Abstract
Low birth weight is associated with various complications, and recent findings rely on the fact that micronized progesterone supplementation leads to improved birth weight, which is crucial for addressing concerns related to fetal growth.
Objective
This study aimed to assess the impact of micronized progesterone (VMP4) supplementation on pregnancies with low serum pregnancy-associated plasma protein-A (PAPP-A) multiples of the median (MoM) values during first-trimester screening.
Methods
Out of 8933 patients evaluated, 116 pregnant women with low PAPP-A concentrations in their blood and no fetal chromosomal anomalies (CAs) were included. Three groups were formed: group 1 received VMP4 from 11 to 16 weeks (29 women, 25%), group 2 received VMP4 from 11 to 36 weeks (25 women, 21.5%), and group 3 (62 women, 53.5%) served as controls without receiving progesterone.
Results
Results indicated that group 3 had higher rates of complications, including miscarriages (16.37%), preterm delivery (17.8%), and fetal developmental abnormalities (19.4%). Birthweight variations were elevated in pregnancies without progesterone, contrasting with lower variations in VMP4 groups. Group 2, receiving VMP4 until 36 weeks, reported the lowest incidence of abortion and preterm birth (PB), along with the highest mean birth weight.
Conclusions
The conclusion suggests that 200 mg per day of VMP4 up to 36 weeks of supplementation led to fewer placental-related complications in women with very low PAPP-A at first-trimester screening (0.399 MoM). By reporting lower rates of miscarriages, PBs, and fetal developmental abnormalities in the micronized progesterone-treated groups, the study suggests a potential reduction in complications.
Introduction
One of the most widely used first-trimester combined screenings includes ultrasound examinations at 11 + 0 to 13 + 6 weeks and evaluation of specific biochemical markers, i.e. pregnancy-associated plasma protein-A (PAPP-A) and (β-HCG) [Citation1]. This is an efficient screening tool for chromosomal anomalies (CAs) detection. Moreover, several studies have suggested that physical and biochemical markers in the first trimester can also predict other adverse pregnancy outcomes [Citation2]. In particular, biochemical blood markers have not only been associated with fetal chromosomal abnormalities, but they can also predict other pregnancy disorders [Citation3].
In particular, low maternal serum PAPP-A, detected at 11 + 0 to 13 + 6 weeks of gestation, has been associated with placenta-associated disorders such as stillbirth, fetal growth restriction (FGR), preterm birth (PB), and preeclampsia (PE) in otherwise chromosomally normal fetuses. It is demonstrated that PAPP-A affects the expression of insulin growth factor (IGF) and impairs cellular mitosis and trophoblastic invasion of the decidua [Citation4,Citation5]. Thus, impaired levels of IGF may affect normal placentation and placental function. Far too little attention has been paid to this biomarker since it has been mainly associated with detecting fetal CAs.
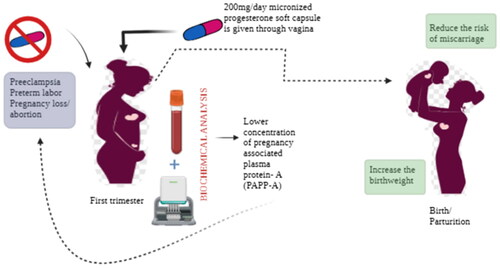
Micronized vaginal progesterone supplementation has been shown to reduce PB incidence in pregnant patients with a previous history of PB and with a short cervix detected by ultrasound at mid-gestation [Citation6,Citation7]. Moreover, vaginal micronized progesterone at a high dosage (800 mg/day) has also been reported to reduce the incidence of miscarriage in women with a history of abortion or with a threatened abortion and at least a previous pregnancy loss. Among the actions of progesterone, the migration of extravillous cytotrophoblast is promoted by the upregulation of an insulin-like growth factor (IGF) binding protein-1 [Citation8]. Therefore, there is a biological plausibility to use progesterone in low PAPP-A cases because progesterone looks to be a pivotal agent that favors placental bed formation [Citation9–11]. This study presents preliminary findings from an investigation where pregnant individuals with extremely low first-trimester PAPP-A levels and chromosomally normal fetuses received progesterone supplementation. While aspirin, statins, and LMWH show promise in this area, our focus on the first-trimester biomarker PAPP-A MoM is distinctive. Unlike its conventional use for chromosomal abnormalities, we explore its link to compromised placentation.
Materials and methods
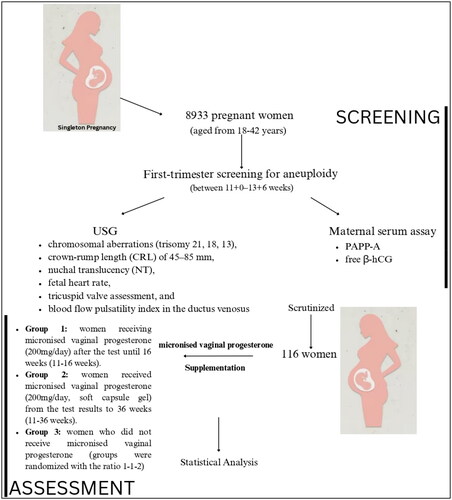
The study was conducted from January 2020 to December 2020 at the Leningrad Region Hospital, Saint Petersburg, Russia. The Ethical Committee approved the study protocol of Leningrad Region Hospital, Saint Petersburg, Russian Federation (No. 114; 14 November 2019). All participants provided written informed consent to participate in the study. The study included 8933 pregnant women aged 18–42 years (mean age of 35.9 years). Participants included in this study had a singleton pregnancy undergoing first-trimester screening for aneuploidy between 11 + 0 and 13 + 6 weeks. The complete patient enrollment data are categorized in .
The risk of chromosomal aberrations was assessed using the routine antenatal scan at 11 + 0 to 13 + 6 gestational weeks, which consisted of fetal ultrasound assessment combined with a maternal serum assay of PAPP-A and free β-hCG. Ultrasound examination included assessment of the risk markers of the most common chromosomal aberrations (trisomy 21, 18, 13), crown-rump length (CRL) of 45–85 mm, nuchal translucency (NT), fetal heart rate (HR), tricuspid valve assessment, and blood flow pulsatility index in the ductus venosus (PI-DV).
Biomarker analysis
Maternal blood serum was obtained at the same time of the US scan between 11 + 0 and 13 + 6 weeks of gestation and stored at a temperature of −20 °C. A technician blinded to clinical outcomes retrospectively measured the levels of PAPP-A, free β-hCG according to the manufacturer’s instructions on a fully automated immunoassays (BRAHMS KRYPTOR compact PLUS system, Thermo Fisher Scientific, Hennigsdorf, Germany). The analyzed data were converted into multiples of the median (MoM) values calculated by the Fetal Medicine Foundation (FMF) Batch MoMs calculator. It was adjusted for maternal characteristics (age, weight, ethnicity, parity, and smoking status), obstetric characteristics (conception method, gestational age at sampling), medical history (diabetes mellitus), and type of assay machine. Low values for PAPP-A (≤5th centile, ≤0.399 MoM) were calculated using the reference values published by Thermo Fisher (Hennigsdorf, Germany) for the BRAHMS KRYPTOR assay machine.
Inclusion criteria: Singleton pregnancy 11 + 0 to 13 + 6, the following PAPP-A MoM ranges were determined: PAPP-А <5th percentile = 0.001–0.399 MoM, chromosomally normal fetuses determined by performing CVS or amniocentesis in all cases with low PAPP-А.
Exclusion criteria: Maternal chronic disease, history of adverse obstetric outcome (miscarriage, stillbirth, PB, and low weight for gestational age), pregnancy obtained by assisted reproductive technology, twin pregnancy, and chromosomally abnormal fetus.
The study population included 116 women whose PAPP-A MoM was low according to the first-trimester screening. This group represent 1.29% of all those screened. The study did not consider PAPP-A MoM more than or equal to 0.4 MoM. The women were divided into three groups according to the supplementation with micronized vaginal progesterone. Group 1 women were receiving micronized vaginal progesterone (200 mg/day) after the test until complete 16 weeks (11–16 weeks). Group 2 women received micronized vaginal progesterone (200 mg/day, soft capsule gel) from the test results to complete 36 weeks (11–36 weeks). Group 3 women did not receive micronized vaginal progesterone (groups were randomized with the ratio 1–1–2).
Statistical analysis
The study utilized IBM SPSS 26.0 software for statistical analysis under the macOS system (IBM, Armonk, NY) platform. The Kolmogorov–Smirnov test determined the median and interquartile range (1st quarter style; 3rd quarter). The quantitative indicators were compared using a nonparametric analysis for assessing Nivali (Mann–Whitney’s U-test). Valid Spearman’s rank correlation coefficient can be used to study the relationship between variables in statistics. Associations were considered statistically significant when p = .05 was used.
Results
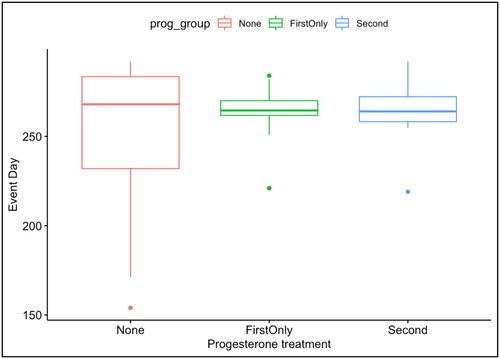
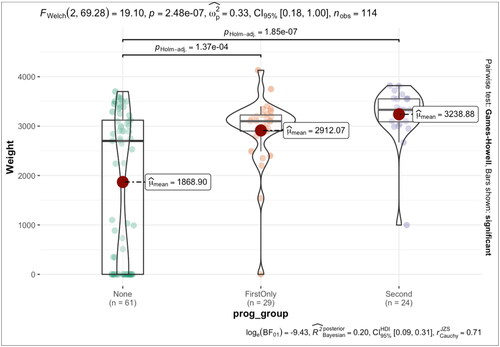
Out of 8933 patients assessed during the first trimester, 116 women with comprehensive medical records and chromosomally normal fetuses (confirmed by CVS or amniocentesis in all cases) were included. After 11 weeks, 25% received 200 mg of vaginal micronized progesterone daily up to 16 completed weeks, while 21.5% received 200 mg daily up to 36 completed weeks. summarizes the results obtained in all groups with low MoM PAPP-A. All women evaluated had an average age of 33 ± 6 years. In all cases, the mean pregnancy duration was 37.4 weeks. In group 3 (no progesterone supplementation), 19 cases (16.37%) out of 62 (53.4% of all women) reported miscarriage, 11 (17.8%) experienced preterm delivery, 12 (19.4%) reported FGR, and the mean birth weight of the offsprings was 1868 g. There are statistically significant differences (p values <.05) in the number of cases, rates of miscarriage, birthweight, pregnancy duration, and PI (DV) between group 1 vs. group 3 and group 2 vs. group 3. The proportion of cases is significantly different between all groups; the miscarriage rates are significantly lower in both group 1 and group 2 compared to group 3 (p = .0039* and p = .0023*, respectively). Similarly, the birthweight and pregnancy duration significantly differ between all groups (p < .0001 for both groups 1 and 2 when compared to group 3). The PI (DV) also significantly differed between group 1 vs. group 3 and group 2 vs. group 3 (p = .0018* and p = .0007*, respectively). Whereas, the characteristics, including NT, HR, and PI (DV), do not show statistically significant differences between the groups.
Table 1. Outcome of pregnancy in patients with a serum concentration of PAPP-A < 0.399 MoM in the first trimester receiving or not natural micronized vaginal progesterone.
Group 2 with supplementation up to 36 weeks, showed a statistically significant lower incidence of abortion, PB, and low birth weight. The median and the mean gestational age estimates appeared close; however, the standard deviation dispersion was significantly greater than average in group 3, as shown in ; Supplement material 1. The birthweight variance was significant in patients who were not receiving progesterone than in groups who had been administered micronized vaginal progesterone. There was a statistically significant (p < .001) association between micronized vaginal progesterone supplementation and higher birthweights, according to the Kruskal–Wallis test as well as a comparison between the two groups by pairwise Wilcoxon’s test as demonstrated in ; Supplement material 1.
Discussion
It is well known that the biochemical markers in the first trimester, particularly PAPP-A, in maternal serum are not only correlated with the risk of specific chromosomal abnormalities but also with the development of one or more outcomes associated with poor placentation and placental perfusion, such as miscarriage, stillbirth, pregnancy-induced hypertension (PIH) PE, and small for gestational age (SGA) [Citation12–17]. It is imperative to acknowledge the evolving landscape of therapeutic regimens aimed at preventing adverse pregnancy outcomes, particularly PIH and PE. Recent interventions, including administering aspirin, statins, and low molecular weight heparin (LMWH), have shown promise in mitigating the risks associated with these conditions. However, despite the expanding armamentarium of therapeutic options, our study uniquely addresses an unexplored avenue in preventing poor placentation-related complications.
To our knowledge, there has been a scarcity of studies investigating the role of a simple biomarker in the first trimester with a clear biological plausibility linked to compromised placentation. Our focus on PAPP-A MoM stands out as a potential risk indicator, extending beyond its conventional association with chromosomal abnormalities. Using progesterone as a preventive tool, guided by the PAPP-A MoM, adds a novel dimension to the field of prenatal care. While aspirin, statins, and LMWH have garnered attention for their prophylactic effects in high-risk pregnancies, our study sheds light on the possibility of tailoring preventive measures based on a specific biomarker indicative of early placental dysfunction.
We found that the administration of 200 mg/day of vaginal micronized progesterone in women found to have a very low serum level of PAPP-A (<5th centile) at the first-trimester screening was beneficial for prolonging pregnancy, decreasing the risk of a miscarriage of PB and increasing the offspring birth weight. All fetuses were shown to be without chromosomal abnormalities. The most plausible etiology relies on an alteration of the physiological trophoblast invasion and the transformation of the small muscular walls of the maternal spiral arteries into larger, more elastic vessels to increase blood flow to the placenta as in the normal pregnancy. PAPP-A, a protease to insulin-like growth factor binding protein-4 (IGFBP-4), facilitates the breakdown of this protein, resulting in a release of free IGF [Citation18]. IGFs are believed to play an important role in regulating trophoblast invasion of the decidua.
Impaired release of free IGFs may cause poor placenta perfusion, affecting fetal growth and other adverse pregnancy conditions [Citation19–21]. In an in vitro study carried out by Wang et al. [Citation22], PAPP-A resulted in significantly up-regulated by progesterone, which promotes the adhesion and proliferation potential of trophoblastic cells. Also, the serum levels of progesterone and PAPPA were closely correlated [Citation22]. Two high-quality RCT studies, the PROMISE trial [Citation23] and the PRISM trial [Citation24], have shown that treatment with vaginal micronized progesterone (400 mg twice daily) was associated with increasing live birth rates according to the number of previous miscarriages. These studies underlined that the crucial role of reaching advanced gestational age is linked with the multifunctional progesterone effect on the placental bed formation. Progesterone has been shown to induce high levels of cyclic adenosine monophosphate (cAMP) and time-dependent stimulation of nitric oxide synthetase, as well as inhibiting the formation of myometrial gap junctions. Progesterone and its metabolites induce uterine quiescence through interactions between nuclear and membrane progesterone receptors and maintain low levels of inflammatory prostaglandins, oxytocin, and intracellular calcium. These and many other functions of progesterone on the placenta and myometrium contribute to its role in preventing miscarriages and PB [Citation6,Citation25].
This event is accomplished through two stages of trophoblast migration under the angiogenic and immune modulation of progesterone. The first stage is the interstitial trophoblast invasion, and the second stage is the endovascular trophoblast invasion in the spiral arteries and incorporation into the arterial wall. The failure of their transformation is considered a cause of reduced perfusion to the intervillous space in women who subsequently may develop PE, FGR, preterm labor, preterm premature rupture of membranes, abruption of placentae, and stillbirth [Citation26]. Moreover, progesterone relaxes the uterus during pregnancy by inhibiting the expression of estrogen receptor alfa and reducing sensitivity to estrogens. Also, it has a vasodilatory effect on the uterine vessels before the 10th week of gestation. This feature, along with the decreased resistance of the placental bed, contributes to reducing systemic blood pressure until 28 weeks of gestation. Similarly, in the present study, we report that miscarriages were more common in those patients who did not receive progesterone supplementation, highlighting the importance of progesterone’s immune, endocrinological, and angiogenic functions.
Limitation of our study
The study’s limitation lies in its small sample size. Still, the total number of low PAPP-A patients comes from nearly 9000 pregnancies screened and dealing only with pregnancies in which the CVS or amniocentesis excluded chromosomal abnormalities.
Conclusions
Micronized vaginal progesterone appears to have a positive impact on reducing miscarriage rates and improving birthweight and pregnancy duration, especially when administered until 36 weeks. Group 2 shows more favorable outcomes than group 1 and group 3 regarding miscarriage rates, birth weight, and pregnancy duration. From these preliminary outcomes, the study demonstrates that 200 mg daily vaginal micronized progesterone supplementation up to 36 weeks in patients with low PAPP-A at first-trimester screening reduces placental-related problems, particularly abortion and PB, and increases offspring birthweight. This unique approach addresses a critical gap in the current literature, providing a foundation for further investigations into personalized and targeted interventions for pregnant individuals at risk of placenta-associated complications.
Ethical approval
The Ethical Committee approved the study protocol of Leningrad Region Hospital, Saint Petersburg, Russian Federation (No. 114; 14 November 2019). All participants provided written informed consent to participate in the study.
Author contributions
GCDR and VT conceptualized the article. AF, IG, and VT wrote the original draft. AF, IG, VT, and AM performed the literature search and selected bibliographic sources, AF, IG, and VT performed all clinical results. GCDR, VT, BAS, and AM performed review and editing. All authors read and agreed to the final version of the manuscript.
Supplemental Material
Download MS Word (40 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available upon request.
Additional information
Funding
References
- Nicolaides KH. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat Diagn. 2011;31(1):1–8. dosi:10.1002/pd.2637.
- Spencer K, Yu CKH, Cowans NJ, et al. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free beta-hCG and with second-trimester uterine artery Doppler. Prenat Diagn. 2005;25(10):949–953. doi:10.1002/pd.1251.
- Kumar M, Singh S, Sharma K, et al. Adverse fetal outcome: is first trimester ultrasound and Doppler better predictor than biomarkers? J Matern Fetal Neonatal Med. 2017;30(12):1410–1416.
- Aplin JD, Myers JE, Timms K, et al. Tracking placental development in health and disease. Nat Rev Endocrinol. 2020;16(9):479–494.
- Fisher SJ, McMaster M, Roberts JM. The placenta in normal pregnancy and preeclampsia. In: Taylor RT, Cunningham FG, Roberts JM, Lindheimer MD, editors. Chesley’s hypertensive disorders in pregnancy. 4th ed. London (UK): Academic Press; 2015. p. 81–112.
- Fonseca EB, Celik E, Parra M, et al. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–469. doi:10.1056/NEJMoa067815.
- Klein K, Rode L, Nicolaides KH, et al. Vaginal micronized progesterone and risk of preterm delivery in high-risk twin pregnancies: secondary analysis of a placebo-controlled randomized trial and meta-analysis. Ultrasound Obstet Gynecol. 2011;38(3):281–287. doi:10.1002/uog.9092.
- Halasz M, Szekeres-Bartho J. The role of progesterone in implantation and trophoblast invasion. J Reprod Immunol. 2013;97(1):43–50. doi: 10.1016/j.jri.2012.10.011.
- Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol. 2006;107(2 Pt 1):399–413.
- Tal RT. Endocrinology of pregnancy. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. South Dartmouth (MA): MDText.com, Inc.; 2000.
- Baulieu EE. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989;245(4924):1351–1357. doi:10.1126/science.2781282.
- Spencer K, Cowans NJ, Nicolaides KH. Low levels of maternal serum PAPP-A in the first trimester and the risk of pre-eclampsia. Prenat Diagn. 2008;28(1):7–10. doi:10.1002/pd.1890.
- Tul N, Pusenjak S, Osredkar J, et al. Predicting complications of pregnancy with first-trimester maternal serum free-betahCG, PAPP-A and inhibin-A. Prenat Diagn. 2003;23(12):990–996. doi:10.1002/pd.735.
- Smith GCS, Shah I, Crossley JA, et al. Pregnancy-associated plasma protein A and alpha-fetoprotein and prediction of adverse perinatal outcome. Obstet Gynecol. 2006;107(1):161–166. doi:10.1097/01.AOG.0000191302.79560.d8.
- Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER trial). Am J Obstet Gynecol. 2004;191(4):1446–1451. doi:10.1016/j.ajog.2004.06.052.
- Kavak ZN, Basgul A, Elter K, et al. The efficacy of first-trimester PAPP-A and free beta hCG levels for predicting adverse pregnancy outcome. J Perinat Med. 2006;34(2):145–148.
- Morssink LP, Kornman LH, Hallahan TW, et al. Maternal serum levels of free beta-hCG and PAPP-A in the first trimester of pregnancy are not associated with subsequent fetal growth retardation or preterm delivery. Prenat Diagn. 1998;18(2):147–152. doi:10.1002/(SICI)1097-0223(199802)18:2<147::AID-PD231>3.0.CO;2-W.
- Lawrence JB, Oxvig C, Overgaard MT, et al. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci U S A. 1999;96(6):3149–3153. doi:10.1073/pnas.96.6.3149.
- van Kleffens M, Groffen C, Lindenbergh-Kortleve DJ, et al. The IGF system during fetal–placental development of the mouse. Mol Cell Endocrinol. 1998;140(1–2):129–135. doi:10.1016/s0303-7207(98)00041-0.
- Conover CA, Bale LK, Overgaard MT, et al. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131(5):1187–1194. doi:10.1242/dev.00997.
- Irwin JC, Suen LF, Martina NA, et al. Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum Reprod. 1999;14(Suppl. 2):90–96. doi:10.1093/humrep/14.suppl_2.90.
- Wang J, Liu S, Qin HM, et al. Pregnancy-associated plasma protein A up-regulated by progesterone promotes adhesion and proliferation of trophoblastic cells. Int J Clin Exp Pathol. 2014;7(4):1427–1437.
- Coomarasamy A, Williams H, Truchanowicz E, et al. PROMISE: first-trimester progesterone therapy in women with a history of unexplained recurrent miscarriages – a randomised, double-blind, placebo-controlled, international multicentre trial and economic evaluation. Health Technol Assess. 2016;20(41):1–92. doi:10.3310/hta20410.
- Coomarasamy A, Harb HM, Devall AJ, et al. Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT. Health Technol Assess. 2020;24(33):1–70. doi:10.3310/hta24330.
- Piette PCM. The pharmacodynamics and safety of progesterone. Best Pract Res Clin Obstet Gynaecol. 2020;69:13–29.
- Devall AJ, Coomarasamy A. Sporadic pregnancy loss and recurrent miscarriage. Best Pract Res Clin Obstet Gynaecol. 2020;69:30–39.