?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Chronic hypertension (CHTN) causes vascular damage and resistance in the pregnant person and malperfusion in the placenta which may worsen the endothelial dysfunction of hypertensive disorders of pregnancy (HDP). These conditions frequently co-exist. A cumulative effect has been inconsistently demonstrated in prior studies, and it is unclear how co-existing hypertensive conditions affect pregnancy outcomes. We sought to examine maternal and neonatal outcomes in pregnancies affected by co-existing CHTN and HDP and compare these outcomes to those of pregnancies which were unaffected or affected by either condition alone.
Methods
This is a retrospective cohort study of singleton deliveries at a single institution 1 October 2013 to 1 October 2021. Data were extracted from the electronic medical record using standardized definitions and billing and diagnosis codes. Pregnant people with no evidence of hypertensive condition were compared to those with CHTN only, HDP only, and co-existing CHTN and HDP. Demographics, baseline clinical data, and use of aspirin or antihypertensive medications were assessed. Maternal outcomes included cesarean delivery, critical range blood pressure, intensive care unit (ICU) admission, and death. Neonatal outcomes included preterm birth <37 weeks’ gestation, small for gestational age (SGA) birthweight, ICU admission, and a morbidity composite. Bivariate tests of association were performed using Chi-square test. Crude and adjusted odds ratios (aORs) were calculated using logistic regression for three maternal and four neonatal outcomes. Descriptive statistics and multivariable analyses were performed.
Results
Of 40,840 eligible people, 1451 (3.6%) had CHTN only; 5213 (12.8%) had HDP only; and 1890 (4.6%) had co-existing CHTN and HDP. Though odds of adverse maternal and neonatal outcomes were significantly increased for all hypertensive groups relative to the unaffected referent group, co-existing CHTN and HDP had the highest odds of cesarean delivery (aOR 1.60; 95% confidence interval (CI) 1.45–1.77), critical blood pressure (OR 41.54; 95% CI 35.96–47.99), maternal ICU admission or death (aOR 3.52; 95% CI 2.65–4.67), preterm birth (aOR 2.76; 95% CI 2.41–3.16), and SGA birthweight (aOR 1.61; 95% CI 1.39–1.87).
Conclusions
Hypertensive disorders of pregnancy in the setting of CHTN are associated with the highest odds of serious consequences on the pregnant person and neonate independent of maternal comorbidities and prematurity. Antihypertensive medication use lowers the odds of some adverse outcomes. Patients should be informed of heightened risks, but optimal management remains unclear.
Introduction
The prevalence of pregnant people in the United States diagnosed with chronic hypertension (CHTN) increased dramatically from 0.11% in 1970 to 1.52% in 2010, a change largely driven by increasing rates of obesity and advanced maternal age [Citation1,Citation2]. CHTN in pregnancy is defined as hypertension present prior to pregnancy, before 20 weeks’ gestation, or unresolving in the typical postpartum period. Traditional criteria are systolic blood pressure (SBP) 140 mm Hg, diastolic blood pressure (DBP)
90 mm Hg, or both with such elevations present over multiple measurements at least 4 h apart [Citation3]. As the lower diagnostic threshold of the 2017 American College of Cardiology/American Heart Association definition of CHTN in nonpregnant adults is more widely applied (SBP
130 mm Hg or DBP
80 mm Hg), the prevalence of CHTN in pregnancy will increase further [Citation4–6]. The potential risks of CHTN during pregnancy are well-described and include hypertensive urgency or crisis, renal injury, stroke, myocardial infarction, fetal growth restriction (FGR), and small for gestational age (SGA) birthweight, preterm birth, cesarean delivery, placental abruption, postpartum hemorrhage, stillbirth, maternal/neonatal intensive care unit (ICU) admission, and maternal/neonatal death [Citation2,Citation3,Citation7–12]. Contemporary management of CHTN during pregnancy includes baseline assessment for end-organ involvement at entry to care, daily low-dose aspirin for preeclampsia risk reduction, antihypertensive therapy to maintain SBP <140 and DBP <90 [Citation13–15], fetal surveillance with growth monitoring and consideration of antenatal fetal testing, timed delivery (generally, 36–39 weeks’ gestation), and careful monitoring for superimposed preeclampsia.
Hypertensive disorders of pregnancy (HDP) occur in roughly a quarter of pregnant people with CHTN, nearly eightfold higher than the general population [Citation7]. People with a longer duration of CHTN, history of HDP, or chronic kidney disease are at particular risk, and preterm HDP is common in CHTN [Citation8,Citation16]. The linked pathophysiology is incompletely understood. Broadly, CHTN causes systemic vascular damage, remodeling, and noncompliance which results in maternal malperfusion at the placenta, and a placental stress response of pro-inflammatory and anti-angiogenic factors into maternal circulation produces maternal endothelial dysfunction which manifests as the clinical syndrome of HDP [Citation17–25]. Typically, HDP are diagnosed by the new onset of hypertension after 20 weeks’ gestation; however, diagnosis in the setting of CHTN is more difficult especially in those with preexisting renal impairment or other comorbidities. In general, superimposed HDP is distinguished by new proteinuria; acute, severe, and persistent blood pressure elevations which are otherwise unexplained; suggestive symptomatology (e.g. neurologic symptoms), or new laboratory derangements (e.g. thrombocytopenia, elevated liver enzymes, or renal injury) [Citation26]. In cases of diagnostic uncertainty, uric acid measurement and prolonged blood pressure monitoring may be useful. Management of HDP must carefully balance fetal maturity with disease progression. Contemporary management in high resource settings relies upon careful clinical, laboratory, and fetal monitoring and timed delivery based on the severity of disease and ranging from delivery at diagnosis with disease features precluding expectant management up to 37 weeks’ gestation if no serious maternal or fetal compromise. Emerging understanding about the lifelong risks of HDP (especially in the setting of underlying CHTN) highlights the importance of long-term attention to cardiovascular and renal health, but the ideal strategy is not well-established [Citation27–30].
Though CHTN and HDP share risk factors and associate with similar adverse outcomes, it is unclear whether the co-existence of these conditions increases risk. We aimed to examine pregnancies affected by isolated CHTN or HDP and those affected by co-existing CHTN and HDP to assess maternal and neonatal outcomes compared to pregnancies unaffected by hypertension. We hypothesized that pregnancies affected by any hypertensive condition would experience more frequent adverse maternal and neonatal outcomes than unaffected pregnancies, and that pregnancies affected by co-existing CHTN and HDP would have the greatest burden of adverse outcomes.
Methods
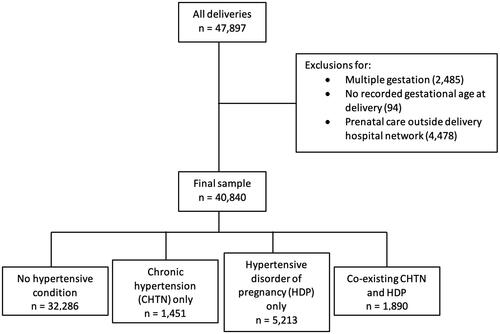
This is a retrospective cohort study of singleton deliveries at a single urban tertiary care hospital from 1 October 2013, when our electronic medical record (EMR) was introduced, through 1 October 2021. There were no significant changes to the EMR during the study period, and all data were extracted from the EMR. All singleton gestations were included. Singleton gestation was defined as one child born in a single delivery ≥20 weeks’ gestation. Multiple gestations that spontaneously or purposefully reduced to one fetus at <20 weeks’ gestation were included. Patients missing documented gestational age at delivery were excluded. Patients who did not have any prenatal care or who received prenatal care outside the delivery hospital network were excluded given our inability to confidently ascertain the presence or absence of hypertensive disorders prior to delivery hospitalization. Observations missing newborn birthweight were included in the cohort as most of those cases (>80%) occurred in the setting of fetal or neonatal death.
Demographic and baseline clinical data examined included maternal age; race and ethnicity; insurance type; body mass index (BMI); parity; tobacco use; medical comorbidities including renal disease, systemic lupus erythematosus, autoimmune disease, and pre-gestational or gestational diabetes; in vitro fertilization; and use of aspirin, antihypertensive medication, or magnesium sulfate infusion during pregnancy. Maternal age was classified as advanced if ≥35 years at delivery. Maternal race and ethnicity were categorized as documented in the EMR. Insurance type was determined by the primary payor for the delivery hospitalization. BMI was calculated using pre-pregnancy weight. Nulliparity was defined as no prior births ≥20 weeks’ gestation. Tobacco use was classified as present if there was any self-reported use of any tobacco-containing product during pregnancy. Medical comorbidities were defined using billing and diagnosis codes documented any time before pregnancy and up to 4 weeks after delivery (Supplemental Table). At our institution, the typical dose of low-dose aspirin prescribed for the indication of preeclampsia risk reduction is 81 mg. Antihypertensive medication use was defined as any prescription for oral labetalol, nifedipine, hydralazine, or methyldopa before the delivery date inclusive of outpatient and inpatient encounters. At our institution, medications are typically reviewed and reconciled within the EMR at each visit type encounter, and all medications are entered, modified, and electronically prescribed in the EMR.
Each delivery was exclusively classified into one of four groups: no documented hypertensive condition (referent group), CHTN only, HDP only, or co-existing CHTN and HDP. Diagnoses of CHTN and HDP were determined based on billing and diagnosis codes (Supplemental Table). CHTN included primary or secondary hypertension documented any time before pregnancy and up to 4 weeks after the delivery. HDP included gestational hypertension, preeclampsia with or without severe features, eclampsia, or hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome documented any time from last menstrual period for the pregnancy of interest up to 4 weeks after delivery. Deliveries which met both criteria were classified as co-existing CHTN and HDP to try and capture patients with CHTN with superimposed HDP.
The maternal outcomes examined were cesarean delivery, critical blood pressure during delivery hospitalization, and ICU admission or death within 30 days of delivery. Critical blood pressure was defined as persistent severe hypertension by ≥2 SBP ≥160 mm Hg or DBP ≥110 mm Hg or both, measured within 1 h of each other during the delivery hospitalization. The neonatal outcomes examined were preterm birth <37 weeks’ gestation, SGA defined as birth weight <10th percentile with sex specificity [Citation31], admission to the neonatal ICU, and a perinatal morbidity composite. Gestational age is calculated in the EMR by estimated delivery date based on patient’s last menstrual period, earliest ultrasound, or best clinical estimate [Citation32]. The perinatal morbidity composite included stillbirth, necrotizing enterocolitis, seizures, respiratory distress syndrome, meconium aspiration, hypoxic ischemic encephalopathy, any intraventricular hemorrhage, and hypoglycemia and/or hyperbilirubinemia requiring ICU admission. All outcome data were extracted from EMR using standardized definitions with billing and diagnosis codes (Supplemental Table).
We compared people with no documented hypertensive condition, versus those with a documented diagnosis of CHTN only, versus those with documented diagnosis of HDP only, versus those with documented diagnoses of co-existing CHTN and HDP. Bivariable analyses were performed using Chi-square tests as all variables were categorical.
Unadjusted logistic regression models were conducted for all seven outcomes. For each outcome, except for critical blood pressure (for which regression was not performed due to multicollinearity), two multivariable logistic regressions were also performed. In model 1, except for antihypertensive medication use, all descriptive variables which significantly associated with hypertensive group membership (p < .05) in the bivariable analyses were included. Model 2 included all covariates from model 1 with additional adjustment for antihypertensive medication use prior to delivery to separately examine the effect of this intervention. Regression for NICU admission and perinatal morbidity were further adjusted for gestational age at birth. For all regressions, patients with no documented hypertensive condition comprised the referent group. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). This study had institutional review board approval with a waiver for informed consent.
Results
Forty thousand eight hundred and forty people met eligibility criteria and were included in the study cohort (). Thirty-two thousand two hundred and eighty-six (79.1%) had no hypertensive condition; 1451 (3.6%) had CHTN only; 5213 (12.8%) had HDP only; and 1890 (4.6%) had co-existing CHTN and HDP. Within the HDP only group, the most frequent final HDP diagnosis was gestational hypertension (56.7%) followed by preeclampsia (40.2%). Within the co-existing CHTN and HDP group, the most frequent final HDP diagnosis was preeclampsia (51.5%) followed by gestational hypertension (43.7%).
Significant differences were observed across the four exposure categories for each baseline characteristic (). Notably high frequencies of characteristics observed in the CHTN and co-existing CHTN and HDP groups included advanced maternal age (36.7% CHTN only and 36.6% co-existing CHTN and HDP versus 23.6% referent and 24.3% HDP only), non-Hispanic black race (31.6% CHTN only and 35.4% co-existing CHTN and HDP versus 15.4% referent and 22.0% HDP only), Medicaid/Medicare insurance (49.6% CHTN only and 49.4% co-existing CHTN and HDP versus 39.1% referent and 43.0% HDP only), obesity (51.1% CHTN only and 52.2% co-existing CHTN and HDP versus 20.9% referent and 35.4% HDP only), tobacco use (12.9% CHTN only and 12.7% co-existing CHTN and HDP versus 7.2% referent and 8.7% HDP only), and comorbid medical conditions (e.g. pre-gestational diabetes in 7.6% CHTN only and 9.2% co-existing CHTN and HDP versus 1.1% referent and 3.5% HDP only). Low-dose aspirin was used in roughly one third of the CHTN only and co-existing CHTN and HDP groups. Antihypertensive medications were used more frequently with CHTN: 23.2% of CHTN only and 32.3% of co-existing CHTN and HDP compared with 4.9% of HDP only (p < .001). Magnesium sulfate for any indication was used in 1.9% of the referent group; 5.2% of CHTN only; 22.1% of HDP only; and 32.1% of co-existing CHTN and HDP (p < .001).
Table 1. Characteristics of study population.
In unadjusted model, all study outcomes were increased in the hypertensive groups compared with the referent group (). Overall, people with co-existing CHTN and HDP had the highest odds of experiencing cesarean delivery, critical blood pressure, and maternal ICU admission or death, and their neonates had the highest odds of preterm birth, being SGA, and experiencing the composite morbidity outcome. After adjustment in model 1, for maternal ICU admission or death, odds were higher with CHTN only and highest with co-existing CHTN and HDP; for preterm delivery and SGA, odds were higher with HDP only and highest with co-existing CHTN and HDP. Though odds of neonatal ICU admission and perinatal morbidity were also highest with co-existing CHTN and HDP even after adjustment for comorbid conditions, additional adjustment for gestational age at delivery altered the trend so that the odds were highest with HDP only.
Table 2. Outcomes for hypertensive conditions versus controls (no hypertensive condition).
Further adjustment for antihypertensive medication use during pregnancy in model 2 reduced the magnitude of ORs across all groups without altering trend for cesarean delivery, maternal ICU admission or death, preterm birth, and SGA. This adjustment eliminated the statistical significance of the effect for CHTN only relative to the referent group for neonatal ICU admission. Adjustment for antihypertensive medication use had no effect on the perinatal morbidity composite.
Discussion
The adjusted odds of experiencing certain adverse maternal and neonatal outcomes were significantly increased across hypertensive groups compared to the control group. Even after adjustment for maternal comorbidities, the odds of experiencing cesarean delivery, critical blood pressure, maternal ICU admission or death, preterm birth, and SGA were the highest in people with co-existing CHTN and HDP. Antihypertensive medication use appeared to mitigate some adverse outcomes.
The maternofetal risks of hypertensive disease are well-established. However, the details of HDP superimposed on CHTN have been difficult to understand and quantify. Prior studies have suggested that the vascular dysfunction and resultant end-organ compromise of CHTN are the basis of increased maternal morbidity and dysfunction and that the added physiologic stress of HDP compounds this effect [Citation33–35]. This is consistent with the results of our study, as we demonstrate that the odds of maternal ICU admission or death were higher with CHTN relative to HDP and highest when HDP occurred in the setting of CHTN.
Identifying the fetal and neonatal risks for co-existing conditions has been more controversial with conflicting results in various reports. International reports suggested increased risk of FGR and SGA with co-existing CHTN and HDP compared with CHTN alone [Citation8,Citation36]. Perinatal morbidity and neonatal ICU admission seemed to be more often driven by HDP rather than CHTN, but uneven inclusion of maternal comorbidities in study populations and variable adjustment for the effect of prematurity has made the relative effects of CHTN and HDP difficult to ascertain [Citation10,Citation33,Citation34]. In our study, which has broad inclusion criteria and is adjusted for maternal comorbidities, the co-existence of CHTN and HDP does appear to impart higher risk for SGA, supporting the presence of longer-term placental dysfunction, but the shorter term outcomes of neonatal ICU admission and perinatal morbidity appear to be driven primarily by gestational age.
This study supports a cumulative effect for CHTN and HDP which increases serious risks for both pregnant person and fetus independent of underlying comorbidities. Patients with co-existing CHTN and HDP have the highest adjusted odds of cesarean delivery, maternal ICU admission or death, preterm birth, and SGA. Odds of neonatal ICU admission (but not perinatal morbidity) remains increased after adjustment for both gestational age and underlying comorbidities. The pattern of the differences in morbidities observed across the hypertensive groups suggests that maternal morbidity is driven largely by CHTN presumably through underlying (and perhaps often subclinical) organ compromise whereas fetal morbidity (apart from FGR) is driven by HDP presumably through placental dysfunction and indicated preterm birth. People with these conditions should be counseled on this heightened risk and advised to employ risk reducing strategies such as aspirin, exercise, and optimization of comorbid conditions, and they may warrant more frequent surveillance and higher-level care to detect and manage. The structural inequities leading to overrepresentation of non-Hispanic black and publicly insured patients within this highest risk group must also be addressed [Citation37,Citation38].
In our cohort, antihypertensive medications were used primarily by those with an underlying diagnosis of CHTN (23.2% of CHTN only and 32.3% of co-existing CHTN and HDP) rather than HDP only (4.9%). It is our institutional practice to use oral antihypertensive medications for HDP undergoing expectant management. However, most HDP cases occur at or near term when delivery is the preferred management. We also suspect that clinicians may have a different (and higher) threshold to introduce antihypertensive medications with a diagnosis of HDP only due to concerns about masking disease progression, particularly in the outpatient setting. The difference in antihypertensive medication use in the CHTN only versus co-existing CHTN and HDP groups is unclear: it may reflect more severe underlying disease in those people with medication-requiring hypertension and their higher tendency to develop HDP, but it could also result from higher use of antihypertensive medications in those with CHTN for the expectant management of superimposed HDP.
The differences observed after adjustment for antihypertensive medication use with the apparent reduction or elimination of effect of CHTN on cesarean delivery, maternal ICU admission or death, preterm birth, SGA, and neonatal ICU admission are particularly interesting in the setting of emerging evidence to support lower treatment threshold for treatment in CHTN [Citation14,Citation15]. The reason for this finding in our population is unclear. It may reflect improvement in maternofetal environment as a result of medication effect and presumed improved blood pressure control throughout pregnancy. However, it also could represent a marker of engagement in prenatal care, a strategy to allow longer period of expectant management with increased gestational age at delivery, or other factors.
This is a large diverse contemporary cohort with broad generalizability. Study limitations include the single-center retrospective design with reliance on recorded data from the EMR and billing and diagnosis codes [Citation39–41]. However, this is a standardized system with a single reliable EMR for both outpatient and inpatient care. The validity of ICD coding for data of interest is high in prior studies [Citation40–42] but has not been determined in this cohort. We had comprehensive information about maternal comorbidities and were able to adjust for such unlike many prior studies. Information about aspirin use and antihypertensive medication exposure allowed for novel exploration of these effects on the outcomes of interest across various categories of hypertension. However, we are limited by lack of information about the timing of initiation, dose, and duration of medications. Documented aspirin utilization is low. Though use may be underreported as aspirin is available as an over-the-counter medication that does not need to be necessarily prescribed, incomplete utilization of indicated aspirin is a concerning possibility [Citation43–46].
Our population has a higher prevalence of CHTN (8.2%) than the national average, though substantial variation in rates of CHTN by race [Citation1] and region [Citation47] are well-established. Other high risk conditions such as advanced maternal age and obesity occur more frequently in our population. We note also that our rate of HDP diagnosis in patients with CHTN (56.6%) is higher than previously published estimates [Citation8,Citation10,Citation34]. This rate may represent a true elevated incidence in our high risk population. It could also represent under-recognition of CHTN in the cohort, regional differences in diagnosis of HDP with CHTN, or miscoding of the respective diagnoses. There is an absence in our data of detailed information about the hypertensive diagnosis including gestational age at HDP diagnosis, how the diagnosis of HDP was established in the setting of CHTN, and the details of the HDP diagnosis (e.g. with versus without severe features). The interval from diagnosis to delivery and rationale for delivery timing are also unavailable but of interest given the high odds of preterm birth and the prominent influence of gestational age on perinatal morbidity in the co-existing CHTN and HDP group.
The pathophysiology for the potentially cumulative effects of CHTN and HDP remains unclear. Additional research is needed to understand how the underlying maternal and placental vascular dysfunction interact with and contribute to aberrant angiogenic and endothelial signaling and how this process might be stalled, arrested, and prevented. More study is needed into the role of antihypertensive medications including the ideal timing, dosing, and agents and the effect of their use on HDP incidence and outcomes in patients with CHTN. The diagnosis and management of HDP in the setting of CHTN also remains inconsistent and without robust data.
Conclusions
Pregnancies affected by co-existing CHTN and HDP have increased rates of adverse maternal and neonatal outcomes. Odds of cesarean delivery, maternal ICU admission or death, preterm birth, and SGA are higher than with either condition alone, even after controlling for comorbid conditions. NICU admission and perinatal morbidity appear to be strongly driven by prematurity and HDP whereas maternal morbidity is more closely linked to CHTN. Antihypertensive medication use in CHTN seems to mitigate likelihood of adverse outcomes, even in cases with superimposed HDP. Further explanation for these differences remains unclear with possible contributions from alterations in underlying maternal physiology, severity/timing of HDP, and management of superimposed HDP.
Supplemental Material
Download MS Word (14.1 KB)Acknowledgements
Presented as poster at 42nd Annual Pregnancy Meeting of the Society for Maternal Fetal Medicine; virtual meeting; January 31–February 5, 2022.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from Yale University and restrictions apply to their availability. The data are not publicly available due to their containing information that could compromise patient privacy.
Additional information
Funding
References
- Ananth CV, Duzyj CM, Yadava S, et al. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension. 2019;74(5):1–9. doi: 10.1161/hypertensionaha.119.12968.
- Bateman BT, Bansil P, Hernandez-Diaz S, et al. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206(2):134.e1–134.e8. doi: 10.1016/j.ajog.2011.10.878.
- ACOG Practice Bulletin No. 203. Chronic hypertension in pregnancy. Obstet Gynecol. 2019;133(1):e26–e50. doi: 10.1097/aog.0000000000003020.
- Bello NA, Zhou H, Cheetham TC, et al. Prevalence of hypertension among pregnant women when using the 2017 American College of Cardiology/American Heart Association Blood Pressure guidelines and association with maternal and fetal outcomes. JAMA Netw Open. 2021;4(3):e213808. doi: 10.1001/jamanetworkopen.2021.3808.
- Greenberg VR, Silasi M, Lundsberg LS, et al. Perinatal outcomes in women with elevated blood pressure and stage 1 hypertension. Am J Obstet Gynecol. 2021;224(5):521.e1–521.e11. doi: 10.1016/j.ajog.2020.10.049.
- Norton E, Shofer F, Schwartz H, et al. Adverse perinatal outcomes associated with stage 1 hypertension in pregnancy: a retrospective cohort study. Am J Perinatol. 2023;40(16):1781–1788. doi: 10.1055/s-0041-1739470.
- Bramham K, Parnell B, Nelson-Piercy C, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348(7):g2301. doi: 10.1136/bmj.g2301.
- Chappell LC, Enye S, Seed P, et al. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: a prospective study. Hypertension. 2008;51(4):1002–1009. doi: 10.1161/hypertensionaha.107.107565.
- Ankumah NE, Sibai BM. Chronic hypertension in pregnancy: diagnosis, management, and outcomes. Clin Obstet Gynecol. 2017;60(1):206–214. doi: 10.1097/grf.0000000000000255.
- Roberts CL, Algert CS, Morris JM, et al. Hypertensive disorders in pregnancy: a population-based study. Med J Aust. 2005;182(7):332–335. doi: 10.5694/j.1326-5377.2005.tb06730.x.
- Page EW, Christianson R. Influence of blood pressure changes with and without proteinuria upon outcome of pregnancy. Am J Obstet Gynecol. 1976;126(7):821–833. doi: 10.1016/0002-9378(76)90671-2.
- Hurrell A, Webster L, Chappell LC, et al. The assessment of blood pressure in pregnant women: pitfalls and novel approaches. Am J Obstet Gynecol. 2022;226(2s):S804–S818. doi: 10.1016/j.ajog.2020.10.026.
- Kaimal AJ, Gandhi M, Pettker CM, et al. Clinical guidance for the integration of the findings of the chronic hypertension and pregnancy (CHAP) study. American College of Obstetricians and Gynecologists’ Committee on Clinical Practice Guidelines; 2022.
- Bailey EJ, Tita ATN, Leach J, et al. Perinatal outcomes associated with management of stage 1 hypertension. Obstet Gynecol. 2023;142(6):1395–1404. doi: 10.1097/aog.0000000000005410.
- Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386(19):1781–1792. doi: 10.1056/NEJMoa2201295.
- Sibai BM, Lindheimer M, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;339(10):667–671. doi: 10.1056/nejm199809033391004.
- Burton GJ, Redman CW, Roberts JM, et al. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. doi: 10.1136/bmj.l2381.
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. doi: 10.1016/s0140-6736(05)17987-2.
- Erez O, Romero R, Jung E, et al. Preeclampsia and eclampsia: the conceptual evolution of a syndrome. Am J Obstet Gynecol. 2022;226(2s):S786–S803. doi: 10.1016/j.ajog.2021.12.001.
- Jung E, Romero R, Yeo L, et al. The etiology of preeclampsia. Am J Obstet Gynecol. 2022;226(2s):S844–S866. doi: 10.1016/j.ajog.2021.11.1356.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022;226(2s):S1019–S1034. doi: 10.1016/j.ajog.2020.10.022.
- Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–2869. doi: 10.1161/circulationaha.109.853127.
- Burton GJ, Woods AW, Jauniaux E, et al. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–482. doi: 10.1016/j.placenta.2009.02.009.
- Perni U, Sison C, Sharma V, et al. Angiogenic factors in superimposed preeclampsia: a longitudinal study of women with chronic hypertension during pregnancy. Hypertension. 2012;59(3):740–746. doi: 10.1161/hypertensionaha.111.181735.
- Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl. A):S32–S37. doi: 10.1016/j.placenta.2008.11.009.
- ACOG Practice Bulletin No. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):1. doi: 10.1097/aog.0000000000003018.
- Tassi A, Sala A, Mazzera I, et al. Long-term outcomes of patients with preeclampsia: a review of the literature. Hypertens Pregnancy. 2023;42(1):2217448. doi: 10.1080/10641955.2023.2217448.
- Srialluri N, Surapaneni A, Chang A, et al. Preeclampsia and long-term kidney outcomes: an observational cohort study. Am J Kidney Dis. 2023;82(6):698–705. doi: 10.1053/j.ajkd.2023.04.010.
- Paquin A, Werlang A, Coutinho T. Arterial health after preeclampsia: role of chronic hypertension in the early vascular aging (EVA) study. Am J Hypertens. 2024;37(1):24–32. doi: 10.1093/ajh/hpad079.
- Kivelä A, Heinonen S, Kivinen K, et al. Hypertensive pregnancy complications and maternal characteristics as predictors of cardiovascular health within ten years after delivery. Pregnancy Hypertens. 2023;34:5–12. doi: 10.1016/j.preghy.2023.09.001.
- Aris IM, Kleinman KP, Belfort MB, et al. A 2017 US reference for singleton birth weight percentiles using obstetric estimates of gestation. Pediatrics. 2019;144(1):e20190076. doi: 10.1542/peds.2019-0076.
- Committee Opinion No. 700: methods for estimating the due date. Obstet Gynecol. 2017;129(5):e150–e154. doi: 10.1097/aog.0000000000002046.
- Becker DA, Machemehl HC, Biggio JR, et al. Pregnancy outcomes of exacerbated chronic hypertension compared with superimposed preeclampsia. Am J Perinatol. 2019;36(8):872–878. doi: 10.1055/s-0038-1675160.
- Tuuli MG, Rampersad R, Stamilio D, et al. Perinatal outcomes in women with preeclampsia and superimposed preeclampsia: do they differ? Am J Obstet Gynecol. 2011;204(6):508.e1–508.e7. doi: 10.1016/j.ajog.2011.01.065.
- Valent AM, DeFranco EA, Allison A, et al. Expectant management of mild preeclampsia versus superimposed preeclampsia up to 37 weeks. Am J Obstet Gynecol. 2015;212(4):515.e1–515.e8. doi: 10.1016/j.ajog.2014.10.1090.
- Giannubilo SR, Dell’Uomo B, Tranquilli AL. Perinatal outcomes, blood pressure patterns and risk assessment of superimposed preeclampsia in mild chronic hypertensive pregnancy. Eur J Obstet Gynecol Reprod Biol. 2006;126(1):63–67. doi: 10.1016/j.ejogrb.2005.06.042.
- Leonard SA, Formanowski BL, Phibbs CS, et al. Chronic hypertension in pregnancy and racial-ethnic disparities in complications. Obstet Gynecol. 2023;142(4):862–871. doi: 10.1097/aog.0000000000005342.
- Kern-Goldberger AR, Friedman A, Moroz L, et al. Racial disparities in maternal critical care: are there racial differences in level of care? J Racial Ethn Health Disparities. 2022;9(2):679–683. doi: 10.1007/s40615-021-01000-z.
- Goodin A, Delcher C, Valenzuela C, et al. The power and pitfalls of big data research in obstetrics and gynecology: a consumer’s guide. Obstet Gynecol Surv. 2017;72(11):669–682. doi: 10.1097/ogx.0000000000000504.
- Sigakis MJ, Leffert LR, Mirzakhani H, et al. The validity of discharge billing codes reflecting severe maternal morbidity. Anesth Analg. 2016;123(3):731–738. doi: 10.1213/ane.0000000000001436.
- Stanhope KK, Joseph NT, Platner M, et al. Validation of ICD-10 codes for gestational and pregestational diabetes during pregnancy in a large, public hospital. Epidemiology. 2021;32(2):277–281. doi: 10.1097/ede.0000000000001311.
- Labgold K, Stanhope KK, Joseph NT, et al. Validation of hypertensive disorders during pregnancy: ICD-10 codes in a high-burden southeastern United States hospital. Epidemiology. 2021;32(4):591–597. doi: 10.1097/ede.0000000000001343.
- Ray JG, Abdulaziz KE, Berger H, et al. Aspirin use for preeclampsia prevention among women with prepregnancy diabetes, obesity, and hypertension. JAMA. 2022;327(4):388–390. doi: 10.1001/jama.2021.22749.
- Phelps AJD, Holmgren C. Relationship between risk factor profile and prescription of low-dose aspirin for preeclampsia prevention. Arch Gynecol Obstet. 2022;308(4):1279–1286. doi: 10.1007/s00404-022-06773-0.
- Ragunanthan NW, Lamb J, Hauspurg A, et al. Assessment of racial disparities in aspirin prophylaxis for preeclampsia prevention. Am J Perinatol. 2022. doi: 10.1055/s-0042-1743142.
- Ayyash M, Goyert G, Pitts D, et al. Provider adherence to aspirin prophylaxis prescription guidelines for preeclampsia. Pregnancy Hypertens. 2023;34:1–4. doi: 10.1016/j.preghy.2023.09.002.
- Butwick AJ, Druzin ML, Shaw GM, et al. Evaluation of US state-level variation in hypertensive disorders of pregnancy. JAMA Netw Open. 2020;3(10):e2018741. doi: 10.1001/jamanetworkopen.2020.18741.