Abstract
Objective
There is uncertainty around the safety of SSRIs for treating depression during pregnancy. Nevertheless, the use of SSRIs has been gradually increasing, especially during the COVID-19 pandemic period. We aimed to (1) characterize maternal depression rate and use of SSRIs in a recent 10-year period, (2) address confounding by indication, as well as socioeconomic and environmental factors, and (3) evaluate associations of the timing of SSRI exposure in pregnancy with risk for preterm birth (PTB), low birthweight (LBW), and small for gestational age (SGA) infants among women with depression before pregnancy.
Methods
We conducted propensity score-adjusted regression to calculate odds ratios (ORs) of PTB, LBW, and SGA. We accounted for maternal/pregnancy characteristics, comorbidity, depression severity, time of delivery, social vulnerability, and rural residence.
Results
There were 50.3% and 40.3% increases in the prevalence rate of prenatal depression and prenatal SSRI prescription rate during the pandemic. We identified women with depression ≤180 days before pregnancy (n = 8406). Women with no SSRI order during pregnancy (n = 3760) constituted the unexposed group. The late SSRI exposure group consisted of women with an SSRI order after the first trimester (n = 3759). The early-only SSRI exposure group consisted of women with SSRI orders only in the first trimester (n = 887). The late SSRI exposure group had an increased risk of PTB of OR = 1.5 ([1.2,1.8]) and LBW of OR = 1.5 ([1.2,2.0]), relative to the unexposed group. Associations between late SSRI exposure and risk of PTB/LBW were similar among a subsample of patients who delivered during the pandemic.
Conclusions
These findings suggest an association between PTB/LBW and SSRI exposure is dependent on exposure timing during pregnancy. Small for gestational age is not associated with SSRI exposure.
Introduction
Depression is one of the most common comorbidities women experience during pregnancy or the postpartum period, with rates ranging from 10% to 24% in the United States (US) in 2018, varying by state, according to the CDC report [Citation1]. The risk of maternal depression has significantly increased during the COVID-19 pandemic period [Citation2,Citation3], and depression is a common symptom of long COVID [Citation4,Citation5]. According to an umbrella review of systemic reviews and meta-analyses up to 2023, which reported on perinatal depression during the COVID-19 pandemic, the global pooled prevalence rate was 29% for prenatal depression and 26% for postpartum depression [Citation3]. In 2013, 6–8% of pregnant women in the US took antidepressants [Citation6–11]. With the rising rate of maternal depression and some studies suggesting a potentially reduced risk of COVID-19 severity associated with SSRIs and SNRIs [Citation12,Citation13], we suspect more prevalent use of antidepressants among pregnant patients.
Despite the high prevalence of prenatal antidepressant use, its safety during pregnancy is not fully understood. There is little consensus on the association between antidepressants and preterm birth (PTB; birth prior to 37 weeks gestation). PTB is a key contributor to perinatal morbidity and mortality in developed and developing countries and occurs in 10% of all live births in the US [Citation14–16]. While studies of varying size and design have reported results supporting the association between antidepressant use and the risk of PTB and related adverse outcomes [Citation8,Citation17–27], findings have been inconsistent, and concerns have been raised about potential confounding-by-indication due to inadequate control for underlying depression [Citation24,Citation28–30]. Additionally, the timing of antidepressant exposure may influence PTB risk [Citation8,Citation17,Citation18,Citation31,Citation32].
Furthermore, uncertainty around the safety of prenatal antidepressant use has potentially led to a high discontinuation rate or reduction in dosage before the second trimester of pregnancy [Citation7,Citation8,Citation25,Citation33]. SSRIs are the most common class of antidepressants [Citation8,Citation18]. This study aims to evaluate the risk of PTB and other relevant adverse outcomes, low birthweight (LBW) and small for gestational age (SGA) infants, following SSRI use during the second or third trimester. To minimize confounding-by-indication, we conducted the study among women with depression onset before the pregnancy. We comprehensively addressed the influences of confounding factors, including pregnancy characteristics, comorbidities, and environmental factors based on the geographical region where the patient resides.
The influence of the timing of SSRI intake was characterized by distinguishing SSRI exposure groups based on exposure status after the first trimester. Multiple sensitivity analyses were performed to assess the influence of the COVID-19 pandemic period, prenatal depressive symptoms, other classes of antidepressants, and misclassification of prescription records. Additionally, SSRI drug-specific analyses and additional analyses on the other classes of antidepressants were conducted.
Methods
Study setting and study population
We conducted a retrospective study among women who delivered between 1 January 2013 and 31 December 2022 (n = 543,408) at Providence St. Joseph Health (PSJH) across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington. Figure S1 shows the study population selection. We selected mothers who were continuously enrolled from 180 days before the last menstrual period (LMP) to the date of delivery. We confirmed the date of delivery was at least 20 weeks apart from the LMP and “living” delivery status of the baby. GA was limited to 20 weeks or greater because ascertainment bias is particularly high for EHR data earlier in pregnancy. We limited our cohort to singleton pregnancies. From this source population (n = 365,075), women with depression onset before pregnancy were selected to avoid confounding-by-indication (n = 8816). We first identified women diagnosed with depression any time before the pregnancy. From this population of women with a history of depression, we selected women with a diagnosis of depression or any antidepressant prescription order during the six-month pre-pregnancy period (LMP-180 days ∼ LMP). Women with bupropion or trazodone prescriptions and no other antidepressants were excluded unless they had a diagnosis of depression because these medications are often prescribed for smoking cessation or insomnia [Citation34,Citation35]. We excluded women with a diagnosis of psychosis or bipolar disorder during the two-year pre-pregnancy period (LMP-730 days ∼ LMP) and fetal sex of “other” category. The final analytic population consisted of 8406 women.
Measures
Outcome
Our primary outcome was PTB, determined by gestational age at birth (GA; GA < 37 weeks). Gestational age at birth was a proxy outcome of PTB, calculated based on the expected date of delivery and actual delivery date. Secondary outcomes were LBW (birthweight <2500 g) and SGA (birthweight <10th percentile of the same gestational age).
Exposure
The main exposure of interest was SSRI exposure after the first trimester (GA ≥ 13 weeks). The secondary exposure of interest was SSRI exposure only in the first trimester (Figures S2 and S3). Study participants were divided into three groups based on SSRI exposure status during pregnancy. The control group (no SSRI exposure) was women with no SSRI prescribed during the pregnancy. The late SSRI exposure group was women who had any SSRI order after the first trimester. The early-only exposure group was of women with SSRI orders only in the first trimester.
Covariates
We extracted information on maternal characteristics, pre-pregnancy/prenatal mental health conditions, and pre-pregnancy comorbidities from the EHR (Table S1). Pregnancy and maternal characteristics were collected during prenatal care or at the time of delivery. These included parity, preterm history, delivery year, fetal sex, age, race, ethnicity, insurance, pregravid body mass index (BMI) category, smoking status, illegal drug use status, ethanol consumption status, and Centers for Disease Control and Prevention Social Vulnerability Index (CDC-SVI). CDC-SVI represents the percentile ranking of each census tract on 15 social factors. Social factor themes include socioeconomic status, household composition, race/ethnicity/language, and housing/transportation [Citation36]. Pre-pregnancy mental health conditions were determined based on prescription orders (Table S2), clinical diagnoses (Table S2), and Patient Health Questionnaire-9 (PHQ-9) scores [Citation37,Citation38]. We collected the pre-pregnancy exposure status of N06AA, N06AB (SSRI), N06AF, N06AG, and N06AX six months before the pregnancy. We extracted diagnoses of adjustment disorders and anxiety disorders from the two-year pre-pregnancy period. Prenatal mental health was determined based on depression diagnosis and Patient Health Questionnaire-4 (PHQ-4) score during late pregnancy [Citation39,Citation40]. Other comorbid diagnoses (anemia, asthma, cardiovascular diseases, cystic fibrosis, chronic lung diseases, diabetes, renal diseases, leukemia, pneumonia, sepsis, and sickle cell diseases) were included when active during the two-year pre-pregnancy period (Table S2).
Statistical analyses
Descriptive statistics
We calculated the prevalence rate of maternal depression, use of antidepressants, and use of SSRI from 2013 to 2022 (). We also compared these rates of the pandemic (6 March 2020 to 31 December 2022) and control period (6 March 2017 to 31 December 2019). Descriptive statistics for maternal characteristics, outcomes, covariates, and prenatal mental health conditions are presented by exposure group (Table S3). We examined the distribution of covariates across exposure groups (no, early-only, late SSRI) and outcome groups (PTB and non-PTB). We used Chi-squared test for categorical variables. For continuous variables, we used one-way ANOVA test and Welch’s t-test. Multiple testing was corrected using Benjamin–Hochberg method (Table S4).
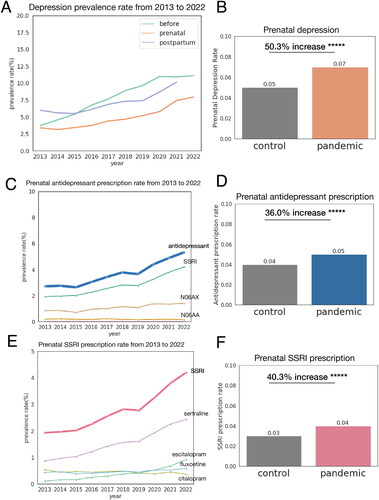
Figure 1. Increase in rate of maternal depression, prenatal antidepressant prescription, and prenatal SSRI prescription from 2013 to 2022. (A) Maternal depression rate from 2013 to 2022. Green line indicates prevalence rate of women who had depression diagnosis before the start of the pregnancy. Orange line indicates prevalence rate of prenatal depression. Blue line indicates prevalence of postpartum depression. As the definition of postpartum depression indicates depression up to one-year postpartum period, we did not evaluate the rate of postpartum depression of patients who delivered in 2022. (B) Comparison of prenatal depression rate in control period (6 March 2017 to 31 December 2019) and pandemic period (6 March 2020 to 31 December 2022). There was 50.3% increase in prenatal depression rate during pandemic period. (C) Prenatal antidepressant prescription rate from 2013 to 2022. Antidepressant with prescription rate below 0.01% is not displayed. (D) Comparison of prenatal antidepressant prescription rate in control period (6 March 2017 to 31 December 2019) and pandemic period (6 March 2020 to 31 December 2022). There was 36.0% increase in prenatal antidepressant prescription rate during pandemic period. (E) Prenatal SSRI prescription rate from 2013 to 2022. SSRI with prescription rate below 0.01% is not displayed. (F) Comparison of prenatal SSRI prescription rate in control period (6 March 2017 to 31 December 2019) and pandemic period (6 March 2020 to 31 December 2022). There was 36.0% increase in prenatal SSRI prescription rate during pandemic period. +p < .1; *p < .05; **p < .01; ***p < .001; ****p < .0001; *****p < .00001.
Main analysis
We used propensity score adjusted log-binomial and linear regression to calculate the odds ratio (OR) of PTB, LBW, and SGA and the difference in the mean of GA corresponding to exposure to SSRIs. We applied multivariable imputation for missing values. We selected the treatment group and calculated the propensity score using logistic regression for each comparison. Covariates of the main model included pregnancy and maternal characteristics, pre-pregnancy mental health conditions, and pre-pregnancy comorbid conditions as defined above.
We compared the late SSRI exposure group to the non-late, early-only, and no SSRI exposure groups (Figure S3). We compared the early-only SSRI group to the no SSRI exposure group. Additionally, any SSRI exposure group was compared with the no SSRI exposure group. These associations were further assessed as follows.
First, we subdivided the late SSRI exposure group into the late-only and both SSRI exposure groups to evaluate the effect of the late-only SSRI exposure (Figure S3). Both SSRI exposure groups consisted of women who had SSRI orders during and after the first trimester. Late-only SSRI exposure group consisted of women who had SSRI orders only after the first trimester. Second, drug-specific analyses were conducted on individual SSRI medications: citalopram, escitalopram, fluoxetine, paroxetine, sertraline, and fluvoxamine.
Sensitivity analysis
First, we evaluated the impact of the COVID-19 pandemic period (n = 3217). Second, the influence of late SSRI exposure was evaluated among women who had depression diagnoses during late pregnancy (n = 5368). This approach was adopted to adjust for the prenatal depression severity. We assumed women with severe prenatal depression symptoms were likely to have their depression diagnosis during pregnancy. Third, we assessed the impact of pre-pregnancy depression severity on a subsample of women who completed the PHQ-9 during the two-year pre-pregnancy period (n = 3532). Similarly, we assessed the impact of prenatal depression severity using the PHQ-4 score [Citation39,Citation40] (n = 3515). Fifth, we conducted an analysis by excluding women who were exposed to other categories of antidepressants (n = 6603). Finally, we restricted the analysis to women with more than one prescription during pregnancy (n = 5986).
Additional analysis
In addition, we examined the effect of other antidepressants (N06AA, N06AG, N06AF, N06AX) exposure on PTB, SGA, and LBW. This approach was to further distinguish associations resulting from SSRI and other antidepressant exposures. We additionally separated serotonin and norepinephrine reuptake inhibitor (SNRI) from N06AX, as it is the most common class of N06AX antidepressants and shares some similarities to SSRIs.
Results
Descriptive statistics
shows the trend in the prevalence rate of maternal depression and prenatal use of SSRIs from 2013 to 2022. There were 50.3% and 40.3% increases in prenatal depression and SSRI prescriptions during the COVID-19 pandemic period. Table S3 presents the descriptive statistics of covariates, outcomes, and maternal mental health. Of 365,075 women, 8406 were eligible as our cohort of interest (study participants, Figure S1). The study cohort was divided into three groups (Figures S2 and S3): 3760 women in the no SSRI exposure group, 887 women in the early-only SSRI exposure group, and 3759 women in the late SSRI exposure group. Among these three exposure groups, the late SSRI exposure group had the highest rate of PTB (11.7%), the no SSRI exposure had a rate of 9.1%, and the early-only SSRI exposure group had the lowest PTB prevalence rate (7.0%). Among the subpopulation with pre-pregnancy PHQ-9 score (n = 3532), PHQ-9 score was not significantly different across outcome groups (p = .8, Table S4).
Main analysis
Increased risk of preterm birth and low birthweight for late SSRI exposure
Late SSRI exposure was associated with elevated risk of PTB and LBW after controlling for confounders ( and Table S5). The ORs of PTB were 1.4 ([1.2,1.7], p < .0001), 1.5 ([1.2,1.8], p < .0001), and 1.4 ([1.1,1.9], p < .05) and those of LBW were 1.6 ([1.3,2.0], p < .0001), 1.5 ([1.2,2.0], p < .01), and 1.8 ([1.3,2.7], p < .01) when comparing late SSRI exposure group with no-late, no, and early-only SSRI exposure groups, respectively.
Figure 2. Increased risk of preterm birth and decreased gestational days at birth for women exposed to SSRI in second or third trimester. CI: confidence interval; GA: gestational age. The odds ratio of preterm birth and difference in the mean of GA at birth were calculated using log-binomial regression and linear regression adjusting for propensity score (top). Exposure groups were defined as follows. No: women with no SSRI exposure during pregnancy. Early only: women with SSRI exposure only in the first trimester. Late: women with SSRI exposure in the second or third trimester. Any: women with any SSRI exposure during pregnancy (early only + late). No-late: women with no SSRI exposure in the 2nd nor 3rd trimester (no + early only). This increased risk of the late SSRI exposure group was similar among subsample of patients who delivered during COVID-19 pandemic period (bottom). Each comparison (a–e) is defined in the table on the left. +p < .1; *p < .05; **p < .01; ***p < .001; ****p < .0001; *****p < .00001.
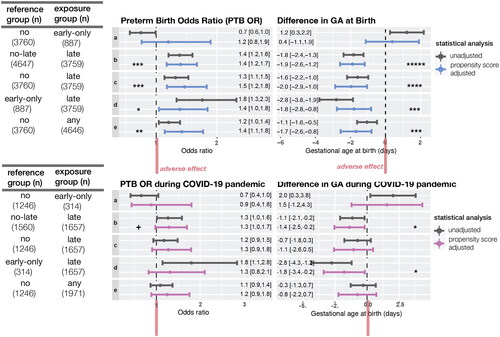
Early-only SSRI exposure was not associated with either PTB (OR = 1.2, [0.7,1.9]) and LBW (OR = 0.7, [0.4,1.3]). Women exposed to SSRI at any time during pregnancy had 1.4 ([1.1,1.8], p < .01) fold higher risk of PTB, 1.4 ([1.1,1.9], p < .01) fold higher risk of LBW than the unexposed to SSRI group ( and Table S5). Most (both SSRI exposure group, n = 3328, 88.5%) of the late SSRI exposure group took SSRIs throughout the pregnancy. The late-only SSRI exposure group (n = 431) had similar PTB and LBW risk (PTB OR = 1.1 [0.7,1.6]; LBW OR = 1.0 [0.6,1.7]) compared with both SSRI exposure group (Table S6).
Drug-specific analysis
In relation to PTB, drug-specific analyses demonstrated consistent results as the main SSRI analysis and remained statistically significant, except for paroxetine (Table S7). There was an increased risk of PTB with early-only exposure to paroxetine (OR = 2.7 [1.1,6.8]). In relation to LBW, all drugs showed similar results as the main SSRI analysis.
Sensitivity analysis
The strong and significant association between late SSRI exposures and PTB/LBW persisted throughout sensitivity analyses (Table S8).
Discussion
In this study, we characterized the prevalence rate of maternal depression and the prescription rate of antidepressants over a recent 10-year period. Our objective was to examine the relationship between the timing of prenatal SSRI exposure and three outcomes (PTB, LBW, and SGA) among women with a history of depression. We adjusted for confounding factors known to be associated with PTB, including parity, preterm history, maternal mental health, and comorbidities. Notably, we observed a 50.3% increase in the prevalence rate of prenatal depression and a 40.3% increase in prescription rate of prenatal SSRI prescription rate during the COVID-19 pandemic. We found that women prescribed SSRIs in late pregnancy were 1.5 times more likely to deliver preterm compared to women who were not prescribed SSRIs. These findings remained robust across several sensitivity analyses, which accounted for factors such as the COVID-19 pandemic period, depression severity and exposure to other categories of antidepressants (N06AA and N06AX).
Our observation of an increased rate of prenatal depression rate during the pandemic aligns with findings from previous studies [Citation2,Citation3,Citation41–44]. An observational study conducted on pregnant women during the pandemic in US Northern California reported that these patients were twice as likely to have possible depression (Edinburgh Postnatal Depression Scale [Citation45] score ≥11; EPDS) compared to those who were pregnant before the pandemic [Citation41]. In their study, King et al. [Citation41] identified key factors contributing to the rise in the prevalence rate of prenatal depression during the pandemic, categorizing them into objective adversity and subjective stress related to COVID-19. Objective adversity encompassed changes in financial and employment situations and disruptions in prenatal care. Subjective stress involved women’s concern and fear about the infection, along with a lack of support during labor and delivery. Our sensitivity analysis on a subsample of patients who delivered during the pandemic period aims to assess the impact of these collective factors on the association between SSRI exposure and the risk of PTB. The outcome of this sensitivity analysis closely paralleled that of the main analysis.
Our findings are consistent with several prior observational studies that have investigated the timing of prenatal antidepressant or SSRI-specific exposures and PTB [Citation8,Citation17,Citation19–21,Citation24,Citation25]. A systematic review and meta-analysis comprising 41 observational studies reported that second and third trimester antidepressant exposure increased the risk of PTB; notably, 22 (53.6%) of studies examined SSRI exposure [Citation17]. Additionally, a retrospective study on a similar-sized singleton pregnancy cohort covered by Tennessee Medicaid also suggested an association between second trimester antidepressant (SSRI or non-SSRI antidepressant) exposures and PTB, though no such association was found with first trimester exposures [Citation8].
In contrast with our study, some studies reported an elevated risk for PTB for first trimester SSRI or antidepressant (non-SSRI antidepressant) exposures [Citation18,Citation31,Citation32]. However, the first trimester exposure in their studies included women who continued the use throughout the pregnancy. Their definition of first-trimester exposure differs from ours, making it challenging to compare our findings with theirs directly. A population-based cohort study conducted in Finland reported a lower risk of PTB for women who purchased SSRIs during pregnancy; the risk of PTB was 16% lower even when accounting for the underlying condition [Citation46]. However, they compared women exposed to SSRIs to unexposed ones with psychiatric diagnoses during the pregnancy. Our study compared only those participants experiencing depression before the pregnancy.
The precise mechanisms of preterm labor and birth are not yet fully understood, making it challenging to speculate on the association between late-pregnancy SSRI exposure and an increased risk of PTB. However, insights from studies on animals suggest a potential link [Citation26]. When pregnant individuals take SSRI, it results in higher levels of serotonin in both the mother and the fetus. This increased serotonin concentration and elevated serotonin signaling rates likely induce vasoconstriction in uterine and placental blood vessels, consequently reducing blood flow to the fetus and placenta. These changes are associated with a smaller placental size/weight and a higher likelihood of PTB. Throughout pregnancy, serotonin metabolism undergoes changes, with fetal serotonin synthesis increasing as gestation progresses. This results in a decrease in placental serotonin synthesis and the transfer of fetal serotonin into the placenta. Considering the time-related changes in serotonin metabolism observed during a physiological pregnancy, variations in the timing of SSRI exposure may impact serotonin signaling metabolism related to pregnancy outcomes.
Surprisingly, pre-pregnancy depression severity, indicated by PHQ-9 scores, did not appear to be relevant to the risk of PTB. Among the patients who met our eligibility criteria for the analytic cohort, we collected the most recent PHQ-9 score before conception to reflect the patient’s pre-pregnancy depression severity. These findings are contrary to earlier studies suggesting a correlation between preconception stress, mental health, and PTB [Citation28,Citation47,Citation48]. However, it is important to note that our study was restricted to participants with a diagnosis of depression before the pregnancy. Therefore, within this group of patients who had depression before pregnancy, pre-pregnancy depression severity did not correlate with an increased risk of PTB. We speculate that the presence of pre-pregnancy depression may be a more significant contributor to PTB than the severity of depressive symptoms. One possible explanation for this result is the saturation of maternal stress-induced response. Maternal stress may induce a response, such as hypothalamic-pituitary-adrenal (HPA) axis dysfunction [Citation49,Citation50] or inflammatory immune response [Citation51], which potentially activates a mechanism leading to PTB. However, this response may saturate and plateau once stress hits the level of depression.
Strengths and limitations
In this study, we minimized confounding-by-indication by analyzing women with depression onset before pregnancy. Depression is an acknowledged risk factor for PTB [Citation52]. In our study, we aimed to differentiate the impact of the depression itself from the association between prenatal SSRI use and the risk of PTB. To achieve this, we limited our analytic cohort to include patients who were diagnosed with depression before pregnancy and showed indications of depression within six months before conception. Notably, pre-pregnancy depression severity was not correlated with an elevated risk of PTB. Furthermore, we conducted multiple sensitivity analyses to assess the impact of both pre-pregnancy and prenatal depression severity. Our key finding, the association between late SSRI exposure and an increased risk of PTB, remained robust across these sensitivity analyses. This strengthens our confidence that the depression did not confound our main finding.
We analyzed a large sample to provide sufficient statistical power to examine PTB, SGA, and LBW. This allowed observations of statistically significant results in subgroup analyses and sensitivity analyses. We applied rigorous sensitivity analyses to test the robustness of associations observed in the main analysis. We accounted for the COVID-19 pandemic, depression severities before and during the pregnancy, and exposure to antidepressant polytherapy. Many prior studies on the association between SSRI and PTB were limited due to lack of severity data [Citation8,Citation21,Citation31,Citation53–57]. We included the CDC social vulnerability index as a covariate. CDC uses U.S. Census data to calculate the social vulnerability of each census tract, a subdivision of counties [Citation36]. This index allows us to account for social disparity based on the location of residence. To our knowledge, this is the first study that accounted for social vulnerability when assessing the impact of SSRIs on the risk of adverse pregnancy outcomes. Additionally, we assessed SSRI drug-specific associations and N06AA/N06AX associations with PTB, SGA, and LBW. This helps us understand the magnitude of individual drug association with PTB, SGA, and LBW and to discern the contributions of other antidepressants (N06AA/N06AX).
A potential area for improvement in this study was the potential misclassification of exposures. Prescription orders may not necessarily indicate actual exposures. Non-differential misclassification of exposure biases toward the null potentially attenuates the strength of the association. This is an inherent limitation of EHR data; however, we assessed the impact of exposure misclassification in sensitivity analysis.
While our study benefited from a large sample size to assess the association between SSRI exposure and the elevated risk of PTB, this sample size was inadequate for stratified analyses or for drawing definitive conclusions from the stratified analyses we conducted. First, we faced limitations in the number of patients for whom depression severity was assessed. Utilizing standardized scoring systems such as the PHQ-9 and PHQ-4, we performed sensitivity analyses on a subpopulation that took the assessments (3532 out of 8406; 42%). However, the sample size was insufficient for stratified analyses based on the depression severity. Moving forward, stratified analysis based on pre-pregnancy and prenatal depression severity should be conducted to elucidate the impact of SSRI on PTB further. Such analyses would strengthen our findings on the association between late SSRI exposure and the risk of PTB, particularly if a similar relative risk of PTB was observed between these subgroups (non-severe and severe).
Second, a primary limitation of our analysis was the assumption of independence among individual pregnancy cases, disregarding potential correlations between multiple pregnancies within the same population. A stratified analysis will enhance the accuracy of our findings, acknowledging the unique characteristics of nulliparous pregnancies.
Third, we conducted a drug-specific analysis assessing different SSRI compounds, observing a contrary observation for paroxetine compared to the entire SSRI group. However, we refrained from an in-depth discussion on this observation due to the considerably smaller sample size of paroxetine compared to other SSRI compounds. The early-only and late paroxetine groups were comprised of 56 and 55 patients, respectively, while the sample size of other SSRI compound exposure groups ranged from hundreds to thousands. Instead, we presented the sample size, relative risk, and confidence interval, anticipating that this observation could be valuable for future meta-analysis studies.
Unfortunately, conducting a stratified analysis based on dosage was not feasible for this study. Our primary focus was to characterize the timing of SSRI exposure during pregnancy and its correlation with the risk of PTB. We faced limitations in sample size, preventing further categorizing our timing-specific exposure groups by dosage. Future retrospective or prospective studies that investigate the impact of the dosage on the association between SSRI exposure and risk of PTB would be valuable.
Conclusions
Among women with depression onset prior to pregnancy, SSRI exposure after the first trimester is associated with an increased risk for PTB and LBW. Although our findings suggest that there is an association between SSRI exposures after the first trimester of pregnancy and the risk of PTB and LBW, depression should not be left untreated during pregnancy. Untreated depression increases the chance of adverse pregnancy outcomes and high-risk health behaviors [Citation58,Citation59]. Both patients and physicians should be informed on both the risks and benefits of all treatment options during pregnancy to make optimal patient-specific decisions given the patient’s health conditions.
Author contributions
Yeon Mi Hwang, Samantha N. Piekos, Mary F. Hebert, Alison G. Paquette, Nathan D. Price, Leroy Hood, and Jennifer J. Hadlock contributed to study conception and design. Yeon Mi Hwang and Ryan T. Roper conducted data cleaning and transformation. Yeon Mi Hwang performed data analyses. Yeon Mi Hwang, Samantha N. Piekos, Daniel A. Enquobahrie, and Mary F. Hebert were involved in data interpretation. Jennifer J. Hadlock supervised study implementation. Yeon Mi Hwang prepared the manuscript with critical revision of the manuscript for important intellectual content provided by Samantha N. Piekos, Daniel A. Enquobahrie, Mary F. Hebert, Alison G. Paquette, Priyanka Baloni, and Jennifer J. Hadlock. All authors reviewed and approved the final version of the manuscript. Funding provided by Nathan D. Price.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. All procedures were reviewed and approved by the Institutional Review Board at the PSJH through expedited review (study number STUDY2020000196).
Consent form
Consent was waived because disclosure of protected health information for the study was determined to involve no more than a minimal risk to the privacy of individuals.
Supplemental Material
Download MS Word (341.3 KB)Acknowledgements
We thank PSJH for sharing their data engineering expertise and computational resources. We acknowledge SNOMED International for developing and maintaining SNOMED-CT©.
Preliminary results for SGA were orally presented at the 2022 Annual Meeting for Society for Reproductive Investigation, and the abstract was published in the proceedings. This manuscript constitutes one of the chapters of Yeon Mi Hwang’s PhD Dissertation.
Disclosure statement
YH, RTR, SNP, DAE, MFH, AGP, and PB declare no conflict of interest. JJH has received funding (paid to the Institute for Systems Biology) from Pfizer, Novartis, Janssen, Gilead, and Bristol Myers Squibb for research unrelated to this study or any of its findings. LH and NDP are scientific advisors for Sera Prognostics, a pregnancy diagnostics company, and NDP holds stock options. Sera Prognostics is not associated with this study or any of the findings.
Data availability statement
Results have been aggregated and reported within this paper to the extent possible while maintaining privacy from personal health information as required by law. Data are archived within Providence St. Joseph Health systems in a HIPAA-secure audited compute environment. All clinical logic for data extraction has been shared within the paper and supplemental materials. Code is publicly available at https://github.com/Hadlock-Lab/YH_SSRI_PTB.
Additional information
Funding
References
- Bauman BL, Ko JY, Cox S, et al. Vital signs: postpartum depressive symptoms and provider discussions about perinatal depression – United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(19):1–11. doi: 10.15585/mmwr.mm6919a2.
- Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022;35(20):4014–4021. doi: 10.1080/14767058.2020.1843155.
- Caffieri A, Gómez-Gómez I, Barquero-Jimenez C, et al. Global prevalence of perinatal depression and anxiety during the COVID-19 pandemic: an umbrella review and meta-analytic synthesis. Acta Obstet Gynecol Scand. 2023;103(2):210–224. doi: 10.1111/aogs.14740.
- Kyzar EJ, Purpura LJ, Shah J, et al. Anxiety, depression, insomnia, and trauma-related symptoms following COVID-19 infection at long-term follow-up. Brain Behav Immun Health. 2021;16:100315. doi: 10.1016/j.bbih.2021.100315.
- Goodman ML, Molldrem S, Elliott A, et al. Long COVID and mental health correlates: a new chronic condition fits existing patterns. Health Psychol Behav Med. 2023;11:2164498.
- Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205(1):51.e1–51.e8. doi: 10.1016/j.ajog.2011.02.029.
- Huybrechts KF, Palmsten K, Mogun H, et al. National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry. 2013;35(3):265–271. doi: 10.1016/j.genhosppsych.2012.12.010.
- Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol. 2012;207(1):49.e1–49.e9. doi: 10.1016/j.ajog.2012.04.028.
- Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–194.e5. doi: 10.1016/j.ajog.2007.07.036.
- Ailes EC, Simeone RM, Dawson AL, et al. Using insurance claims data to identify and estimate critical periods in pregnancy: an application to antidepressants. Birth Defects Res. 2016;106(11):927–934. doi: 10.1002/bdra.23573.
- Hanley GE, Mintzes B. Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC Pregnancy Childbirth. 2014;14(1):242. doi: 10.1186/1471-2393-14-242.
- Oskotsky T, Marić I, Tang A, et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4(11):e2133090. doi: 10.1001/jamanetworkopen.2021.33090.
- Nakhaee H, Zangiabadian M, Bayati R, et al. The effect of antidepressants on the severity of COVID-19 in hospitalized patients: a systematic review and meta-analysis. PLOS One. 2022;17(10):e0267423. doi: 10.1371/journal.pone.0267423.
- Rush RW, Keirse MJNC, Howat P, et al. Contribution of preterm delivery to perinatal mortality. Obstet Gynecol Surv. 1977;32(10):643–645. doi: 10.1097/00006254-197710000-00003.
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4.
- Hamilton B, Martin J, Osterman M. VSRR 028: births: provisional data for 2022. U.S.: National Center for Health Statistics; 2023.
- Huybrechts KF, Sanghani RS, Avorn J, et al. Preterm birth and antidepressant medication use during pregnancy: a systematic review and meta-analysis. PLOS One. 2014;9(3):e92778. doi: 10.1371/journal.pone.0092778.
- Sujan AC, Rickert ME, Öberg AS, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. Obstet Gynecol Surv. 2017;72(9):523–524. doi: 10.1097/01.ogx.0000524510.66825.09.
- Yonkers KA, Norwitz ER, Smith MV, et al. Depression and serotonin reuptake inhibitor treatment as risk factors for preterm birth. Epidemiology. 2012;23(5):677–685. doi: 10.1097/EDE.0b013e31825838e9.
- Reis M, Källén B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med. 2010;40(10):1723–1733. doi: 10.1017/S0033291709992194.
- Källén B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158(4):312–316.
- Nörby U, Forsberg L, Wide K, et al. Neonatal morbidity after maternal use of antidepressant drugs during pregnancy. Pediatrics. 2016;138(5):e20160181. doi: 10.1542/peds.2016-0181.
- Zhao X, Liu Q, Cao S, et al. A meta-analysis of selective serotonin reuptake inhibitors (SSRIs) use during prenatal depression and risk of low birth weight and small for gestational age. J Affect Disord. 2018;241:563–570.
- Chang Q, Ma X-Y, Xu X-R, et al. Antidepressant use in depressed women during pregnancy and the risk of preterm birth: a systematic review and meta-analysis of 23 cohort studies. Front Pharmacol. 2020;11:659. doi: 10.3389/fphar.2020.00659.
- Bandoli G, Chambers CD, Wells A, et al. Prenatal antidepressant use and risk of adverse neonatal outcomes. Pediatrics. 2020;146(1):e20192493. doi: 10.1542/peds.2019-2493.
- Domingues RR, Wiltbank MC, Hernandez LL. Maternal serotonin: implications for the use of selective serotonin reuptake inhibitors during gestation. Biol Reprod. 2023;109(1):17–28.
- Rosenfeld CS. Placental serotonin signaling, pregnancy outcomes, and regulation of fetal brain development. Biol Reprod. 2020;102(3):532–538. doi: 10.1093/biolre/ioz204.
- Phillips GS, Wise LA, Rich-Edwards JW, et al. Prepregnancy depressive symptoms and preterm birth in the black women’s health study. Ann Epidemiol. 2010;20(1):8–15. doi: 10.1016/j.annepidem.2009.09.009.
- Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Hum Reprod. 2008;24(1):146–153. doi: 10.1093/humrep/den342.
- Amit G, Yanover C, Bivas-Benita M, et al. Antidepressant use during pregnancy and the risk of preterm birth – a cohort study. Research Square; 2023.
- Lennestål R, Källén B. Delivery outcome in relation to maternal use of some recently introduced antidepressants. J Clin Psychopharmacol. 2007;27(6):607–613. doi: 10.1097/jcp.0b013e31815ac4d2.
- Ericson A, Källén B, Wiholm B. Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol. 1999;55(7):503–508.
- Bandoli G, Kuo GM, Sugathan R, et al. Longitudinal trajectories of antidepressant use in pregnancy and the postnatal period. Arch Womens Ment Health. 2018;21(4):411–419. doi: 10.1007/s00737-018-0809-2.
- Wilkes S. The use of bupropion SR in cigarette smoking cessation. Int J Chron Obstruct Pulmon Dis. 2008;3:45–53. doi: 10.2147/COPD.S1121.
- Jaffer KY, Chang T, Vanle B, et al. Trazodone for insomnia: a systematic review. Innov Clin Neurosci. 2017;14:24–34.
- CDC/ATSDR SVI fact sheet [Internet]; 2023 [cited 2023 Dec 18]. Available from: https://www.atsdr.cdc.gov/placeandhealth/svi/fact_sheet/fact_sheet.html
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x.
- Levis B, Sun Y, He C, et al. Accuracy of the PHQ-2 alone and in combination with the PHQ-9 for screening to detect major depression: systematic review and meta-analysis. JAMA. 2020;323(22):2290–2300.
- Löwe B, Wahl I, Rose M, et al. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord. 2010;122(1–2):86–95. doi: 10.1016/j.jad.2009.06.019.
- Kroenke K, Spitzer RL, Williams JBW, et al. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–621.
- King LS, Feddoes DE, Kirshenbaum JS, et al. Pregnancy during the pandemic: the impact of COVID-19-related stress on risk for prenatal depression. Psychol Med. 2023;53(1):170–180. doi: 10.1017/S003329172100132X.
- Lantigua-Martinez M, Trostle ME, Torres AM, et al. Perinatal depression before and during the COVID-19 pandemic in New York city. AJOG Glob Rep. 2023;3(3):100253. doi: 10.1016/j.xagr.2023.100253.
- Ghazanfarpour M, Bahrami F, Rashidi Fakari F, et al. Prevalence of anxiety and depression among pregnant women during the COVID-19 pandemic: a meta-analysis. J Psychosom Obstet Gynaecol. 2022;43(3):315–326. doi: 10.1080/0167482X.2021.1929162.
- Arzamani N, Soraya S, Hadi F, et al. The COVID-19 pandemic and mental health in pregnant women: a review article. Front Psychiatry. 2022;13:949239. doi: 10.3389/fpsyt.2022.949239.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Br J Psychiatry. 1987;150:782–786.
- Malm H, Sourander A, Gissler M, et al. Pregnancy complications following prenatal exposure to SSRIs or maternal psychiatric disorders: results from population-based national register data. Am J Psychiatry. 2015;172(12):1224–1232. doi: 10.1176/appi.ajp.2015.14121575.
- Gavin AR, Chae DH, Mustillo S, et al. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA study. J Womens Health. 2009;18(6):803–811. doi: 10.1089/jwh.2008.0984.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111.
- Stephens MAC, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34:468–483.
- Najafzadeh A. Stress and preterm birth: biological and vascular mechanisms affecting the feto-placental circulation and the length of gestation. Sonography. 2016;3(3):95–102. doi: 10.1002/sono.12073.
- Liu Y-Z, Wang Y-X, Jiang C-L. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. doi: 10.3389/fnhum.2017.00316.
- Grigoriadis S. Antenatal depression: pregnancy and neonatal outcomes [Internet]. UpToDate; 2023 [cited 2024 Jan 3]. Available from: https://www.uptodate.com/contents/antenatal-depression-pregnancy-and-neonatal-outcomes
- Oberlander TF, Warburton W, Misri S, et al. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63(8):898–906.
- Calderon-Margalit R, Qiu C, Ornoy A, et al. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. Obstet Anesth Dig. 2011;31(1):46–47. doi: 10.1097/01.aoa.0000393180.98348.f6.
- Lund N, Pedersen LH, Henriksen TB. Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Arch Pediatr Adolesc Med. 2009;163(10):949–954. doi: 10.1001/archpediatrics.2009.164.
- Colvin L, Slack-Smith L, Stanley FJ, et al. Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Res. 2011;91(3):142–152. doi: 10.1002/bdra.20773.
- Davis RL, Rubanowice D, McPhillips H, et al. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug. 2007;16(10):1086–1094. doi: 10.1002/pds.1462.
- Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience. 2017;342:154–166.
- Chan J, Natekar A, Einarson A, et al. Risks of untreated depression in pregnancy. Can Fam Physician. 2014;60:242–243.

