 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
The study aims to investigate the levels of serum NLRP3 along with its effector molecules (Caspase-1, IL-1β, and IL-18) in the mid-pregnancy in pregnant women with hyperglycemia, and explore the relationship between NLRP3, along with its effector molecules (Caspase-1, IL-1β, and IL-18) and insulin resistance, as well as pregnancy outcomes.
Methods
The levels of serum NLRP3 along with its effector molecules (Caspase-1, IL-1β, and IL-18) in three groups of pregnant women with gestational diabetes mellitus (GDM), pregestational diabetes mellitus (PGDM) and normal glucose tolerance (NGT) were measured in mid-pregnancy, and their relationship with insulin resistance and pregnancy outcomes was analyzed. The ROC curve was also used to evaluate the predictive value of serum NLRP3 inflammasome and its effector molecules for pregnancy outcomes.
Results
There were no statistical differences in the general clinical data of the three groups, and the concentrations of serum NLRP3 along with its effector molecules were higher in the GDM and PGDM groups than in the NGT group, and NLRP3 along with its effector molecules were positively correlated with fasting blood glucose, fasting insulin, and insulin resistance index in both groups (r > 0, p < .05). The incidence of preterm delivery, hypertensive disorders of pregnancy, premature rupture of membranes, neonatal hypoglycemia and macrosomia was significantly higher in both groups than in the NGT group (p < .05). The value of the combined serum NLRP3 and its effector molecules in mid-pregnancy to predict adverse pregnancy outcomes was highest, and the AUCs for the combined prediction of late hypertensive disorders of pregnancy, premature rupture of membranes, preterm delivery, neonatal hypoglycemia and macrosomia were 0.84 (95% CI 0.79–0.88, p < .001), 0.81 (95% CI 0.75–0.85, p < .001), 0.76 (95% CI 0.70–0.81, p < .001), 0.76 (95% CI 0.70–0.81, p < .001) and 0.72 (95% CI 0.63–0.81, p < .001), respectively.
Conclusions
Increased serum NLRP3 along with its effector molecules in pregnant women with hyperglycemia are associated with the levels of insulin resistance and the subsequent development of adverse pregnancy outcomes.
Introduction
Hyperglycemia in pregnancy, including three different types of glucose metabolism abnormalities: pregestational diabetes mellitus (PGDM), pre-diabetes mellitus and gestational diabetes mellitus (GDM), can cause various maternal and infant complications and seriously affect the health of mother and child [Citation1]. GDM, especially PGDM, not only increases the adverse outcomes of pregnancy and perinatal fetus, but also increases the risk of long-term metabolic diseases such as diabetes mellitus in pregnant women [Citation2]. However, at present, the pathogenesis of hyperglycemia during pregnancy has not been fully clarified. The potential islet cell dysfunction and insulin resistance during pregnancy may be its mechanism and elucidating the pathogenesis is important to improve pregnancy outcomes.
Pyroptosis is a newly discovered inflammatory programmed cell death mode in recent years, which is faster than apoptosis and widely involved in the occurrence of an inflammatory response [Citation3]. The classical pyroptosis pathway is to recognize danger signals through nucleotide binding oligomerization domains like receptor protein-3 (NLRP3), which further causes the activation of cysteine aspartate specific protein-1 (Caspase-1) [Citation4]. On the one hand, the activated Caspase-1 cleaves the downstream gasdermin D protein (GSDMD) and forms 1–2 mm pores on the cell membrane; on the other hand, it causes the maturation and release of inflammatory factor precursors such as Interleukin 1β (IL-1β) and Interleukin-18 (IL-18), which further amplifies the cascade inflammatory response [Citation5,Citation6]. Moderate pyroptosis can maintain the homeostasis of the internal environment, but if it is continuously over activated, it will lead to immune imbalance and excessive inflammatory reactions, and lead to the injury of tissues and organs and the occurrence of various diseases to a certain extent [Citation7,Citation8].
Previous studies have shown that maternal inflammation and the combined action of multiple cytokines play an important role in the imbalance of insulin resistance [Citation9,Citation10]. There are few studies on the levels of the serum NLRP3 inflammasome along with its effector molecules, Caspase-1, IL-1β, and IL-18 and their relationship with insulin resistance in pregnant women with hyperglycemia. Therefore, the main purpose of this study is to analyze the levels of serum NLRP3 along with its effector molecules, in PGDM pregnant women, GDM pregnant women and NGT pregnant women, and to explore the relationship between the levels of serum NLRP3 along with its effector molecules and insulin resistance and pregnancy outcomes. It provides a new research direction for the mechanism of hyperglycemia during pregnancy, which is of great significance for predicting the occurrence of adverse pregnancy outcomes.
Materials and methods
Study population
A total of 265 pregnant women with single pregnancy who were periodically examined and delivered in the Third Affiliated Hospital of Zhengzhou University from June 2021 to June 2022 were randomly divided into the normal glucose tolerance (NGT) group (123 cases), GDM group (82 cases) and PGDM group (60 cases) randomly. (1) Inclusion criteria: ①Carry out perinatal health care in our hospital system; ②Blood glucose was normal during early filing (11–13 + 6 weeks); ③18 years old < The age of pregnant women ≤ 35 years old; ④Singleton pregnancy; No gestational hypertension, gestational thyroid disease or other gestational diseases; ⑤The basic and clinical data of pregnant women are complete; ⑥Able to cooperate with follow-up, high compliance. (2) Exclusion criteria: ①Suffering from hypertension and thyroid disease before pregnancy; ②Taking drugs that affect blood glucose metabolism during pregnancy, such as glucocorticoids; ③Complicated with serious heart, lung, liver, kidney, and other important organ diseases or benign and malignant tumors; ④Hepatitis B, Acquired Immune Deficiency Syndrome (AIDS) and other blood transmitted diseases and acute and chronic infectious diseases in the near future; ⑤Combined with autoimmune diseases, polycystic ovary syndrome, obese patients, assisted reproductive technology pregnancy, etc. (3) Diagnostic criteria: According to the guidelines for diagnosis and treatment of hyperglycemia during pregnancy [Citation1], the blood glucose levels of fasting 75 g Oral Glucose Tolerance Test (OGTT), 1 h and 2 h after taking sugar at 24–28 weeks of pregnancy were at 5.1, 10.0, and 8.5 mmol/L, respectively, and any one blood sugar value reached or exceeded the corresponding boundary values, which could be diagnosed as GDM. PGDM was diagnosed as fasting plasma glucose ≥7.0 mmol/L or blood glucose ≥ 11.1 mmol/L 2 h after taking sugar among pregnant women with undiagnosed pre-pregnancy and whose blood glucose was found to meet these criteria in mid-pregnancy (24–28 weeks).
Pregnant women with diagnosed GDM and PGDM were given medical nutrition treatment and exercise guidance immediately, and blood glucose monitoring education was carried out. Insulin was used in pregnant women who could not effectively control their blood sugar through diet and exercise alone. Blood glucose control standard during pregnancy [Citation1]: The fasting plasma glucose level is ≤5.3 mmol/L, and HbA1c < 5.5% for GDM. The fasting blood glucose was controlled at 3.3–5.6 mmol/L, and HbA1c <6.0% for PGDM. This study was approved by the ethics committee of our hospital (Ethics number: 2021-056-01), obtained the patient’s informed consent and signed the informed consent form.
Specimen collection
After obtaining informed consent, about 5 ml of elbow vein blood was drawn from pregnant women on a fasting at 24–28 weeks of gestation (in the second trimester). The blood was naturally coagulated at room temperature for 10–20 min and centrifuged at 3000 r·min−1 for 20 min. The serum was separated, transferred to a cryopreservation tube and stored in a − 80 °C freezer for standby.
Detection of the serum NLRP3 along with its effector molecules
NLRP3, Caspase-1, IL-1β, and IL-18 in serum were determined by ELISA. The kit was purchased from Jiangsu Mei Mian Industry Limited Company and all operations were carried out in strict accordance with the instructions of the kit. The kit catalog numbers are as follows: ①NLRP3:MM-2198H1;②caspase-1:MM-13398H1;③IL-1β:MM-0181H1; and ④IL-18:MM-0139H1.The levels of fasting plasma glucose (FPG) and fasting insulin (FIN) were detected by automatic electrochemiluminescence immunoanalyzer, and the insulin resistance index (HOMA-IR) was calculated and analyzed with homeostasis model assessment. The calculation formula is HOMA‐IR = FPG × FINS/22.5 [Citation11].
Observation items
The basic clinical data of the pregnant women were collected, including age, gravidity, pre-pregnancy Body Mass Index (BMI = weight (kg)/height (m)2), etc.; The results of OGTT at 24–28 weeks of gestation, weight gain during pregnancy, mode of delivery, gestational weeks of delivery, and neonatal birth weight were recorded; The pregnancy outcomes studied in this paper include pregnancy complications and perinatal complications. The incidence of hypertensive disorders of pregnancy, premature rupture of membranes, premature delivery, macrosomia, and neonatal hypoglycemia were recorded. The above diagnoses are based on the diagnostic criteria of the ninth edition of Obstetrics and gynecology [Citation12].
Statistical analysis
All the normally distributed data were expressed as the mean ± SD. Differences among multiple groups were examined by a one-way ANOVA followed by a Bonferroni post hoc test. The Chi-square test was used to test the significance of ratio and percentage data. The Pearson correlation analysis was used to evaluate the association between the indicators. The receiver operator characteristic curve (ROC) was used to evaluate the predictive value of serum NLRP3 along with its effector molecules on pregnancy outcomes. The value was 0.05 as α standard test, p values < .05 were considered to be statistically significant. Statistical tests were performed using SPSS26.0 Statistical Software.
Results
Comparison of clinical data of three groups of pregnant women
There was no significant difference in age, gravidity, pre-pregnancy BMI and gestational weeks among the three groups (p > .05) ().
Table 1. Comparison of clinical data of three groups of pregnant women ( ± s).
Comparison of serological indexes among three groups of pregnant women: OGTT results, FIN, HOMA-IR and NLRP3 inflammasome along with its effector molecules
FPG levels, 1h and 2h after sugar ingestion, as well as fasting insulin, HOMA-IR, NLRP3, Caspase-1, IL-1β, and IL-18 were increased in GDM and PGDM compared to the NTG group (; p < .05), with the PGDM group presenting even higher levels than the GDM (; p < .05).
Table 2. Comparison of serological indexes among three groups of pregnant women( ± s).
Correlation analysis of NLRP3 along with its effector molecules with blood glucose and insulin resistance
The levels of NLRP3, Caspase-1, IL-1β, and IL-18 in pregnant women with hyperglycemia were positively correlated with FPG, FIN, HOMA-IR, blood glucose at 1h and 2h after taking sugar (r > 0, p<.05) ().
Table 3. Correlation analysis of NLRP3 along with its effector molecules with blood glucose and insulin resistance in the second trimester of pregnancy.
Comparison of pregnancy outcomes among three groups of pregnant women
There were significant differences in weight gain during pregnancy, blood glucose level before delivery, HbA1c level and gestational weeks of delivery among the three groups (p < .05), but no significant difference in neonatal birth weight among the three groups (p > .05). The incidence rates of cesarean section, premature delivery, hypertensive disorders of pregnancy, premature rupture of membranes, neonatal hypoglycemia and macrosomia in the GDM group and PGDM group were significantly higher than those in the NGT group (p < .05) ().
Table 4. Comparison of pregnancy outcomes among three groups of pregnant women.
Predictive value of serum NLRP3 along with its effector molecules in the second trimester of pregnancy for adverse pregnancy outcomes
Predictive value of NLRP3 along with its effector molecules in the second trimester for hypertensive disorders of pregnancy. By drawing ROC curve, the predictive value of serum NLRP3 along with its effector molecules for hypertensive disorders of pregnancy was evaluated. The combination of NLRP3 + Caspase-1 + IL-1β + IL-18 has high sensitivity and specificity, the AUC was 0.84 (95% CI 0.79–0.88, p < .001).; The best cutoff values of NLRP3, Caspase-1, IL-1β, and IL-18 in predicting hypertensive disorders of pregnancy were 575.43 pg/ml, 51.35 pg/ml, 48.56 pg/ml, and 65.35 pg/ml ( and ).
Figure 1. ROC curve of serum NLRP3 along with its effector molecules in the second trimester of pregnancy outcomes.
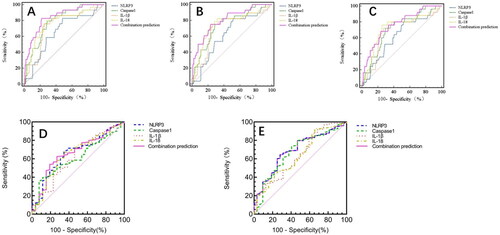
Table 5. Predictive value of NLRP3 along with its effector molecules in the second trimester for pregnancy outcomes.
Predictive value of serum NLRP3 along with its effector molecules in premature rupture of membranes. By drawing ROC curve, the predictive value of serum NLRP3 along with its effector molecules for premature rupture of membranes was evaluated. The predictive value of combination of NLRP3 + Caspase-1 + IL-1β + IL-18 was the highest, the AUC was 0.81 (95% CI 0.75–0.85, p < .001); NLRP3 and IL-18 has the highest sensitivity of 82.14%. The specificity of combined prediction of NLRP3 + Caspase-1 + IL-1β + IL-18 was 76.79%; The best cutoff values of NLRP3, Caspase-1, IL-1β, and IL-18 in predicting premature rupture of membranes were 566.28 pg/ml, 47.24 pg/ml, 50.51 pg/ml, and 56.17 pg/ml ( and ).
Predictive value of NLRP3 along with its effector molecules in premature delivery. By drawing ROC curve, the predictive value of serum NLRP3 along with its effector molecules for premature delivery was evaluated. The AUC of combination of NLRP3 + Caspase-1 + IL-1β + IL-18 was 0.76 (95% CI 0.70–0.81, p < .001); NLRP3 has the highest sensitivity of 80.00%, but the specificity of 47.92% was lowest. The combination of NLRP3 + Caspase-1 + IL-1β + IL-18 has high specificity; The best cutoff values of NLRP3, Caspase-1, IL-1β, and IL-18 in predicting premature delivery were 576.68 pg/ml、45.69 pg/ml、49.87 pg/ml, and 57.38 pg/ml ( and ).
Predictive value of NLRP3 along with its effector molecules in neonatal hypoglycemia. By drawing ROC curve, the predictive value of serum NLRP3 along with its effector molecules for neonatal hypoglycemia was evaluated. The AUC of combination of NLRP3 + Caspase-1 + IL-1β + IL-18 was 0.67 (95% CI 0.56–0.77, p = .005); Caspase-1 has the highest sensitivity of 92.00%, but the specificity of 35.10% was lowest. The best cutoff values of NLRP3, Caspase-1, IL-1β, and IL-18 in predicting premature delivery were 613.40 pg/ml, 30.14 pg/ml, 49.59 pg/ml, and 55.52 pg/ml ( and ).
Predictive value of NLRP3 along with its effector molecules in macrosomia. By drawing ROC curve, the predictive value of serum NLRP3 along with its effector molecules for premature delivery was evaluated. The AUC of combination of NLRP3 + Caspase-1 + IL-1β + IL-18 was 0.72 (95% CI 0.63–0.81, p < .001); IL-18 has the highest specificity of 92.70%. The combination of NLRP3 + Caspase-1 + IL-1β + IL-18 has high sensitivity; The best cutoff values of NLRP3, Caspase-1, IL-1β, and IL-18 in predicting premature delivery were 576.68 pg/ml, 45.69 pg/ml, 49.87 pg/ml, and 57.38 pg/ml ( and ).
Discussion
In this prospective longitudinal study, we had evaluated serum NLRP3, Caspase-1, IL-1β, and IL-18 levels of pregnant women in mid-second trimester, and followed-up maternal and neonatal outcomes, to test whether these metabolic markers can be useful predictors for adverse pregnancy outcomes. Our study found that the levels of serum NLRP3, Caspase-1, IL-1β, and IL-18 of pregnant women with hyperglycemia during pregnancy were higher than those in NGT group in the second trimester, and the levels of pregnant women in PGDM group was the highest. Increased serum NLRP3 along with its effector molecules are associated with the levels of insulin resistance in patients and may even affect the subsequent development of adverse pregnancy outcomes.
At the same time, our research found that in the serum of pregnant women with hyperglycemia during pregnancy, serum NLRP3, Caspase-1, IL-1β, and IL-18 were positively correlated with fasting blood glucose, blood glucose at 1h, blood glucose at 2h, fasting insulin and HOMA-IR, which were independent risk factors of insulin resistance, suggesting that serum NLRP3 along with its effector molecules may promote insulin resistance. Previous study [Citation13] found that NLRP3 levels were significantly higher in T2DM patients than in normal and prediabetic groups, and there was a correlation between NLRP3 and IL-18 levels, which is similar to our study. Jia et al. [Citation14] found that NLRP3 inflammasome activation inhibits insulin-stimulated glucose uptake in hepatocytes by blocking IRS signaling. Jourdan et al. [Citation15] found that endogenous cannabinoids can activate NLRP3 inflammasome, thereby activating Caspase-1, aggravating insulin resistance and leading to abnormal glucose tolerance. While Healy et al. [Citation16] found that supplementation with a casein hydrolysate reduce NLRP3 inflammasome mediated IL-1β secretion and improve insulin resistance. Youm et al. [Citation17] have studied that NLRP3 promotes islet fibrosis and increases insulin resistance in mice. However, knockout of NLRP3 gene in mouse pancreas can protect islet cells from death caused by long-term high-fat diet, significantly increase islet volume and improve glucose metabolism and insulin sensitivity. The possible mechanisms by which cell focal death factors promotes insulin resistance are: (1) Activated NLRP3 increases the release of IL-1β and IL-18, which can inhibit the expression of a variety of glucose transporters (GLUT) and transduction of insulin signal, leading to the occurrence of maternal insulin resistance [Citation15,Citation18,Citation19]. (2) Studies have shown that IL-1β induced the release of inflammatory factors through triggering nuclear transcription factors-κB (NF-κB), mitogen activated protein kinases (MAPK), and Fas signaling pathway [Citation20]. And IL-18 induced the release of inflammatory factors mainly through MAPK, STAT3, PI3K/Akt, NF-κB, and other pathways [Citation21–23]. We speculate that the chronic inflammatory state in pregnant women with hyperglycemia may be caused by the activation of inflammatory related pathways induced by pyroptosis mechanism, which aggravates insulin resistance.
In this study, the incidence rates of hypertensive disorders of pregnancy, premature delivery, premature rupture, neonatal hypoglycemia and macrosomia of membranes in GDM group and PGDM group were significantly higher than those in NGT group. Yang and Zhang [Citation24] found that pyroptosis is involved in the occurrence and development of preeclampsia, NLRP3 and IL-1β are overexpressed in the placenta of pregnant women with preeclampsia. Ingrid et al. [Citation25] demonstrated that the expression of NLRP3, Caspase-1, IL-1β, and IL-18 in the peripheral blood of preeclampsia pregnant women was significantly higher than that of normal pregnant women. It may be through the activation of NLRP3 inflammasome to induce the pyroptosis of placental trophoblast, release inflammatory factors, aggregate immunocyte, amplify the inflammatory response and damage vascular endothelial cells.
On the one hand, the inflammatory factors produced by pyroptosis act on the fetal membrane, making the fetal membrane edema and brittle, and finally the fetal membrane breaks down; On the other hand, excessive pyroptosis can also increase the release of high mobility probability box-1 protein (HMGB-1). Cui et al. [Citation26] found that overexpression of HMGB-1 is related to the occurrence and development of premature rupture of membranes. Pregnant women with premature rupture of membranes may also be that the fetal membrane cells, when stimulated by pathogen infection or other dangerous signals, start the pyroptosis pathway, activate Caspase-1, promote the maturation of IL-1β, and act on the gasdermin protein (GSDMD) to perforation the fetal membrane, resulting in swelling and dissolution of fetal membrane cells and rupture of fetal membrane. It may also be caused by the release of a large number of extracellular inflammatory cytokines, such as IL-1β, which stimulates matrix metalloproteinases and leads to the degradation of extracellular matrix [Citation27]. The study found that compared with those of pregnant women without placental lesions, the levels of NLRP3 and activated Caspase-4 in the amniotic membrane of spontaneous preterm pregnant women with acute chorioamnionitis were increased. The increased complex formation of ASC/Caspase-1 is involved in the release of IL-1β and IL-18 and induces chorionic membrane pyroptosis [Citation28]. These pieces of evidence suggest that pyroptosis may also be involved in the pathological inflammation of spontaneous preterm birth.
Our study showed that NLRP3 along with its effector molecules are associated with adverse pregnancy outcomes. So, the ROC curve was drawn to evaluate the predictive value of serum NLRP3, Caspase-1, IL-1β, and IL-18 in the second trimester on perinatal outcomes. It was found that serum NLRP3 along with its effector molecules had certain predictive value on pregnancy induced hypertensive disorders, premature rupture of membranes premature delivery, neonatal hypoglycemia, and macrosomia.
The sensitivity and specificity of combined prediction of NLRP3, Caspase-1, IL-1β, and IL-18 of hypertensive disorders is the highest, with 82.76% and 77.97%, and the area under the ROC curve is the highest, with 0.84. The specificity of combined prediction of premature rupture of membranes and preterm delivery are both the highest, respectively, with 76.79% and 76.25%, the area under the ROC curve is also the highest, with 0.81and 0.76. The AUCs of NLRP3 along with its effector molecules in jointly predicting neonatal hypoglycemia and macrosomia are, respectively, 0.67 and 0.72. Samuel Parry et al. demonstrated the ROC curve area of serum placental protein levels, including vascular endothelial growth factor, endoglin, soluble fms-like tyrosine kinase-1, and alpha-fetoprotein et al. in the second trimester to predict adverse pregnancy outcomes was relatively low, in the range of 0.50–0.64 [Citation29]. The study revealed that the mid-pregnancy HbA1c level was associated with various adverse pregnancy outcomes. Compared with the HbA1c category of 4.5–4.9%, higher HbA1c levels were significantly associated with increased risks of Hypertensive disorders of pregnancy, preterm delivery, low birth weight, and macrosomia (OR ranges of 1.20–9.98, 1.31–5.16, 0.89–4.10, and 2.22–27.86, respectively; all trend p values <.05) [Citation30]. While Carlsen et al. found that Per unit HbA1c within the top quartile, preterm delivery increased by 14%, preeclampsia increased by 20% [Citation31]. Our study suggests mid-trimester NLRP3 along with its effector molecules can serve as clinical biomarkers to predict adverse pregnancy outcomes, and the joint prediction effect is the best.
In conclusion, the levels of serum NLRP3, Caspase-1, IL-1β, and IL-18 of pregnant women with hyperglycemia are increased and may participate in the occurrence of insulin resistance by increasing the chronic inflammatory process in pregnant women; Moreover, serum NLRP3 along with its effector molecules may increase the risk of adverse pregnancy outcomes in pregnant women with diabetes. To the best of our knowledge, this is the first study to assess serum levels of NLRP3 along with its effector molecules, such as Caspase-1, IL-1β, and IL-18, in pregnant women with varying levels of glycemia. We studied the serum levels cytokine of pyroptosis in mid-pregnancy hyperglycemia and their relationship with insulin resistance and provided a new research direction for the mechanism of gestational diabetes mellitus. It is of great significance to intervene and predict the occurrence of adverse pregnancy outcomes by investigating the relationship between gestational diabetes mellitus and pyroptosis. Limitations of our study must be acknowledged. Further basic experiment is warranted to determine whether the activation of the NLRP3 inflammasome can lead to insulin resistance and adverse pregnancy outcomes. The question of whether reducing NLRP3 inflammasome levels will improve adverse pregnancy outcomes also needs to be explored in the future.
Ethics approval
This study was approved by the ethics committee of our hospital (Ethics number: 2021-056-01).
Consent form
This study obtained the patient’s informed consent and signed the informed consent form.
Author contributions
NH acted as guarantor and significantly contributed to the manuscript. HZ, ZY, XC, and YC collect and analyzed the data. NH drafted the manuscript. All the authors contributed to the critical revision of the manuscript for important intellectual content and approved the final version.
Acknowledgments
We thank all the participants who participated in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this research are available on request from the corresponding author.
Additional information
Funding
References
- Obstetrics Group, Division of Obstetrics and Gynecology, Chinese Medical Association, Division of Perinatal Medicine, China Maternal and Child Health Association. Guidelines for the diagnosis and treatment of gestational hyperglycemia. Chin J Obstet Gynecol. 2022;57(1):1–9.
- Yu XY, Wu XF, Qi HB. Interpretation of the key points of the Queensland health organization guidelines for gestational diabetes (2021 version). Chin J Pract Gynecol Obstet. 2021;37(9):933–936.
- Wei X, Xie F, Zhou X, et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19(9):971–992. doi: 10.1038/s41423-022-00905-x.
- Xu J, Núñez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem Sci. 2023;48(4):331–344. doi: 10.1016/j.tibs.2022.10.002.
- Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19(1):107–120. doi: 10.1038/cdd.2011.96.
- Hu YC, Yang S. Advances in the study of cell pyroptosis. J Nanjing Med Univ Sci. 2021;41(8):1245–1251.
- Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. doi: 10.1111/imr.12534.
- Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46(2):269–280. doi: 10.1002/eji.201545839.
- Pragnesh M, Kaplan MJ. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin Immunol. 2017;185(185):59–73. doi: 10.1016/j.clim.2016.08.010.
- Lu F, Lan Z, Xin Z, et al. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J Cell Physiol. 2020;235(4):3207–3221. doi: 10.1002/jcp.29268.
- Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am. 2019;48(3):479–493. doi: 10.1016/j.ecl.2019.05.001.
- Xie X, Kong BH, Duan T. Obstetrics gynecology. Version 9. Beijing: People’s Medical Publishing House; 2018.
- Alfadul H, Sabico S, Ansari MGA, et al. Differences and associations of NLRP3 inflammasome levels with interleukins 1α, 1β, 33 and 37 in adults with prediabetes and type 2 diabetes mellitus. Biomedicines. 2023;11(5):1315. doi: 10.3390/biomedicines11051315.
- Jia X, Qiu T, Yao X, et al. Arsenic induces hepatic insulin resistance via mtROS-NLRP3 inflammasome pathway. J Hazard Mater. 2020;399:123034. doi: 10.1016/j.jhazmat.2020.123034.
- Jourdan T, Godlewski G, Cinar R, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19(9):1132–1140. doi: 10.1038/nm.3265.
- Healy NP, Kirwan AM, McArdle MA, et al. A casein hydrolysate protects mice against high fat diet induced hyperglycemia by attenuating NLRP3 inflammasome-mediated inflammation and improving insulin signaling. Mol Nutr Food Res. 2016;60(11):2421–2432. doi: 10.1002/mnfr.201501054.
- Youm YH, Adijiang A, Vandanmagsar B, et al. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152(11):4039–4045. doi: 10.1210/en.2011-1326.
- Liu P, Zhang Z, Wang J, et al. Empagliflozin protects diabetic pancreatic tissue from damage by inhibiting the activation of the NLRP3/caspase-1/GSDMD pathway in pancreatic β cells: in vitro and in vivo studies. Bioengineered. 2021;12(2):9356–9366. doi: 10.1080/21655979.2021.2001240.
- Zhang J, Chi H, Xiao H, et al. Interleukin 6(IL-6) and tumor necrosis factor α (TNF-α) single nucleotide polymorphisms (SNPs), inflammation and metabolism in gestational diabetes mellitus in Inner Mongolia. Med Sci Monit. 2017;23:4149–4157. doi: 10.12659/msm.903565.
- Tran HT, Liong S, Lim R, et al. Resveratrol ameliorates the chemical and microbial induction of inflammation and insulin resistance in human placenta, adipose tissue and skeletal muscle. PLoS One. 2017;12(3):e0173373. doi: 10.1371/journal.pone.0173373.
- Park YJ, Warnock GL, Ao Z, et al. Dual role of IL-1β in islet amyloid formation and its β-cell toxicity: implications in type 2 diabetes and islet transplantation. Diabetes Obes Metab. 2017;19(5):682–694. doi: 10.1111/dom.12873.
- Chen X, Zhang D, Li Y, et al. NLRP3 inflammasome and IL-1beta pathway in type 2 diabetes and atherosclerosis: friend or foe?. Pharmacol Res. 2021;173:105885. doi: 10.1016/j.phrs.2021.105885.
- Alboni S, Montanari C, Benatti C, et al. Interleukin 18 activates MAPKs and STAT3 but not NF-κB in hippocampal HT-22 cells. Brain Behav Immun. 2014;40:85–94. doi: 10.1016/j.bbi.2014.02.015.
- Yang Y, Zhang H. TXNIP activates NLRP3 inflammasome in the pathogenesis of preeclampsia. J Chongqing Med Univ. 2016;41(7):658–662.
- Ingrid CW, Mariana RV, Mariana LM, et al. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J Reprod Immunol. 2017;123:40–47.
- Cui SH, Shen LN, Zhi YX, et al. Expression and significance of HMGB-1 and MMP-9 in fetal membranes of patients with premature rupture of chorioamnionitis membranes. Adv Modern Obstet Gynecol. 2016;25(8):582–585.
- Xiao WX, Wang HZ, Zhou GJ, et al. Study on the correlation between premature rupture of membranes and fetal membrane cell coke death. Chinese J Reproduct Health. 2019;30(3):281–283.
- Gomez-Lopez N, Romero R, Xu Y, et al. A role for the inflammasome in spontaneous preterm labor with acute histologic chorioamnionitis. Reprod Sci. 2017;24(10):1382–1401. doi: 10.1177/1933719116687656.
- Parry S, Carper BA, Grobman WA, et al. Placental protein levels in maternal serum are associated with adverse pregnancy outcomes in nulliparous patients. Am J Obstet Gynecol. 2022;227(3):497.e1–497.e13. doi: 10.1016/j.ajog.2022.03.064.
- Ho YR, Wang P, Lu MC, et al. Associations of mid-pregnancy HbA1c with gestational diabetes and risk of adverse pregnancy outcomes in high-risk Taiwanese women. PLoS One. 2017;12(5):e0177563. doi: 10.1371/journal.pone.0177563.
- Carlsen EØ, Harmon Q, Magnus MC, et al. Glycated haemoglobin (HbA1c) in mid-pregnancy and perinatal outcomes. Int J Epidemiol. 2022;51(3):759–768. doi: 10.1093/ije/dyab270.

