were plotted, and parameter analysis was made to evaluate the diagnostic efficacy of FIB for PPH.
To have 90% power to detect a significant interaction between low/normal (<4 g litre−1/≥4 g litre−1) FIB concentrations and vaginal/cesarean delivery regarding probability of severe bleeding (significance level .05) with PASS2021 (NCSS, LLC, Kaysville, UT), a calculation showed that 46 subjects were needed. The actual sample size is substituted into the calculation, and the calculation efficiency is 99.984%.
Results
Among all the 128 valuable samples in this study, 38 (30%) patients were non-severe PPH (500–1000 mL), 66 (51%) developed severe PPH, and seven (5%) of them met the diagnostic criteria of refractory PPH. Besides, 24 (19%) recorded no PPH (less than 500 mL). In the terms of delivery modes, 70 patients underwent cesarean section, 57 cases of vaginal delivery, and seven cases of critical bleeding underwent interventional surgery. No dead case was reported.
Patient characteristics of every sample in this study are presented in . Generally, the concentration of plasma FIB will rise to 4–6 g/L in the late pregnancy. It was therefore that the samples were divided into two groups as per FIB levels: low FIB (<4 g/L) and normal FIB (≥4 g/L). The mean value of the low FIB group was 3.15 and 4.94 of the normal FIB group. In addition, the mean age of patients with normal FIB was 30.37, and 30.00 for those with low FIB (p = .661). The median gestational weeks of patients were 37.27 and 37.00 weeks, respectively (p = .650).
Table 1. Patient characteristics (χ2 test).
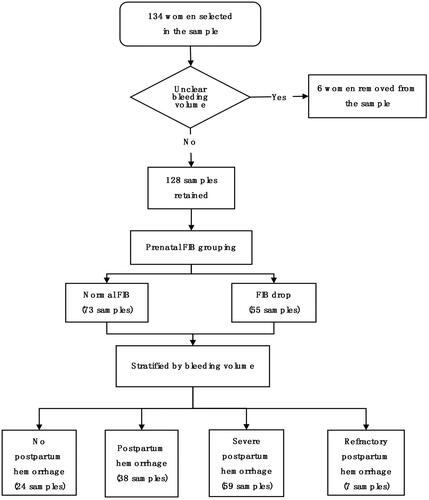
The two diagrams in presented the correlation between prenatal FIB and the amount of PPH. The amount of PPH included the estimated blood loss during delivery and suction drainage 24 h after delivery. Thereinto, exhibits that the amount of blood loss had a downward trend as prenatal FIB increased. indicates the mean value of blood loss for each phase of prenatal FIB. compared the prenatal laboratory examination of patients in two groups. The results illustrated that prenatal fibrinogen was positively related to TT (p < 0.01) whereas it was not related to the additional factors (p > 0.01).
Figure 2. The relationship between prenatal fibrinogen and the amount of blood loss (mL). (A) Scatter diagram of prenatal fibrinogen concentration and volume of blood loss. (B) Histogram of prenatal fibrinogen subsection and the mean value of blood loss.
Table 2. Prenatal laboratory examination.
The mean value of blood loss in the lower FIB group (<4 g/L) was 1 316.36 mL and 932.26 mL in the higher FIB group (≥4 g/L), p = .020. The mean values of FIB in non-PPH, PPH, severe PPH, and refractory PPH are 4.5088, 4.3761, 3.9959, and 3.3771, respectively. The p value of this test was .033, which indicated the differences of prenatal FIB between each degree of PPH were statistically significant. presents a linear regression equation of the six major factors related to PPH. There were four factors including placenta previa, prenatal FIB, placental abruption, and prenatal INR, which were intimately correlated with this equation.
Table 3. Multi-factor linear regression equation about the mean value of PPH and six major factors for PPH.
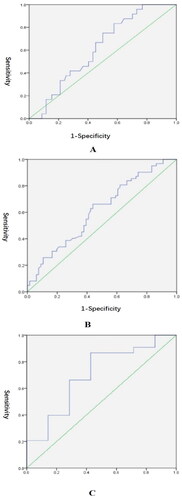
ROC curves in presented the sensitivity (true positive rate) and specificity (1 – false positive rate) of prenatal FIB for each degree of PPH. reflects the value of prenatal FIB to predict non-severe PPH (AUC = 0.614; p = .082). depicts the value of prenatal FIB to predict severe PPH (AUC = 0.613; p = .027). The maximum Youden index of was 0.222, with a corresponding prenatal FIB of 4.030. illustrates the value of prenatal FIB to predict refractory PPH (AUC = 0.701; p = .074).
Figure 3. ROC curves of prenatal FIB for the degree of PPH. (A) Test variables: prenatal FIB; state variable: PPH or not; area under curve = 0.614 (p = .082); Standard error = 0.054; 95% CI = 0.507–0.721. (B) Test variable: prenatal FIB; state variable: severe PPH or not; area under curve = 0.613 (p = .027); standard error = 0.050; 95% CI = 0.516–0.710. (C) Test variable: prenatal FIB; state variable: refractory PPH or not; area under curve = 0.701 (p = .074); standard error = 0.107; 95% CI = 0.492–0.910.
Discussion
The major aim of this study was to investigate whether prenatal FIB or other related factors could evaluate the risk of PPH. Our findings revealed that prenatal FIB was significantly related to TT, and prenatal FIB plays an important role in predicting refractory PPH (AUC = 0.701; p = .074). Prenatal FIB served as an independent factor to reflect the coagulation state of prenatal pregnancy.
In recent years, the incidence of PPH has been on the rise worldwide [Citation2]. Due to its complicated etiology and pathogenesis, it is difficult to prevent the occurrence of PPH. Early detection and treatment are urgently needed for PPH prevention. Some investigations have reported that blood clotting disorders are considered to be one of the four major reasons of postpartum bleeding [Citation3,Citation8,Citation12]. FIB is an important component of physiological coagulation and an important biomarker for evaluating coagulation function in the body. Too high or too low levels of FIB can lead to coagulation disorders.
Multivariate regression analysis in our study suggested that prenatal FIB was intimately associated with the volume of bleeding in PPH. A low prenatal FIB may act as a potential biomarker in assessing the risk of massive bleeding in PPH. Besides, we discovered that prenatal FIB was not affected by the remaining risk factors including APTT, PT, D-dimer, and PLT. TT was a coagulation indicator that reflected fibrinolytic status, and it was closely related to FIB. Therefore, prenatal FIB may be considered as a potential independent factor to reflect the coagulation state of prenatal pregnancy, which has also been confirmed by previous studies.
Our study focused on the association between prenatal FIB and various levels of PPH. The results of statistics indicated that prenatal FIB definitely connected with several indicators of PPH. Compared with normal prenatal patients, FIB (≥4 g/L), the bleeding of patients with low prenatal FIB increased markedly. Furthermore, all patients with prenatal FIB lower than 2 g/L came out with an emergent condition of more than 1000 mL hemorrhage. Moreover, we found that prenatal FIB did not help predict non-severe PPH. Several previous studies have reported the value of FIB in estimating early PPH and rectifying the coagulation disorder in PPH [Citation7, Citation9,Citation10, Citation13], and the value of prenatal FIB in predicting severe PPH, which is more complicated in cause and difficult to be diagnosed. Coagulation disorder caused by massive blood loss in PPH is widely considered to result in more serious situations namely severe PPH and refractory PPH. Cumulative studies have indicated that decreased plasma FIB levels increase the risk of severe postpartum bleeding, and recommend early detection of plasma FIB levels to prevent its occurrence. Furthermore, a study on the difference of blood coagulation material level between the severe and non-severe groups, including FIB, platelet count, FII, FV, D-dimer, protein C antigen, and plasma FIB levels in the non-severe group remained stable in the PPH, performed by Collins et al. has suggested that the lower the FIB value, the higher the diagnostic value of severe PPH [Citation14], which is in agreement with the research findings of Cortet et al. [Citation10].
In the analysis of the characteristics of patients, we found that gestational diabetes mellitus was also a protective factor for PPH (p < .05). We believe that this is related to the thrombotic transformation caused by hyperglycemia. At present, many mechanisms have been proposed to explain this thrombotic transition in hyperglycemia, such as the direct effect of oxidative stress induced by hyperglycemia on the transcription of coagulation factor gene, the loss of endothelial glycocalyx layer containing coagulation factors, and the direct glycosylation of coagulation factors [Citation15]. Thrombotic transformation caused by diabetes reduces the risk of PPH.
Currently, FIB determination by the Clauss method can be obtained within 10–20 min, which is the fastest test of all coagulation functions [Citation16–18]. More importantly, emerging studies have shown that FIB is an independent biomarker that cannot be affected by other factors in PPH, namely hemoglobin concentration, clotting factors, and delivery style. Therefore, plasma FIB measurement is well-recognized as a rapid and accurate method to evaluate the severity of PPH.
Conclusions
Our study proposed an assured association between prenatal FIB and PPH. Low levels of prenatal FIB indicated a risk of massive bleeding after delivery. Furthermore, prenatal FIB could be adopted to predict severe PPH with the critical threshold of 4.030 g/L in this study. In clinical practice, a comprehensive assessment of prenatal FIB and other risks of PPH could guide us to make a more efficient plan to avoid the risk of massive blood loss and reduce the use of blood products. However, it still needs more population-based studies to prove the value of prenatal FIB in the synthetic evaluation of PPH and provide guidelines for the application of FIB in clinical work.
Supplemental Material
Download MS Excel (131 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the supplementary materials.
Additional information
Funding
References
- Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):1–6. doi:10.1016/j.ajog.2013.07.007.
- World Health Organization. Maternal mortality. World Health Organization [Internet]; 2018. Available from: http://www.who.int/en/news-room/fact sheets/detail/maternal-mortality
- Anderson JM, Etches D. Prevention and management of postpartum hemorrhage. Am Fam Physician. 2007;75(6):875–882.
- McNamara H, Mallaiah S. Managing coagulopathy following PPH. Best Pract Res Clin Obstet Gynaecol. 2019;61:106–120. doi:10.1016/j.bpobgyn.2019.04.002.
- Collins PW, Bell SF, de Lloyd L, et al. Management of postpartum haemorrhage: from research into practice, a narrative review of the literature and the Cardiff experience. Int J Obstet Anesth. 2019;37:106–117. doi:10.1016/j.ijoa.2018.08.008.
- Mavrides E, Allard S, Chandraharan E, et al. Prevention and Management of Postpartum Haemorrhage. BJOG. 2016;124:e106–e149.
- Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5(2):266–273. doi:10.1111/j.1538-7836.2007.02297.x.
- Gayat E, Resche-Rigon M, Morel O, et al. Predictive factors of advanced interventional procedures in a multicentre severe postpartum haemorrhage study. Intensive Care Med. 2011;37(11):1816–1825. doi:10.1007/s00134-011-2315-0.
- de Lloyd L, Bovington R, Kaye A, et al. Standard haemostatic tests following major obstetric haemorrhage. Int J Obstet Anesth. 2011;20(2):135–141. doi:10.1016/j.ijoa.2010.12.002.
- Cortet M, Deneux-Tharaux C, Dupont C, et al. Association between fibrinogen level and severity of postpartum haemorrhage: secondary analysis of a prospective trial. Br J Anaesth. 2012;108(6):984–989. doi:10.1093/bja/aes096.
- Snegovskikh D, Souza D, Walton Z, et al. Point-of-care viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J Clin Anesth. 2018;44:50–56. doi:10.1016/j.jclinane.2017.10.003.
- Geeraedts LMJr., Kaasjager HA, van Vugt AB, et al. Exsanguination in trauma: a review of diagnostics and treatment options. Injury. 2009;40(1):11–20. doi:10.1016/j.injury.2008.10.007.
- Collis RE, Collins PW. Haemostatic management of obstetric haemorrhage. Anaesthesia. 2015;70(Suppl. 1):78–86, e27–e28. doi:10.1111/anae.12913.
- Collins PW, Lilley G, Bruynseels D, et al. Fibrin-based clot formation as an early and rapid biomarker for progression of postpartum hemorrhage: a prospective study. Blood. 2014;124(11):1727–1736. doi:10.1182/blood-2014-04-567891.
- Atila C, Benjamin Loughrey P, Garrahy A, et al. Central diabetes insipidus from a patient’s perspective: management, psychological co-morbidities, and renaming of the condition: results from an International Web-Based Survey. Lancet Diabetes Endocrinol. 2022;10(10):700–709. doi:10.1016/S2213-8587(22)00219-4.
- Matsunaga S, Takai Y, Seki H, et al. Fibrinogen for the management of critical obstetric hemorrhage. J Obstet Gynaecol Res. 2019;45(1):13–21. doi:10.1111/jog.13788.
- Collins PW, Cannings-John R, Aawar N, et al. Viscoelastometry guided fresh frozen plasma infusion for postpartum haemorrhage: OBS2, an observational study. Br J Anaesth. 2017;119(3):422–434. doi: 10.1093/bja/aex245.
- Spasiano A, Matellon C, Orso D, et al. Functional fibrinogen (FLEV-TEG) versus the Clauss method in an obstetric population: a comparative study. BMC Anesthesiol. 2019;19(1):90. doi:10.1186/s12871-019-0769-8.