Abstract
Objective
The relationship between placental location in pregnancies without previa and adverse pregnancy outcomes has not been well studied. Additionally, the impact of abnormal cord insertion sites remains controversial. Therefore, the objective of this study was to explore the adverse outcomes associated with placental location and abnormal cord insertion in nulliparous women and to assess their impact on pregnancy outcomes.
Methods
This retrospective cohort study was conducted at a single tertiary hospital between January 2019 and June 2022. The study included nulliparous women with singleton pregnancies who delivered live infants and had available data on placental location and umbilical cord insertion site from a second- or third-trimester ultrasound. Placental location was categorized as anterior or posterior using transabdominal ultrasonography. The association between placental location/cord insertion site and pre-eclampsia was evaluated using multivariate logistic regression analysis. We compared the area under the curve to evaluate the impact of placental location and cord insertion site on pre-eclampsia.
Results
A total of 2219 pregnancies were included in the study. Pre-eclampsia occurred significantly more frequently in the anterior group than in the posterior group (8.21% vs. 3.04%, p < .001). In multivariate analysis investigating the association between placental location and pre-eclampsia, anterior placenta and marginal cord insertion showed increased odds ratios for pre-eclampsia of 3.05 (95% confidence interval [CI] 1.68–6.58) and 3.64 (95% CI 1.90–6.97), respectively. Receiver operating characteristic (ROC) curves were constructed to predict pre-eclampsia using independent factors from multivariate analyses. Model I, including maternal age, pre-pregnancy body mass index, in vitro fertilization, chronic hypertension, overt diabetes, kidney disease, and hematologic diseases, achieved an area under the ROC curve of 0.70 (95% CI 0.65–0.75). Adding cord insertion site and placental location to the model (Model II) improved its predictive performance, resulting in an area under the ROC curve of 0.749 (95% CI 0.70–0.79, p = .02).
Conclusions
Anterior placenta and marginal cord insertion were associated with an increased risk of pre-eclampsia. Further studies on prospective cohorts are necessary to validate these findings.
Introduction
Both the placenta and umbilical cord are temporary organs, but they play key roles in fetal health support, which can have lifelong consequences [Citation1,Citation2]. The placenta not only functions as an endocrine organ and natural immunological barrier but also facilitates the exchange of multiple gases, nutrients, and waste products between the mother and developing fetus [Citation3–5]. Therefore, abnormal placental development has been associated with significant obstetric complications, such as pre-eclampsia, gestational diabetes mellitus (GDM), fetal growth restriction (FGR), recurrent miscarriage, and preterm birth [Citation6,Citation7]. Pregnancies with placenta previa are reportedly associated with poor obstetric outcomes, including massive bleeding, placenta accreta syndrome, preterm labor, or FGR [Citation8–10]. In a previous study on placenta previa, anterior placental location was suggested as a risk factor for maternal vascular malperfusion lesions, which is a marker of placental insufficiency [Citation10]. However, only a few studies have investigated the association between the placental location and perinatal outcomes in pregnant women without previa [Citation11–13]. The condition within the uterus may differ depending on its location, potentially affecting the placenta that is implanted in the uterus. Hence, placental positions other than previa could also be associated with pregnancy outcomes. In addition, there is no clear evidence whether marginal cord insertion is associated with adverse perinatal outcomes, although some studies have suggested an increased risk of FGR, postpartum bleeding, or preterm birth [Citation14–16]. Therefore, this study aimed to investigate whether the position of the placenta and umbilical cord in nulliparous women is associated with adverse pregnancy outcomes, including pre-eclampsia.
Methods
This retrospective cohort study included women who delivered live infants after 24-week gestation between January 2019 and June 2022 at a single tertiary hospital. Nulliparous women with singleton pregnancies with available data on placental location and cord insertion sites from second- or third-trimester ultrasound scans were included. Baseline information, including age, gravidity, parity, body mass index (BMI), medical and obstetric histories, delivery details, and neonatal outcomes, was obtained by reviewing medical records. Underlying diseases, such as diabetes mellitus; chronic hypertension; rheumatologic diseases, including systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS); kidney diseases, including immunoglobulin A nephropathy, chronic kidney disease, and glomerulonephritis; and hematologic diseases, such as aplastic anemia, immune thrombocytopenia, or chronic myeloid leukemia, were evaluated. All pregnant women with SLE or APS were administered low-dose aspirin and low molecular weight heparin during pregnancy to prevent pre-eclampsia.
The placental locations were determined as anterior, posterior, lateral, and fundal using transabdominal sonography. For the anterior or posterior placenta, the majority were positioned entirely on the anterior or posterior. However, if not, they were included in the respective category if positioned on more than two-thirds. A fundal placenta indicated an even distribution across the top of the anterior and posterior walls of the uterus. Placentas with more than two-thirds on one side of the middle were classified as lateral. For the study, women were divided into anterior and posterior groups, and patients with placentas located in the fundal or lateral positions were excluded from the study. Additional exclusion criteria were placenta previa, low-lying placenta (<20 mm between the placenta and the internal os of the cervix), vasa previa, or insufficient information about the placenta. Cord insertion was classified as normal, marginal (<2 cm from the placental edge), or velamentous (inserted into the fetal membranes before reaching the placental disc). In all cases of suspected marginal or velamentous cord insertion on ultrasonography, the final postpartum diagnosis of cord insertion was performed and recorded by the attending physician and pathologists.
Adverse pregnancy outcomes included gestational hypertension, pre-eclampsia, placental abruption, preterm premature rupture of membrane (PPROM), spontaneous preterm delivery (SPD), GDM, and postpartum hemorrhage. Neonatal outcomes of interest were evaluated as follows: 5-min Apgar score <7, admission to the neonatal intensive care unit (NICU) within 48 h after birth, respiratory distress syndrome (RDS), need for a mechanical ventilator, and small for gestational age (SGA). In accordance with the American College of Obstetricians and Gynecologists’ Hypertension in Pregnancy Task Force guidelines as a new-onset elevation in blood pressure >140/90 mmHg and proteinuria, or in the absence of proteinuria, new-onset hypertension in conjunction with any of the following: thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or persistent neurologic symptoms [Citation17]. Pre-eclampsia included eclampsia and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. SPD was defined as delivery at 24 0/7 to 36 6/7 weeks of gestation resulting from preterm labor. SGA was defined as a birth weight below the 10th percentile for a given gestational age based on the reference population [Citation18]. Postpartum hemorrhage was defined as a cumulative blood loss of ≥1000 ml or blood loss accompanied by signs or symptoms of hypovolemia within 24 h after birth [Citation19].
Statistical analyses
Analysis was performed using the Wilcoxon rank-sum test for nonparametric continuous variables. The chi-square test and Fisher’s exact test were used for categorical variables, where appropriate. After comparing adverse obstetric outcomes according to the location of the placenta, the association between placental location, including the cord insertion site, and pre-eclampsia, which showed a significant difference between the two groups, was analyzed by logistic regression analysis. The independent variables that were significant in the univariate analysis were included in the multivariate logistic regression analysis for pre-eclampsia. Model I evaluated pre-pregnancy maternal factors only, while Model II also included information about placenta and cord locations. The receiver operating characteristic (ROC) curves of the two models were evaluated using independent factors. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at p < .05.
Results
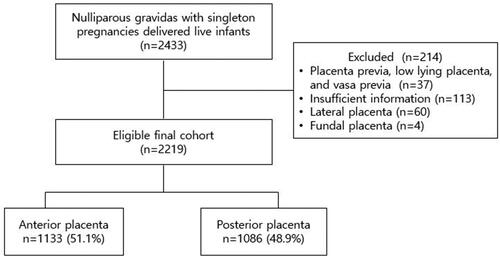
A total of 2219 pregnancies were included in this study, with 1133 (51.1%) patients having an anterior placenta and 1086 (48.9%) patients having a posterior placenta (). The baseline characteristics of the two study groups are presented in . There were no statistically significant differences between the groups in maternal characteristics, except for pre-pregnancy BMI (21.5 ± 3.28 vs. 21.1 ± 3.05, p = .01). Pre-eclampsia occurred significantly more frequently in the anterior group than in the posterior group (8.21% vs. 3.04%, p < .001). Marginal and velamentous cord insertions occurred in 4.32% and 0.59% of cases, respectively. Regarding the cord insertion site, no significant differences were observed between the anterior and posterior placental groups (p = .568). The maternal outcomes of gestational hypertension, placental abruption, PPROM, SPD, GDM, and postpartum hemorrhage and neonatal outcomes, including birth weight, placental weight, NICU admission, RDS, mechanical ventilation, and SGA, were not significantly different between the two groups ().
Table 1. Baseline characteristics according to placental location.
Table 2. Pregnancy and neonatal outcomes according to placental location.
Associations between pre-pregnancy maternal factors with or without placental factors and pre-eclampsia
In the univariate analysis, the pre-pregnancy maternal factors of maternal age, pre-pregnancy BMI, in vitro fertilization (IVF), diabetes, chronic hypertension, kidney disease, and hematologic disease showed significantly increased odds ratios for pre-eclampsia (). Anterior placenta location and marginal cord insertion also showed increased odds ratios for pre-eclampsia. In Model I, which evaluated pre-pregnancy maternal factors, pre-pregnancy BMI, IVF, chronic hypertension, and kidney disease were significantly associated with pre-eclampsia. In Model II, which evaluated pre-pregnancy maternal factors in addition to information about the placenta and cord, anterior placental location, and marginal cord insertion were associated with pre-eclampsia (adjusted odds ratio [aOR] 3.05, 95% confidence interval [CI] 1.68–6.58 and aOR 3.64, 95% CI 1.90–6.97, respectively).
Table 3. Univariate and multivariate logistic regression for prediction of pre-eclampsia.
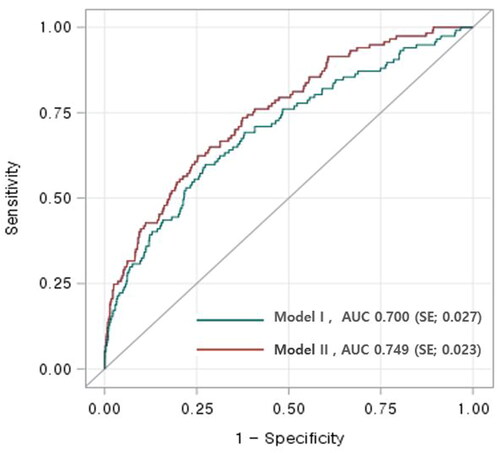
The ROC curves for the prediction of pre-eclampsia, using independent factors in Model I and II, are presented in . Model I achieved an area under the ROC curve of 0.700 (95% CI 0.65–0.75). However, the addition of placental location and cord insertion site to Model II improved its predictive performance, yielding an area under the ROC curve of 0.749 compared to that of Model I (95% CI 0.70–0.79, p = .02).
Figure 2. Receiver operating characteristic (ROC) curves for prediction of pre-eclampsia of the two models.
Comparison of area under ROC curves of two models showed that the addition of placental location and cord insertion site improved its predictive performance: 0.749 (95% CI 0.70–0.79) vs. 0.700 (95% CI 0.65–0.75), p = .02.
Discussion
In this study, nulliparous women with an anterior placenta were associated with pre-eclampsia compared to those with a posterior placenta. Additionally, marginal cord insertion was significantly correlated with a higher risk of pre-eclampsia. The classification of placental location lacks standardization, but it is often divided into anterior, posterior, fundal, and lateral positions. Several authors have suggested an increased risk of pre-eclampsia associated with a lateral placenta, potentially attributed to a higher mean uterine artery pulsatility index and ipsilateral blood supply to the lateral uterine wall. However, these studies merely categorized the placenta into lateral and non-lateral groups (central group); including anterior, posterior, and fundal positions. Moreover, the sample sizes of the studies were relatively small (<500), and the reported percentages of the lateral placenta location varied significantly among different studies. For instance, Kakkara et al. categorized 56% of 150 samples into the lateral group and 44% into the non-lateral group, while Salma et al. reported that 11.4% were in the lateral position [Citation20–22]. However, in a recent large-scale population-based cohort study, over 10,000 participants were categorized into lateral and central groups, with approximately 5.6% in the lateral group. Interestingly, this study found no association between the lateral placental location and pre-eclampsia [Citation23]. Additionally, several other studies have presented findings indicating no significant relationship between a lateral placenta and pre-eclampsia [Citation24,Citation25]. Therefore, we propose that understanding the correlation between pre-eclampsia and a lateral placental position would be more meaningful by considering data from previous large-scale studies with similar proportions of lateral locations.
In a previous large population-based cohort study, the majority of placental positions were either anterior or posterior; 47.8% of women had an anterior placenta and 46.4% had a posterior placenta [Citation11]. The distribution of placental locations observed in our study was similar to the previous findings. The structure and environment vary at different locations within the uterus. The implantation and development of the placenta within the uterus can be influenced by its specific location. Several hypotheses have been proposed to explain the association between an anterior placenta and the increased risk of pre-eclampsia. One hypothesis suggests that the anterior wall of the uterus may be slightly thinner than the posterior wall during the first and second trimesters of pregnancy. Consequently, there is a potential relative disadvantage in the invasion of the spiral arteries when the placenta is implanted into this thinner wall [Citation26,Citation27]. However, acknowledging that the observed difference in thickness is typically minimal and may not have significant clinical implications is important. Another plausible explanation is that an anterior placenta is more exposed to external forces compared to a posterior placenta. Acar et al. reported a higher risk of placental abruption among pregnant drivers with an anterior placenta than among those with other placental positions [Citation28]. This suggests that an anterior placenta, which is exposed to relatively continuous external influences, may experience weaker implantation in the uterine wall. Moreover, an additional hypothesis from placenta migration theory suggests that during pregnancy, the anterior low-lying placenta may migrate more rapidly and extensively toward the fundus compared to the posterior low-lying placenta. In this theory, the concept of dynamic placentation is proposed, where the anterior uterine wall undergoes more substantial expansion than the posterior wall during the uterus growth [Citation29]. Therefore, the dynamic changes experienced by the anterior wall during pregnancy, differing from those of the posterior wall, might lead to an unfavorable position relative to the posterior. Any disruption in the remodeling process can lead to an elevation of uteroplacental vascular resistance, resulting in diminished blood flow to the placenta and subsequent consequences, including coagulation activation, endothelial cell dysfunction, placental thrombosis, and fibrin deposits, ultimately causing placental insufficiency. [Citation30]. However, additional research and comprehensive investigations are essential to validate and refine these hypotheses.
Previous studies have not shown a significant association between abnormal cord insertion and pre-eclampsia, and recent meta-analyses have suggested that marginal and velamentous cord insertions are linked to a higher risk of pre-eclampsia [Citation31–34]. In this study, we specifically found that a marginal cord insertion was associated with an increased risk of developing pre-eclampsia. Velamentous cord insertion can lead to blood vessels that lack the protective properties of Wharton’s jelly, which may be associated with several adverse perinatal outcomes [Citation35]. In our study, we found no significant association between velamentous cord insertion and pre-eclampsia. This lack of association could be attributed to the low incidence of velamentous cord insertion in our study sample. However, although not included in the table, the velamentous cord insertion group showed a statistically significantly higher incidence of PPROM when compared to the central cord insertion group (3.64% vs. 23.08%, p = .03) consistent with findings observed in other previous studies [Citation14,Citation32].
This study had several limitations. First, it was a retrospective study conducted at a single tertiary hospital, which limits the generalizability of the findings to a broader population. Additionally, the absence of a standardized classification system for placental location may have implications for the accuracy and consistency of the reported placental positions. Second, the study did not analyze the placental pathology that could support the relationship between placental location and pre-eclampsia, specifically the presence of maternal vascular malperfusion [Citation36,Citation37]. In addition, the study did not include predictive markers for pre-eclampsia, such as maternal serum pregnancy-associated plasma protein A (PAPP-A), placental growth factor (PLGF), and the uterine artery pulsatility index. The reason for this is that a considerable number of pregnant women opted for noninvasive prenatal testing instead of undergoing maternal serum PAPP-A measurements, or there were missing records of results from other hospitals [Citation38].
However, the strengths of this study include its relatively large sample of women with nulliparous singleton pregnancies. This specific inclusion criterion helped eliminate the potential influence of multiple pregnancies or prior adverse pregnancy outcome histories. After adjusting for confounding variables, anterior placental location and marginal cord insertion were associated with pre-eclampsia.
The specific mechanisms underlying the association between placental location and placenta-mediated disorders such as pre-eclampsia remain unclear, indicating the need for further research in this area. Recent studies have evaluated placental function using advanced imaging tools, such as magnetic resonance imaging, as well as methods for integrating ultrasonographic information about the morphology of tissue layers with optical information on blood flow to investigate their relationship with pregnancy complications [Citation39–42]. These recent advanced technologies for placental investigation may help identify high-risk populations for pre-eclampsia. However, it is time-consuming to investigate useful biomarkers and ultrasonography markers that are clinically useful and cost-effective. Observation of the placental location and umbilical cord insertion using antenatal ultrasound is a simple, easy method that can be applied in low-resource settings. Therefore, it may be worth investigating its value in a prospective setting along with other parameters.
When placental location and cord insertion site were added to the pre-pregnancy risk factors for pre-eclampsia, there was a statistical improvement in predictive capability, but it did not notably enhance it. Since this current investigation is in its early stages, altering clinical approaches based on the results of the present study is not advisable. This is a development study using a retrospective cohort technique that requires validation. Further studies, including testing for serum markers such as PAPP-A, sFlt-1, or PLGF, may be necessary. An essential examination of placental pathology is important to substantiate the findings of the study, contributing to a better understanding of the results and providing insights into potential mechanisms or physiological processes. Furthermore, high-quality prospective studies could offer more reliable effect estimates, free from biases that may predominate in retrospective studies.
Conclusion
This study showed an association between placental location and cord insertion site with pre-eclampsia. However, the specific mechanisms underlying the association between placental location and placenta-mediated disorders remain unclear. This investigation is preliminary and specific prospective studies are needed to further investigate the correlation between placental location and pre-eclampsia.
Acknowledgments
Statistical analysis was performed by biostatisticians (MSc Youn-Ju Lee and Hana Kim) employed by a contract research organization, Medical Excellence Inc.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Bauer MK, Harding JE, Bassett NS, et al. Fetal growth and placental function. Mol Cell Endocrinol. 1998;140(1–2):1–8. doi: 10.1016/s0303-7207(98)00039-2.
- Öztürk HNO, Türker PF. Fetal programming: could intrauterin life affect health status in adulthood? Obstet Gynecol Sci. 2021;64(6):473–483. doi: 10.5468/ogs.21154.
- Hong SH, Kim SC, Park MN, et al. Expression of steroidogenic enzymes in human placenta according to the gestational age. Mol Med Rep. 2019;19(5):3903–3911.
- Nazari L, Salehpour S, Hosseini S, et al. Effect of autologous platelet-rich plasma for treatment of recurrent pregnancy loss: a randomized controlled trial. Obstet Gynecol Sci. 2022;65(3):266–272. doi: 10.5468/ogs.21261.
- Soncin F, Parast MM. Role of hippo signaling pathway in early placental development. Proc Natl Acad Sci USA. 2020;117(34):20354–20356. doi: 10.1073/pnas.2013559117.
- Jung E, Romero R, Yeo L, et al. The etiology of preeclampsia. Am J Obstet Gynecol. 2022;226(2S):S844–S866. doi: 10.1016/j.ajog.2021.11.1356.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics and the Society for Maternal-Fetal Medicine. ACOG Practice Bulletin No. 204: fetal growth restriction. Obstet Gynecol. 2019;133(2):e97–e109.
- Lal AK, Hibbard JU. Placenta previa: an outcome-based cohort study in a contemporary obstetric population. Arch Gynecol Obstet. 2015;292(2):299–305. doi: 10.1007/s00404-015-3628-y.
- Jang DG, We JS, Shin JU, et al. Maternal outcomes according to placental position in placental previa. Int J Med Sci. 2011;8(5):439–444. doi: 10.7150/ijms.8.439.
- Tairy D, Weiner E, Schreiber L, et al. Placental lesions and pregnancy outcome in anterior as compared to posterior placenta previa. Reprod Sci. 2021;28(11):3241–3247. doi: 10.1007/s43032-021-00558-7.
- Granfors M, Stephansson O, Endler M, et al. Placental location and pregnancy outcomes in nulliparous women: a population-based cohort study. Acta Obstet Gynecol Scand. 2019;98(8):988–996. doi: 10.1111/aogs.13578.
- Granfors M, Sandström A, Stephansson O, et al. Placental location and risk of retained placenta in women with a previous cesarean section: a population-based cohort study. Acta Obstet Gynecol Scand. 2020;99(12):1666–1673. doi: 10.1111/aogs.13943.
- Belachew J, Eurenius K, Mulic-Lutvica A, et al. Placental location, postpartum hemorrhage and retained placenta in women with a previous cesarean section delivery: a prospective cohort study. Ups J Med Sci. 2017;122(3):185–189. doi: 10.1080/03009734.2017.1356405.
- Ebbing C, Johnsen SL, Albrechtsen S, et al. Velamentous or marginal cord insertion and the risk of spontaneous preterm birth, prelabor rupture of the membranes, and anomalous cord length, a population-based study. Acta Obstet Gynecol Scand. 2017;96(1):78–85. doi: 10.1111/aogs.13035.
- Ebbing C, Kiserud T, Johnsen SL, et al. Prevalence, risk factors and outcomes of velamentous and marginal cord insertions: a population-based study of 634,741 pregnancies. PLoS One. 2013;8(7):e70380. doi: 10.1371/journal.pone.0070380.
- Ebbing C, Kiserud T, Johnsen SL, et al. Third stage of labor risks in velamentous and marginal cord insertion: a population-based study. Acta Obstet Gynecol Scand. 2015;94(8):878–883. doi: 10.1111/aogs.12666.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133(1):e26–e50. doi: 10.1097/AOG.0000000000003020.
- Chou JH, Roumiantsev S, Singh R. PediTools electronic growth chart calculators: applications in clinical care, research, and quality improvement. J Med Internet Res. 2020;22(1):e16204. doi: 10.2196/16204.
- Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 183: postpartum hemorrhage. Obstet Gynecol. 2017;130(4):e168–e186. doi: 10.1097/AOG.0000000000002351.
- Kakkar T, Singh V, Razdan R, et al. Placental laterality as a predictor for development of preeclampsia. J Obstet Gynaecol India. 2013;63(1):22–25. doi: 10.1007/s13224-012-0241-x.
- Siargkas A, Tsakiridis I, Grammenos P, et al. The impact of lateral placenta on preeclampsia and small for gestational age neonates: a systematic review and meta-analysis. J Perinat Med. 2023;51(4):468–476. doi: 10.1515/jpm-2022-0118.
- Gonser M, Tillack N, Pfeiffer KH, et al. [Placental location and incidence of pre-eclampsia]. Ultraschall Med. 1996;17(5):236–238. doi: 10.1055/s-2007-1003188.
- Doctory N, Romano A, Navon I, et al. Placental location and obstetrical-neonatal outcomes: a retrospective study. Int J Gynaecol Obstet. 2023;160(2):641–645. doi: 10.1002/ijgo.14316.
- Salama-Bello R, Duncan JR, Howard SL, et al. Placental location and the development of hypertensive disorders of pregnancy. J Ultrasound Med. 2019;38(1):173–178. doi: 10.1002/jum.14681.
- Porto L, Aviram A, Jackson R, et al. Lateral placentation and adverse perinatal outcomes. Placenta. 2020;101:1–3. doi: 10.1016/j.placenta.2020.08.012.
- Durnwald CP, Mercer BM. Myometrial thickness according to uterine site, gestational age and prior cesarean delivery. J Matern Fetal Neonatal Med. 2008;21(4):247–250. doi: 10.1080/14767050801926709.
- Degani S, Leibovitz Z, Shapiro I, et al. Myometrial thickness in pregnancy: longitudinal sonographic study. J Ultrasound Med. 1998;17(10):661–665. doi: 10.7863/jum.1998.17.10.661.
- Acar BS, Meric M. The effect of placenta location on the safety of pregnant driver and her fetus. Int J Crashworthiness. 2017;22(2):163–168. doi: 10.1080/13588265.2016.1243609.
- Jansen CHJR, Kastelein AW, Kleinrouweler CE, et al. Development of placental abnormalities in location and anatomy. Acta Obstet Gynecol Scand. 2020;99(8):983–993. doi: 10.1111/aogs.13834.
- Wardinger JE, Ambati S. Placental insufficiency. Treasure Island (FL): StatPearls Publishing; 2022.
- Tsakiridis I, Dagklis T, Athanasiadis A, et al. Impact of marginal and velamentous cord insertion on uterine artery doppler indices, fetal growth, and preeclampsia. J Ultrasound Med. 2022;41(8):2011–2018. doi: 10.1002/jum.15883.
- Sinkin JA, Craig WY, Jones M, et al. Perinatal outcomes associated with isolated velamentous cord insertion in singleton and twin pregnancies. J Ultrasound Med. 2018;37(2):471–478. doi: 10.1002/jum.14357.
- Allaf MB, Andrikopoulou M, Crnosija N, et al. Second trimester marginal cord insertion is associated with adverse perinatal outcomes. J Matern Fetal Neonatal Med. 2019;32(18):2979–2984. doi: 10.1080/14767058.2018.1453798.
- Lutz AB, Young-Lin N, Leon-Martinez D, et al. Measurement of marginal placental cord insertion by prenatal ultrasound was found not to be predictive of adverse perinatal outcomes. J Ultrasound Med. 2021;40(10):2079–2086. doi: 10.1002/jum.15586.
- Siargkas A, Tsakiridis I, Pachi C, et al. Impact of marginal cord insertion on perinatal outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2023;5(4):100876. doi: 10.1016/j.ajogmf.2023.100876.
- Siargkas A, Tsakiridis I, Pachi C, et al. Impact of velamentous cord insertion on perinatal outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2023;5(2):100812. doi: 10.1016/j.ajogmf.2022.100812.
- Falco ML, Sivanathan J, Laoreti A, et al. Placental histopathology associated with pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):295–301. doi: 10.1002/uog.17494.
- Ernst LM. Maternal vascular malperfusion of the placental bed. APMIS. 2018;126(7):551–560. doi: 10.1111/apm.12833.
- O’Gorman N, Wright D, Syngelaki A, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol. 2016;214(1):103.e1–103.e12. doi: 10.1016/j.ajog.2015.08.034.
- Arthuis C, Millischer AE, Bussières L, et al. MRI based morphological examination of the placenta. Placenta. 2021;115:20–26. doi: 10.1016/j.placenta.2021.08.056.
- Stout JN, Rouhani S, Turk EA, et al. Placental MRI: development of an MRI compatible ex vivo system for whole placenta dual perfusion. Placenta. 2020;101:4–12. doi: 10.1016/j.placenta.2020.07.026.
- Wang L, Cochran JM, Ko T, et al. Non-invasive monitoring of blood oxygenation in human placentas via concurrent diffuse optical spectroscopy and ultrasound imaging. Nat Biomed Eng. 2022;6(9):1017–1030. doi: 10.1038/s41551-022-00913-2.