Abstract
Objective
The aim of this study is to assess the clinical efficacy of minimally invasive surgical interventions in addressing spontaneous intracranial hemorrhage among neonates aged 0–3 months.
Methods
A retrospective analysis was conducted on a cohort of 30 neonates diagnosed with spontaneous intracranial hemorrhage, who underwent minimally invasive cranial trepanation and drainage procedures at our department between 2011 and 2015.
Results
A comprehensive follow-up, spanning a duration of 1–5 years, was conducted for all 30 neonates, revealing a 100% survival rate among the pediatric cohort.
Conclusion
The findings suggest that minimally invasive cranial trepanation and drainage exhibit efficacy in neonates aged 0–3 months experiencing spontaneous intracranial hemorrhage, leading to a reduction in both mortality and disability rates. It is recommended that surgery be promptly performed upon definitive diagnosis and identification of operation indications to prevent severe brain damage resulting from prolonged intracranial hypertension and potential fatal outcomes in neonates. Furthermore, the surgical procedure is characterized by its simplicity, involving minimal trauma.
Introduction
Due to the distinctive physiological characteristics of neonates, spontaneous intracranial hemorrhage is characterized by swift onset, rapid development, and consequential harm [Citation1]. Currently, the predominant approach to managing spontaneous intracranial hemorrhage in neonates involves conservative medical interventions, with surgical treatment being approached cautiously. However, clinical outcomes indicate a poor prognosis for neonates, often accompanied by significant sequelae [Citation2]. While certain studies propose craniotomy for neonates exhibiting evident space-occupying symptoms [Citation3], the clinical efficacy is suboptimal due to the vulnerability of neonates and their limited tolerance for surgical interventions. Hence, clinical treatment should not solely focus on the efficacy of hematoma removal but should also prioritize surgical safety to address the limitations of conventional surgical approaches. Minimally invasive surgery not only expeditiously alleviates brain tissue hematoma pressure but also minimizes trauma to brain tissue, fostering accelerated recovery of brain function. This approach proves beneficial in effectively lowering disability and fatality rates, thereby enhancing the overall quality of life [Citation4]. To investigate the clinical impact of minimally invasive surgery on spontaneous intracranial hemorrhage in neonates aged 0–3 months, a retrospective review and analysis of 30 cases treated at our department between 2011 and 2015 was conducted in this study. All cases underwent surgical intervention employing minimally invasive cranial trepanation and drainage.
Materials and methods
Clinical data
A retrospective analysis of the clinical data pertaining to 30 neonates undergoing minimally invasive surgery for spontaneous intracranial hemorrhage at our hospital from 2011 to 2015 was conducted. The study involved a comprehensive review and analysis of the diagnosis and treatment approaches employed, accompanied by a discussion on the challenges and experiences encountered throughout the diagnostic and therapeutic processes. Notably, all guardians of the patients provided informed consent by signing the requisite consent forms.
Inclusion criteria: 1. Confirmation of neonatal intracranial hemorrhage through clinical diagnosis and corroborated by brain imaging examination; 2. Exclusion of cases involving head trauma and birth canal injury; 3. Age within the range of 0 days to 3 months; 4. Gestational age at birth > 37 weeks; 5. Availability of complete clinical data.
Surgical methods
Drilling and drainage procedure for intracerebral hemorrhage Following successful anesthesia, a skin incision of approximately 1.5 cm in length was made at a suitable location on the skull surface. Subsequently, a layered incision and separation process ensued until a thorough exploration of the skull was achieved. Subsequently, a bone hole was meticulously drilled utilizing a skull drill, accompanied by bipolar electrocoagulation cautery and a "cross" incision made in the endocranium. A drainage tube was then strategically positioned at the subdural or brain hematoma site to facilitate the drainage of blood clots, with a subsequent cycle of repeated flushing conducted as necessary. Following these procedures, an indwelling drainage tube was inserted, and hemostasis was accomplished through bipolar coagulation. The incision was then meticulously sutured layer by layer. Additionally, a thorough examination of the anterior fontanel was conducted to ensure discernible flatness and softness. Following the surgical intervention, all neonates were transferred to the intensive care unit and provided assisted ventilation through ventilators for a specified duration. Extubation from the ventilator occurred only upon achieving a stable medical condition. Enteral nutrition was initiated through nasal administration via a gastric tube in the initial phase. The removal of the ventilator occurred within a timeframe of 1–4 days, averaging 1.89 days, while pupil recovery time post-surgery averaged 7.48 days. Subsequent to the operation, all children were initially admitted to the pediatric intensive care unit (PICU) and transitioned to the general ward after a period of 2–3 days of stable weaning.
Laboratory tests
Comprehensive blood tests were conducted for all patients, encompassing parameters such as blood routine, liver function, clotting-related indicators, and electrolyte levels. Additionally, TORCH [an acronym meaning (T)oxoplasmosis, (O)ther Agents, (R)ubella, (C)ytomegalovirus, and (H)erpes Simplex] detection was carried out in applicable cases. It is noteworthy that all these tests were administered by the same nurse, adhering to stringent and standardized conditions to maintain uniformity in the testing environment and procedures.
Imaging examination
CT examinations were conducted both pre- and post-surgery. To mitigate potential interference arising from infant non-cooperation during CT or MRI imaging, it was ensured that all cases were in a sedated state before the imaging examination. Cross-sectional scanning was employed for CT examinations. Prior to MRI examinations, precautions were taken to remove any metal objects, with special attention given to scalp needles. A multi-channel special neonate coil, designed for optimal fitting, was employed during imaging procedures. The infant’s head was positioned for comfort, ensuring alignment of the neck with the bed body to safeguard the infant’s airway. Furthermore, to maintain consistency and precision, all imaging procedures were conducted by the same radiologist.
Results
Clinical data
The study encompassed 21 male and 9 female participants, whose ages spanned from 12 h to 2 months and 6 days, averaging 46 days. Age subcategories included above 6 weeks (n = 11), 2–6 weeks (n = 18), and below 2 weeks (n = 1). The body weight of the participants ranged from 3.0 to 6.0 kg, with an average weight of 4.5 kg. The feeding patterns included exclusive breastfeeding (n = 26), artificial feeding (n = 2), and mixed feeding (n = 2). One neonate experienced a complication of diarrhea. All neonates were full-term births, with a mean time from onset to admission of 2.2 days. The mean time from admission to the operation was 6.1 h, and none of the neonates had a history of trauma.
Clinical manifestations
As reported by family members, the initial symptoms included vomiting (n = 18), convulsions (n = 5), crying and refusal to feed (n = 5), and clouding of consciousness (n = 1). Additionally, all neonates exhibited a bulging anterior fontanel, poor appetite, and rapid disease progression. Some neonates experienced recurrent convulsions and apnea. Furthermore, within this group, 23 neonates presented with signs of cerebral hernia, including bilateral unequal pupil size and coma.
Imaging examination
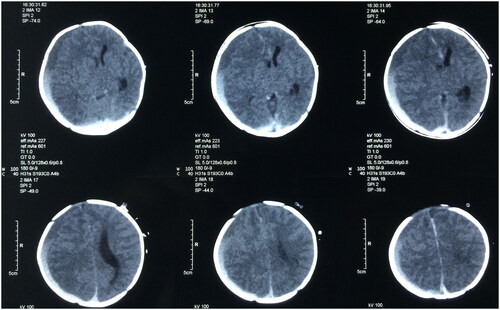
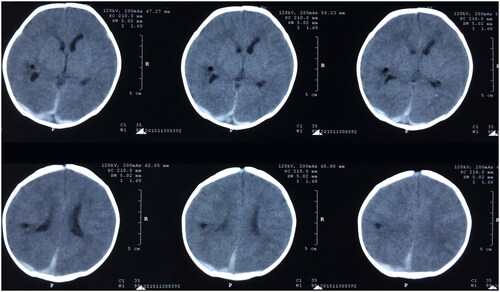
All neonates underwent a head CT scan, revealing a pronounced deviation of the midline and evident compression on one ventricle (). The findings indicated 29 cases of subdural hemorrhage and 1 case of intracerebral hematoma. However, post-operation assessments demonstrated a return to normal midline position and ventricular shape ().
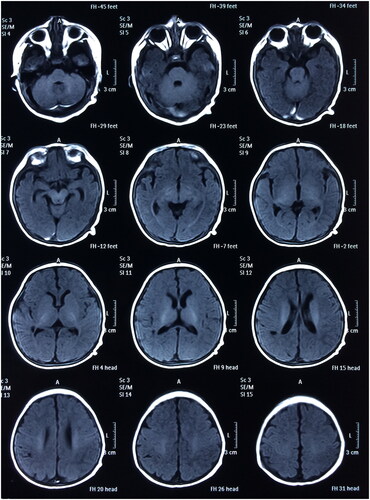
All 30 neonates successfully survived without any recurrence of intracranial hemorrhage. Subsequent to the initial treatment, rehabilitation therapies, including hyperbaric oxygen, were administered to all neonates. Throughout the follow-up period of 1–5 years or more, all children exhibited healthy growth without instances of obstructive hydrocephalus or paralysis. Brain MRI scans ( and ) indicated improved brain development with age in the majority of the children. Detailed results are presented in .
Figure 4. Postoperative head MRI scan (T2) (A boy aged 1 year and 13 days with spontaneous subdural intracranial hemorrhage).
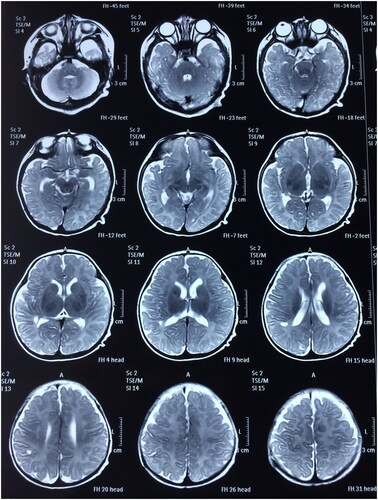
Table 1. Statistical results of neonatal intracranial hemorrhage.
Discussion
Intracranial hemorrhage represents a prevalent brain injury condition in severe neonatal diseases, primarily arising from birth injuries, hypoxia, and vitamin K1 deficiency. It stands as a prominent contributor to perinatal mortality. Timely intervention is imperative to mitigate the mortality and disability rates associated with neonatal intracranial hemorrhage. The primary clinical manifestations of intracranial hemorrhage include vomiting, convulsions, loss of appetite, a pale complexion, irritability, confusion, and coma. Given that infants lack the ability to express themselves verbally, and vomiting frequently appears as one of the initial symptoms, there is a risk of misdiagnosing the condition as a digestive disorder during the initial clinical visit. This misdiagnosis may consequently lead to a delay in administering the appropriate treatment. In addition to a thorough inquiry into the patient’s history, careful attention should be directed toward assessing the anterior fontanel, checking for signs of anemia, observing pupil size, and conducting a head CT scan to ascertain the presence of intracranial hemorrhage. A prompt imaging head CT scan is the preferred method for detecting acute bleeding, as supported by literature [Citation5–11]. This modality aids in comprehending the location, extent, and severity of intracranial bleeding, facilitating the identification of surgical indications. In contrast, a head MRI scan is characterized by its high soft tissue resolution, absence of X-ray radiation, and the provision of clear axial, sagittal, and coronal images. A head MRI scan is essential for diagnosing subacute and chronic hemorrhage. This imaging modality not only reveals the presence of hemorrhage but also provides insights into brain tissue injuries.
All neonates in this cohort exhibited a platelet count within the normal range. Notably, exclusive breastfeeding was observed in 86.67% of cases, anemia was prevalent in 93.33%, and hyponatremia was present in 36.67%, although the mean sodium value was 135.2 mmol/L, hovering at the lower limit of the normal range. Hypocalcemia accounted for 43.33%, and CMV (+) accounted for 69.23% in 13 neonates subjected to the TORCH test. The average age of onset was about 46 days: neonates above 6 weeks of age accounted for 36.67% and those aged 2–6 weeks accounted for 60.00%, indicating that 4–8 weeks was the high-incidence stage. Abnormal liver function accounted for 33.33% of cases, subdural hemorrhage accounted for 96.67% of cases, and there was no recurrence of intracranial hemorrhage after surgical treatment. From the data analysis provided, it is apparent that the intracranial hemorrhage observed in neonates in this group primarily stemmed from vitamin K deficiency. Nevertheless, coagulation disorders were only observed in 23.33% of these cases, representing a relatively lower proportion. This discrepancy can be attributed to the fact that a significant number of patients were referred cases, and the average duration from the onset of the disease to admission to our hospital was notably prolonged, reaching 2.2 days. Consequently, many of these patients had already undergone basic treatments, including vitamin K supplements, at local hospitals before being transferred to our department. This pre-hospital intervention likely contributed to the improvement of their blood coagulation function.
In young infants, spontaneous intracranial hemorrhage predominantly occurs in the subdural region, with subarachnoid hemorrhage, intraventricular hemorrhage, and intraparenchymal hemorrhage following in decreasing order of frequency [Citation12]. The damage to brain tissue encompasses multiple facets. On one hand, hemorrhage exerts compression on brain tissue, inducing brain edema, which in turn results in intracranial hypertension, reduction in cerebral blood supply, cerebral infarction, and cerebral anoxia. On the other hand, conditions such as low sodium, low calcium, and anemia exacerbate cerebral hypoxia and contribute to the aggravation of brain edema. Moreover, the inadequate vascular regulation in the brain contributes to the swift progression of the disease, resulting in a high incidence of cerebral hernia, reaching 76.67% in this infant cohort. Given the immature development of the brains in young infants, the damage incurred due to cerebral hemorrhage, cerebral infarction, and cerebral edema is notably more severe than that observed in adults. Hence, the crux of successful intervention lies in promptly conducting surgical removal of intracranial hematoma with space-occupying effects, ensuring the maintenance of a stable internal environment, actively addressing anemia, and providing vitamin K supplementation [Citation13]. Early surgical intervention is advisable for children exhibiting evident ventricular compression, a substantial deviation of midline structure (exceeding 1 cm), bleeding volume surpassing 20 ml, a bulging anterior fontanel, or cerebral herniation evident in head CT scans. Nonetheless, caution should be exercised when considering surgery for young infants with extensive intracranial hemorrhage.
In accordance with our experience, surgery should be expeditiously conducted for children meeting the criteria for surgical intervention. Moreover, preoperative examinations should be concluded within a timeframe of 2–6 h [Citation14]. For children diagnosed with intracranial hemorrhage, it is imperative to address and correct conditions such as anemia, electrolyte disorders, and coagulation disorders. Additionally, supplementation of vitamin K is recommended, and the use of phenobarbital may be employed as a preventive measure against epilepsy. Timely surgical intervention is crucial and should be carried out as soon as possible. The neonates in this group underwent surgery with an average time from admission to operation of approximately 6.1 h. The open cranial suture and the unclosed anterior and posterior fontanels in young infants, along with the relatively soft nature of their skulls compared to adults, afford a certain buffering capacity against rapid increases in intracranial pressure. These physiological characteristics not only serve to protect the brain tissue of children but also offer valuable time for surgical intervention. Consequently, surgery remains viable even in the presence of a brain hernia, and effective drainage and decompression can significantly mitigate the damage inflicted by cerebral hemorrhage on brain tissue [Citation15]. In general, the recommended approach is minimally invasive cranial drilling and drainage [Citation16]. For infants presenting with ventricular hemorrhage and markedly dilated ventricles, external ventricular drainage is the recommended method. Craniotomy with substantial trauma and severe bleeding is not recommended, as it might render young infants unable to tolerate the surgical intervention due to extensive bleeding and significant brain edema. In cases of spontaneous intracranial hemorrhage of this nature, particular attention must be dedicated to assessing preoperative anemia, blood coagulation function, and electrolyte levels. Anemia was a prevalent manifestation observed in this neonatal group, often accompanied by potential complications such as hyponatremia, hypocalcemia, electrolyte imbalances, and coagulation disorders. Consequently, surgery was undertaken after pertinent indices had been ameliorated through blood transfusion, plasma transfusion, fluid replacement, and vitamin K supplementation. Following the surgical procedure, all infants were transferred to the intensive care unit for further monitoring and care. Infants are susceptible to developing epilepsy and apnea as a result of severe cerebral edema, and it is not recommended to prematurely remove ventilator support. Instead, there should be active measures taken for the prevention and control of epilepsy to mitigate the risk of frequent ventilator usage stemming from the infant’s unstable condition. Ventilators were typically removed within 1–4 days (with a mean of 1.89 days) post-surgery for this group of neonates. Following surgery, early enteral nutrition through a gastric tube was initiated to minimize the need for fluid replacement. Given that the anterior fontanel remained open during this period, it served as a valuable indicator for monitoring changes in intracranial pressure. Consequently, the dosage of mannitol and other dehydrant agents could be adjusted as needed during the course of treatment. The indwelling external drainage tube can be removed once the drainage fluid is reduced and becomes clear. Proactive and effective intervention plays a crucial role in significantly enhancing the survival rate and reducing the disability rate in infants with such conditions.
There are certain limitations inherent in this study. Notably, the sample size was relatively small, and the study focused solely on the period from 2011 to 2015 due to data constraints. In future research endeavors, efforts will be made to address these limitations by expanding the sample size and updating the data, thereby providing a more comprehensive understanding of the subject matter. In future research endeavors, we intend to utilize recent cases as the focus of our investigation. The objective will be to delve deeper into the impact of minimally invasive surgery on the prognosis of infants with spontaneous intracranial hemorrhage, particularly those induced by high-risk factors such as vitamin K deficiency and TORCH infection. The study will also aim to compare the outcomes of minimally invasive surgery with other types of treatments, providing a more nuanced understanding of the therapeutic options for these cases.
Conclusion
Early prevention, timely diagnosis, and prompt intervention play pivotal roles in enhancing the prognosis of neonates with spontaneous intracranial hemorrhage. Additionally, efforts to avoid intrauterine infection and provide early vitamin K supplementation can contribute to reducing the incidence of Vitamin K deficiency bleeding (VKDB) in neonates. Timely surgery may be a valuable treatment option for neonates with spontaneous intracranial hemorrhage, particularly when the diagnosis is definitive, and surgical indications are clear. This approach can prove instrumental in alleviating brain damage and improving overall outcomes for affected neonates. Postoperative rehabilitation therapy, including hyperbaric oxygen, is crucial for promoting the recovery of damaged brain function in infants, considering their ongoing developmental stage. In future studies, expanding the sample size will be a priority, allowing for a more comprehensive exploration of the relationship between minimally invasive surgery and risk factors such as vitamin K deficiency and TORCH infection in infants with spontaneous intracranial hemorrhage.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki(as was revised in 2013). The study was approved by Ethics Committee of the Children’s Hospital of Anhui Medical University (No. EYLL-2019-026). All patient guardians signed a document of informed consent.
Availability of data and material
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgements
We are particularly grateful to all the people who have given us help on our article.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Adil MM, Qureshi AI, Beslow LA, et al. Factors associated with increased in-hospital mortality among children with intracerebral hemorrhage. J Child Neurol. 2015;30(8):1–8. doi: 10.1177/0883073814552191.
- Ferriero DM, Fullerton HJ, Bernard TJ, et al. Management of stroke in neonates and children: a scientific statement from the American heart association/American stroke association. Stroke. 2019;50(3):e51–e96. doi: 10.1161/STR.0000000000000183.
- Sporns PB, Psychogios MN, Fullerton HJ, et al. Neuroimaging of pediatric intracerebral hemorrhage. J Clin Med. 2020;9(5):1518. doi: 10.3390/jcm9051518.
- Zhou CB, Li Q. Etiology and efficacy in children with spontaneous intracerebral hemorrhage. Foreign Medical Treatment. 2015;34(29):73–74.
- Boulouis G, Stricker S, Benichi S, et al. Mortality and functional outcome after pediatric intracerebral hemorrhage: cohort study and meta-analysis. J Neurosurg Pediatr. 2021;27(6):661–667. doi: 10.3171/2020.9.PEDS20608.
- Boulouis G, Hak JF, Kerleroux B, et al. Hemorrhage expansion after pediatric intracerebral hemorrhage. Stroke. 2021;52(2):588–594. doi: 10.1161/STROKEAHA.120.030592.
- Porcari GS, Beslow LA, Ichord RN, et al. Neurologic outcome predictors in pediatric intracerebral hemorrhage: a prospective study. Stroke. 2018;49(7):1755–1758. doi: 10.1161/STROKEAHA.118.021845.
- Guédon A, Blauwblomme T, Boulouis G, et al. Predictors of outcome in patients with pediatric intracerebral hemorrhage: development and validation of a modified score. Radiology. 2018;286(2):651–658. doi: 10.1148/radiol.2017170152.
- Chen CX, Lu JZ, Gui J. Medicine and health guidance news. Therapeutic Effect of Ommaya Reservoir Drainage on Neonatal Hydrocephalus Secondary to Intracranial Hemorrhage. International. 2021;27(18):2892–2894.
- Liu FX. Feasibility and imaging findings of neonatal intracranial hemorrhage diagnosed by CT. Chin J Clinical Rational Drug Use. 2017;10(20):147–148.
- Huang S, Cao LJ, Wang XW, et al. Surgical treatments of intracranial hemorrhages in the neonates and infants under 2 years of age. Chin J Clinical Neurosurgery. 2015;20(09):541–543.
- Wan B, Xiao R, Yang XQ, et al. The value of ultrasound and CT in diagnosing neonatal intracranial hemorrhage. Chin J CT and MRI. 2020;18(07):4–6 + 13.
- Liu J, Wang D, Lei C, et al. Etiology, clinical characteristics and prognosis of spontaneous intracerebral hemorrhage in children: a prospective cohort study in China. J Neurol Sci. 2015;358(1–2):367–370. doi: 10.1016/j.jns.2015.09.366.
- Lai LM, Zhang Z. Diagnosis and treatment on neonates with spontaneous intracranial hemorrhage(SIH) and the effect on intelligence and psychological development. Chin J Women Children Health. 2017;8(01):27–30.
- Yu LJ, Lin ZL. Evaluation of the therapies for neonatal posthaemorrhagic hydrocephalus. J Clinical Pediatrics. 2014;32(03):201–205.
- Zhang J, Liang C, Ding YH, et al. To compare the efficacy of minimally invasive puncture drainage and decompressive craniectomy in the treatment of spontaneous hypertensive intracranial hemorrhage. World Latest Med Inform. 2016;16(96):121–122.