Abstract
Objective
Iron deficiency anemia is a very common health problem during pregnancy and intravenous (IV) iron substitution has become part of routine management. However, recent studies have raised concerns about the association of IV iron infusion and the development of secondary transitory hypophosphatemia (HP) in adults, including pregnant women. We aimed to evaluate the impact of IV iron administration during pregnancy on the phosphocalcic metabolism of newborns.
Methods
A prospective, single-center, observational study was performed from December 2022 to May 2023 at the maternity facility of Geneva University Hospitals. We included women treated with either IV or oral iron during pregnancy. At delivery, a maternal blood sample was collected to assess hemoglobin, hematocrit, and levels of ferritin, phosphate and calcium, as well as an umbilical cord blood sample to assess levels of phosphate and calcium. Univariate and multivariate analyses were performed to evaluate the contribution of IV iron substitution on cord blood phosphatemia and calcemia, considering potential confounding factors. Neonatal HP was defined as a phosphate level <1.3 mmol/L.
Results
Forty-three pregnant women were included in our study. Among these, 22 were treated with ferric carboxymaltose and 21 with oral iron. There were three cases of maternal HP in the IV iron group (13.6%) and one (4.8%) in the control group (p value for the difference= .607). We observed one case (4.5%) of neonatal HP in the IV iron group and no cases in the control group. Median cord blood phosphatemia and calcemia were 1.7 mmol/L vs. 1.71 mmol/L and 2.67 mmol/L vs. 2.64 mmol/L in the IV iron and oral groups, respectively. After adjustment, IV iron administration had no impact on cord blood phosphate (p= .919) and calcium (p= .891) levels.
Conclusion
No impact of IV iron administration during pregnancy was observed on the newborn phosphocalcic metabolism.
Introduction
Iron deficiency anemia (IDA) is a very common health problem during pregnancy and can lead to serious maternal and fetal complications (Citation1). It is usually recommended to measure hemoglobin (Hb) at least two times during pregnancy and serum ferritin once in early pregnancy (Citation2). Currently, oral iron therapy is the first-line treatment recommended to treat IDA during pregnancy. Intravenous (IV) iron therapy should be reserved for patients who do not respond to oral therapy, present adverse effects, have poor compliance, or a severe anemia (<9 g/dL), as well as those who require rapid iron repletion, such as when delivery is imminent (Citation2–4).
IV iron formulations allow for the administration of high doses of iron, thus enabling a rapid correction of IDA. Several studies have shown that IV therapy is more effective and rapid than oral therapy for resolving anemia (Citation5,Citation6). In addition, the use of IV iron therapy reduces the need for blood transfusion and is associated with a shorter duration of hospitalization in the postpartum period (Citation3,Citation7). The IV route is also highly convenient and allows a one-day treatment, while preventing problems related to poor compliance and adverse effects of oral substitution (Citation8,Citation9). Although IV iron incurs a significantly higher cost compared to classic oral treatments, its cost-effectiveness has been demonstrated (Citation10). Several studies support the safety of a single dose of IV iron during pregnancy (Citation8,Citation11) and due to its convenience and effectiveness, its use in this context has significantly increased in recent years (Citation12,Citation13).
In Switzerland, IV iron is available in three distinct forms: ferric carboxymaltose (FCM) (Ferinject®, Vifor Pharma UK Ltd, Staines-upon-Thames, UK); iron hydroxide sucrose complex (Venofer®, Vifor (International) Inc., St Gallen, Switzerland; Fermed®, Fermed GmbH, Wolfhausen, Switzerland); and iron isomaltoside (Monofer®, Pharmacosmos, Holbaek, Denmark). According to Swissmedic, IV iron preparations are contraindicated as a precautionary measure during the first trimester of pregnancy and can be administered in the second and third trimester when the potential benefits outweigh the risks. However, recent studies have raised concerns about the association of IV iron infusion and the development of secondary transient hypophosphatemia (HP) in pregnant and non-pregnant adults (Citation14,Citation15).
Whether IV iron during pregnancy has any impact on the fetus has not yet been evaluated. Phosphate plays essential roles in energy metabolism, cell signaling and skeletal development, and even mild biological changes can be potentially of great concern during pregnancy. The definition of neonatal HP varied among the studies, ranging from serum concentrations of <0.8 mmol/L to <1.6 mmol/L (Citation16–18). In the context of newborns, there is a limited number of studies addressing the incidence of HP, with a predominant focus on premature newborns. Fenton et al. (Citation19) provided cord blood phosphate values based on gestational age at birth. Bustos et al. (Citation20) reported an incidence of HP in 2.4% of all newborns admitted to the neonatal intensive care unit, defining hypophosphatemia as a phosphate level lower than 1.3 mmol/l. HP in newborns has been associated with a higher risk of late onset sepsis and prolonged respiratory problems. In fact, phosphate depletion impacts white blood cell involvement by decreasing granulocyte activity (Citation21). At the muscle level, HP causes hypotonia, leading to prolonged respiratory distress, difficulties in weaning from the ventilator, a longer duration of oxygen requirements and, consequently, the development of bronchopulmonary dysplasia (Citation22–25). In cases of severe HP with heavy adenosine 5′-triphosphate (ATP) depletion, it may lead to respiratory failure, myocardial failure, rhabdomyolysis, anemia, metabolic acidosis and convulsions (Citation26). The goal of our study was to evaluate the impact of maternal IV iron administration during pregnancy on the phosphocalcic metabolism of newborns.
Materials and methods
Study design
This was a prospective, single-center, observational study performed in the Department of Pediatrics, Gynecology and Obstetrics at Geneva University Hospitals (Geneva, Switzerland). The maternity facility is the largest in Switzerland with more than 4000 deliveries per year.
The study was approved by the Swiss Ethics Committees (Swissethics, Project-ID: 2022-00197) and conducted according to the protocol, Swiss legal requirements, the World Medical Association Declaration of Helsinki, and the principles of Good Clinical Practice.
Patient recruitment
Study participation was offered to women during a prenatal visit to the outpatient clinic between December 2022 and May 2023. Inclusion criteria were women with an uncomplicated term (≥37 weeks of gestation) single pregnancy who received IV or oral iron substitution from the second trimester of pregnancy (>12 weeks of gestation) to the day of delivery. Women with chronic medical conditions (diabetes, body mass index [BMI] > 35, metabolic disease, hemoglobinopathy), multiple pregnancies, fetal abnormalities or preterm delivery were excluded. Patients were included after signing an informed consent form.
Data collection
We collected maternal, neonatal, and labor data from the medical records. During labor, a maternal blood sample was taken to assess hemoglobin, hematocrit and levels of ferritin, phosphate and calcium. After delivery, a blood sample on the umbilical cord was taken to assess the level of phosphate and calcium. Samples were analyzed in the hospital laboratories following standardized techniques. Maternal HP was defined as a level of phosphate <0.80 mmol/L and subdivided into mild HP (0.65–0.80 mmol/L), moderate HP (0.32–0.65 mmol/L mmol/L) and severe HP (<0.32 mmol/L) (Citation27). We considered neonatal HP when phosphate levels were lower than 1.3 mmol/l, and further classified the severity as follows: mild, 1.26–1.3 mmol/L; moderate, 0.97–1.25 mmol/l; severe 0.65–0.96 mmol/l; and critical lower than 0.65 mmol/l (Citation19,Citation20). Data were collected in a secure database “REDCap,” which is specifically designed to support online and offline data capture for research studies.
Statistical analyses
Sample size
Based on a previous study, we expected a standard deviation (SD) between 0.2 and 0.3 for cord blood phosphatemia (Citation19). We defined a minimal clinically important difference in the level of phosphate of 0.3 mmol/L based on our clinical experience. Conservatively assuming a SD of 0.3, 17 patients in each group (total, 34) were required to detect a significant difference between groups with 80% power and a 5% two-sided significance level. As we planned to include three confounding variables, the recruitment of 20 patients in each group (total, 40) was required. No follow-up was planned, and therefore, no sample size inflation to adjust for a dropout rate was needed. Sample size was calculated using G*Power 3.1.9.7.
Descriptive statistics
Continuous variables were described using median and quartiles (Q1-Q3), and categorical variables were expressed as n and percentages. Differences between groups were determined by the chi-squared test, Mann–Whitney test or Fisher’s exact test, as appropriate. The association between cord blood phosphate and maternal phosphate was tested using a Spearman correlation.
Multivariate analyses
We used multiple linear regression to test the independent contribution of ferric IV substitution on cord blood phosphatemia and calcemia, considering potential confounding factors. Adjustment was made for gestational age (in weeks), maternal BMI (by increment of 1 point on the BMI scale), and neonatal weight (in g). Results were reported with corresponding 95% confidence intervals (CIs). A p-value <.05 was considered statistically significant. CIs were estimated using the robust Huber-White method. Using Cook’s distances, no outlier was identified. No multicollinearity was identified using variance inflation factors. Statistical analyses were performed using the statistical software R (version 4.1.0).
Results
We included 43 pregnant women: 22 were treated with IV FCM and 21 with oral iron.
The dose of FCM used in the IV group was 1000 mg for 10 participants (45.5%), 500 mg for three participants (13.6%), another dose for 3 participants (13.6%) and unknown for 6 participants (27.3%). We lacked information about the precise timing of FCM administration and, consequently, the time interval between IV administration and phosphate measurement. Due to the substantial diversity in the available forms of oral iron supplementation and compliance issues, we did not collect information regarding both the cumulative and daily dose of oral iron, as well as the type of supplementation used.
Sociodemographic characteristics, biological and medical data of participants are shown in .
Table 1. Descriptive statistics of mothers and newborns per type of iron treatment.
Maternal hemoglobin, phosphate and calcium levels were similar between groups, but ferritin levels were significantly higher in mothers treated with IV iron (p = .029). There were four cases of mild maternal HP (between .68 and .79 mmol/L), three in the IV iron group (13.6%) and one (4.8%) in the control group (p value for the difference= .607). We observed one case (4.5%) of moderate neonatal HP in the IV iron group and no cases in the control group. The only case of neonatal HP was observed in a 2930 g neonate, born at 40 weeks’ gestation with cord phosphatemia of 1.16 mmol/L and cord calcemia of 2.57 mmol/L. The mother of this infant presented an HP with a phosphate level of 0.72 mmol/L and a calcium level of 2.18 mmol/L. Median umbilical cord levels of phosphate and calcium were not significantly different betwen groups (). The route of administration had no significant impact on cord blood phosphatemia (p= .919) or calcemia (p= .891).
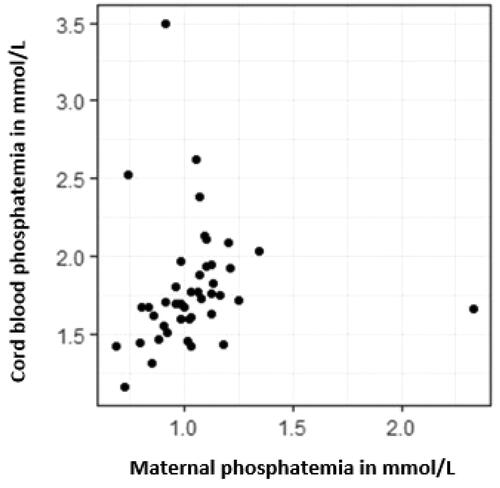
Adjusted analyses showed that the maternal BMI had a statistically significant impact on cord blood phosphatemia, with an increase of 0.03 mmol/l of phosphate for every 1 kg/m2 increment (). Gestational age and birth weight had no significant impact on cord blood phosphatemia or calcemia ( and ). Maternal phosphatemia and cord blood phosphatemia were related with the Spearman correlation coefficient of 0.412 (95% CI 0.100–0.667). No comparison between FCM and other iron formulations could be made due to the exclusive use of FCM in the recruited population.
Table 2. Multiple linear regression predicting cord phosphate by iron administration route.
Table 3. Multiple linear regression predicting cord calcium by iron administration route.
Discussion
Recent studies have raised concerns about the risk of IV iron-induced HP in adults (Citation14,Citation15). The hypothesized physiopathological mechanism involves an acute increase in the concentration of active fibroblast growth factor 23 (FGF-23) following IV iron infusion. FGF-23 is normally secreted by osteoblasts and osteoclasts and induces HP by stimulating urinary phosphate excretion and reducing the serum active vitamin D level (Citation14). The incidence of HP may vary depending on the type of iron formulation used, with FCM appearing to induce significantly higher rates of HP compared to iron isomaltoside (Citation28). A recent study revealed that up to 51% of FCM-treated patients developed HP, including 13% with severe HP. The severity of HP was directly correlated with the administered dose of iron (Citation29).
The association between IV iron administration and the development of HP has also been observed during pregnancy (Citation8,Citation9,Citation15). The incidence and severity of HP appeared to be less important with a faster normalization of serum phosphate levels in pregnant women compared to non-pregnant adults receiving the same IV iron formulations (Citation8,Citation15). Fetal bone development is mostly regulated by parathyroid hormone (PTH) and PTH-related protein (PTHrP), but not calcitriol, FGF-23, calcitonin, or sex steroids (Citation30,Citation31). Furthermore, a placental perfusion study reported that FCM does not pass to the fetus via the placenta (Citation32). Therefore, it is unlikely that IV iron would directly affect fetal phosphatemia. However, during fetal life, the mother is the unique source of minerals, and the fetus relies on the active transport of phosphate ions through the placenta (Citation30). Consequently, maternal phosphate deficiency could significantly affect fetal metabolism.
In our study, all women in the IV substitution group received FCM (Ferinject®). The incidence of maternal HP in the IV group was 13.6%, while the incidence found in previous studies with pregnant populations ranged between 4% and 8% (Citation8,Citation9). However, there was a significant heterogeneity in the reported incidence of IV iron-induced HP depending on various factors, such as the timing of phosphate measurement, dose of FCM, HP definition, and the populations studied (Citation33).
The incidences of maternal and neonatal HP were higher in the IV iron group compared to the control group. However, these differences were not statistically significant. In addition, HP severity was mild or moderate, and all cases were asymptomatic. We found no difference in phosphate and calcium cord blood levels of newborns exposed vs. non-exposed to IV iron infusion during pregnancy. Maternal and cord blood phosphatemia were correlated (), which aligns with existing knowledge about placental phosphate transport and supports our hypothesis that maternal HP could have an impact on the fetus.
Our findings showed that mean ferritin levels were significantly higher in mothers treated with IV iron (77 μg/L) compared to those treated with oral iron (44 μg/L), which is consistent with the current literature (Citation9). Interestingly, Hb levels were similar in the two groups whereas in other studies, patients substituted with IV iron tended to have higher Hb levels compared to those substituted with oral iron (Citation5,Citation9). Unfortunately, we do not have information about the pretreatment Hb levels of participants, but we can speculate that baseline Hb levels might have been lower in patients who received IV iron compared to those who received oral iron.
To our knowledge, this is the first study to assess newborn phosphocalcic changes due to IV iron administration during pregnancy. As IV iron formulations are very commonly used during pregnancy, we considered that it was important to assess its safety in relation to the fetus/newborn. The inclusion of a control group consisting of pregnant women treated with oral iron helped to minimize bias as both groups shared an indication for iron substitution. Additionally, to address confounding factors that have the potential to modify phosphate and calcium levels, such as gestational age, maternal BMI, and neonatal weight, we included them as covariates in our statistical analyses. Notably, maternal BMI had a statistically significant impact on cord blood phosphatemia.
This study’s methodology, while robust, encounters several limitations that merit consideration. First, the use of a single timepoint for sampling newborn cord blood provides a limited snapshot of the metabolic state, potentially overlooking transient or delayed alterations in phosphocalcic metabolism. Indeed, IV iron-induced HP in pregnant and non-pregnant adults is a transient state and if the fetus undergoes the same phenomenon, it would likely be also transient. Transient HP, if not too severe or repeated, is supposed to have no consequences in adults, but knowledge is lacking about the possible impact on fetuses. Second, the exclusive deployment of FCM restricts the generalizability of our findings to other IV iron formulations, underscoring the need for research incorporating a broader spectrum of treatments. Third, the study’s sample size, though adequate to demonstrate the primary outcomes, may lack the statistical power necessary to discern subtle differences in HP incidence and severity. This point is further questioned by the observation that the HP cases were found to predominantly occur in the IV iron group. This limitation calls for larger-scale studies to confirm or refute our observations. Furthermore, the absence of detailed baseline data on participants’ iron levels and the specific timing of IV iron administration introduces potential biases in interpreting the impact of IV iron therapy. This underscores the importance of comprehensive baseline assessments in future research to accurately gauge treatment effects. The selection of a control group treated with oral iron, while logical, introduces variables that may affect comparative analyses. Differences in baseline characteristics between groups could influence outcomes, highlighting the need for careful control group selection in future studies. Another limitation of our study is the absence of maternal PTH and vitamin D levels, which could have had an impact on post-FCM administration phosphate levels as vitamin D and PTH are crucial in phosphate metabolism. Indeed, the FCM-induced increase in FGF-23 impedes conversion of 25 (OH) vitamin D to calcitriol while promoting 24,25 (OH)2 vitamin D metabolism. Calcitriol decline leads to mild hypocalcemia, elevating PTH. PTH surge protects against severe hypocalcemia but extends hypophosphatemia beyond FGF23 elevation (Citation34).
In response to the growing utilization of IV iron formulations for IDA during pregnancy, our findings underscore the absence of a significant impact on newborn phosphocalcic metabolism. However, to comprehensively delineate the safety and efficacy profiles of these interventions, further investigations are warranted. A critical avenue for future research involves comparative studies across different IV iron formulations beyond FCM, which was the focus of this study. Such research could illuminate potential variances in outcomes, informing safer and more effective clinical practices. Longitudinal studies are essential to trace the long-term effects of prenatal exposure to IV iron on children, particularly concerning their growth, development, and metabolic parameters. These studies would offer invaluable insights into the developmental trajectory of these children and any latent effects of maternal IV iron therapy. Moreover, mechanistic studies aimed at elucidating the physiological pathways affected by IV iron administration during pregnancy could provide a deeper understanding of its impact on both maternal and fetal health. Specifically, the role of FGF-23 in mediating phosphocalcic metabolism changes warrants detailed exploration. Evaluation of the dose-response relationship between IV iron administration and its metabolic consequences is imperative. Such research could refine dosing guidelines to mitigate potential risks while ensuring the therapeutic efficacy of IV iron for IDA in pregnant women. In addition, the exploration of maternal health outcomes related to IV iron therapy, especially its long-term effects on phosphocalcic metabolism and bone health, is crucial. This line of inquiry could inform strategies to optimize maternal health postdelivery. Finally, the development of comprehensive, evidence-based guidelines for the use of IV iron in pregnancy is necessary. These guidelines should integrate findings from upcoming studies to balance efficacy with safety, ensuring optimal maternal and fetal health outcomes.
By pursuing these future directions and addressing the outlined limitations, subsequent research can build on our findings to enhance our understanding of IV iron therapy during pregnancy, ultimately informing safer and more effective clinical practices.
In conclusion, IV iron is a valuable and safe option for the treatment of IDA during pregnancy. Our findings provide reassurance concerning the potential impact of IV iron on the phosphocalcic metabolism of newborns. However, more studies evaluating the impact and safety of IV iron in infants exposed to IV iron in utero are needed.
Acknowledgements
The authors are grateful for the support of Ms Sonia Campelo for her precious collaboration in patient recruitment and data collection. We are indebted to the Laboratory Medicine Division of Geneva University Hospitals for performing biochemical analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, F.S., upon reasonable request.
Additional information
Funding
References
- World Health Organization. Preconception care to reduce maternal and childhood mortality and morbidity. 6-7 february 2012: meeting report. Geneva: World Health Organization; 2013 (https://iris.who.int/handle/10665/78067).
- Api O, Breyman C, Çetiner M, et al. Diagnosis and treatment of iron deficiency anemia during pregnancy and the postpartum period: iron deficiency anemia working group consensus report. Turk J Obstet Gynecol. 2015;12(3):1–7. doi: 10.4274/tjod.01700.
- Auerbach M, Goodnough LT, Picard D, et al. The role of intravenous iron in anemia management and transfusion avoidance. Transfusion. 2008;48(5):988–1000. doi: 10.1111/j.1537-2995.2007.01633.x-i2.
- Breymann C, Honegger C, Holzgreve W, et al. Diagnosis and treatment of iron-deficiency anaemia during pregnancy and postpartum. Arch Gynecol Obstet. 2010;282(5):577–580. doi: 10.1007/s00404-010-1532-z.
- Al RA, Unlubilgin E, Kandemir O, et al. Intravenous versus oral iron for treatment of anemia in pregnancy: a randomized trial. Obstet Gynecol. 2005;106(6):1335–1340. doi: 10.1097/01.AOG.0000185260.82466.b4.
- Bhandal N, Russell R. Intravenous versus oral iron therapy for postpartum anaemia. BJOG. 2006;113(11):1248–1252. doi: 10.1111/j.1471-0528.2006.01062.x.
- Peebles G, Fenwick S. Intravenous iron administration in a short-stay hospital setting. Nurs Stand. 2008;22(48):35–41. doi: 10.7748/ns2008.08.22.48.35.c6635.
- Jose A, Mahey R, Sharma JB, et al. Comparison of ferric carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy- randomised controlled trial. BMC Preg Childbirth. 2019;19(1):54. doi: 10.1186/s12884-019-2200-3.
- Breymann C, Milman N, Mezzacasa A, FER-ASAP investigators., et al. Ferric carboxymaltose vs. oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP). J Perinat Med. 2017;45(4):443–453. doi: 10.1515/jpm-2016-0050.
- Aksan A, Schoepfer A, Juillerat P, et al. Iron formulations for the treatment of iron deficiency anemia in patients with inflammatory bowel disease: a cost-effectiveness analysis in Switzerland. Adv Ther. 2021;38(1):660–677. doi: 10.1007/s12325-020-01553-1.
- Wesström J. Safety of intravenous iron isomaltoside for iron deficiency and iron deficiency anemia in pregnancy. Arch Gynecol Obstet. 2020;301(5):1127–1131. doi: 10.1007/s00404-020-05509-2.
- Seeho SKM, Morris JM. Intravenous iron use in pregnancy: ironing out the issues and evidence. Aust N Z J Obstet Gynaecol. 2018;58(2):145–147. doi: 10.1111/ajo.12794.
- Biétry FA, Hug B, Reich O, et al. Iron supplementation in Switzerland – a bi-national, descriptive, observational study. Swiss Med Wkly. 2017;147:w14444. doi: 10.4414/smw.2017.14444.
- Zoller H, Schaefer B, Glodny B. Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens. 2017;26(4):266–275. doi: 10.1097/MNH.0000000000000329.
- Huang LL, Lee D, Troster SM, et al. A controlled study of the effects of ferric carboxymaltose on bone and haematinic biomarkers in chronic kidney disease and pregnancy. Nephrol Dial Transplant. 2018;33(9):1628–1635. doi: 10.1093/ndt/gfx310.
- Nelson N, Finnström O, Larsson L. Neonatal reference values for ionized calcium, phosphate and magnesium. Selection of reference population by optimality criteria. Scand J Clin Lab Invest. 1987; 47(2):111–117. doi: 10.1080/00365518709168878.
- Bradford CV, Cober MP, Miller JL. Refeeding syndrome in the neonatal intensive care unit. J Pediatr Pharmacol Ther. 2021;26(8):771–782. Epub 2021 Nov 10. PMID: 34790066; doi: 10.5863/1551-6776-26.8.771.
- Mihatsch W, Fewtrell M, Goulet O, ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition., et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: calcium, phosphorus and magnesium. Clin Nutr. 2018; 37(6 Pt B):2360–2365. Epub 2018 Jun 18. doi: 10.1016/j.clnu.2018.06.950.
- Fenton TR, Lyon AW, Rose MS. Cord blood calcium, phosphate, magnesium, and alkaline phosphatase gestational age-specific reference intervals for preterm infants. BMC Pediatr. 2011;11(1):76. doi: 10.1186/1471-2431-11-76.
- Bustos Lozano G, Hidalgo Romero Á, Melgar Bonis A, et al. Hipofosforemia precoz en recién nacidos de riesgo. Frecuencia y magnitud. An Pediatr (Engl Ed). 2018;88(4):216–222. doi: 10.1016/j.anpedi.2017.04.010.
- Moltu SJ, Strømmen K, Blakstad EW, et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia – a randomized, controlled trial. Clin Nutr. 2013;32(2):207–212. doi: 10.1016/j.clnu.2012.09.004.
- Kilic O, Demirkol D, Ucsel R, et al. Hypophosphatemia and its clinical implications in critically ill children: a retrospective study. J Crit Care. 2012;27(5):474–479. Epub 2012 May 15. doi: 10.1016/j.jcrc.2012.03.005.
- Al-Wassia H, Lyon AW, Rose SM, et al. Hypophosphatemia is prevalent among preterm infants less than 1,500 grams. Am J Perinatol. 2019;36(13):1412–1419. Epub 2019 Jan 21. doi: 10.1055/s-0039-1677713.
- Ross JR, Finch C, Ebeling M, et al. Refeeding syndrome in very-low-birth-weight intrauterine growth-restricted neonates. J Perinatol. 2013; 33(9):717–720. Epub 2013 Mar 28. doi: 10.1038/jp.2013.28.
- Sung SI, Chang YS, Choi JH, et al. Increased risk of refeeding syndrome-like hypophosphatemia with high initial amino acid intake in small-for-gestational-age, extremely-low-birthweight infants. PLoS One. 2019; 14(8):e0221042. doi: 10.1371/journal.pone.0221042.
- Marinella MA. The refeeding syndrome and hypophosphatemia. Nutr Rev. 2003;61(9):320–323. doi: 10.1301/nr.2003.sept.320-323.
- García Martín A, Varsavsky M, Cortés Berdonces M, et al. Phosphate disorders and clinical management of hypophosphatemia and hyperphosphatemia. Endocrinol Diabetes Nutr (Engl Ed). 2020;67(3):205–215. Epub 2019 Sep 26. doi: 10.1016/j.endinu.2019.06.004.
- Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323(5):432–443. doi: 10.1001/jama.2019.22450.
- Hardy S, Vandemergel X. Intravenous iron administration and hypophosphatemia in clinical practice. Int J Rheumatol. 2015;2015:468675–468676. doi: 10.1155/2015/468675.
- Ryan BA, Kovacs CS. Calciotropic and phosphotropic hormones in fetal and neonatal bone development. Semin Fetal Neonatal Med. 2020;25(1):101062. doi: 10.1016/j.siny.2019.101062.
- Ma Y, Samaraweera M, Cooke-Hubley S, et al. Neither absence nor excess of FGF23 disturbs murine fetal-placental phosphorus homeostasis or prenatal skeletal development and mineralization. Endocrinology. 2014;155(5):1596–1605. doi: 10.1210/en.2013-2061.
- Malek A. In vitro studies of ferric carboxymaltose on placental permeability using the dual perfusion model of human placenta. Arzneimittelforschung. 2010;60(6a):354–361. doi: 10.1055/s-0031-1296300.
- Glaspy JA, Lim-Watson MZ, Libre MA, et al. Hypophosphatemia associated with intravenous iron therapies for iron deficiency anemia: a systematic literature review. Ther Clin Risk Manag. 2020;16:245–259. PMID: 32308402; doi: 10.2147/TCRM.S243462.
- Schaefer B, Tobiasch M, Wagner S, et al. Hypophosphatemia after intravenous iron therapy: comprehensive review of clinical findings and recommendations for management. Bone. 2022; 154:116202. Epub 2021 Sep 15. doi: 10.1016/j.bone.2021.116202.