Abstract
Objectives
There have been significant advances in the medical management of severe postpartum hemorrhage (sPPH) over recent decades, which is reflected in numerous published guidelines. To date, many of the currently available national and international guidelines recommend recombinant factor VIIa (rFVIIa) to be used only at a very late stage in the course of sPPH, as a “last resort”, before or after hysterectomy. Based on new safety data, rFVIIa has recently been approved by the European Medicines Agency (EMA) and Swissmedic for use in sPPH, if uterotonics are insufficient to achieve hemostasis, which in fact is significantly earlier in the course of postpartum hemorrhage (PPH). We therefore aimed to develop expert consensus guidance as a step toward standardizing care with the use of rFVIIa for clinicians managing women experiencing life-threatening sPPH.
Methods
The consensus process consisted of one face-to-face meeting with a group of nine experts, including eight obstetrician-gynecologists and a hematologist highly experienced in sPPH care in tertiary care perinatal centers. The panel was representative of multidisciplinary expertise in the European obstetrics community and provided consensus opinion in answer to pre-defined questions around clinical practice with rFVIIa in the management of sPPH. Recommendations have been based on current national and international guidelines, extensive clinical experience, and consensus opinion, as well as the availability of efficacy and new safety data.
Results
The expert panel developed 17 consensus statements in response to the 13 pre-defined questions on the use of rFVIIa in the management of sPPH including: available efficacy and safety data and the need for interdisciplinary expertise between obstetricians, anesthesiologists, and hematologists in the management of sPPH. Based on novel data, the experts recommend: (1) earlier administration of rFVIIa in patients with sPPH who do not respond to uterotonic administration to optimize the efficacy of rFVIIa; (2) the importance of hematological parameter prerequisites prior to the administration of rFVIIa to maximize efficacy; and (3) continued evaluation or initiation of further invasive procedures according to standard practice. Furthermore, recommendations on the timing of rFVIIa treatment within the sPPH management algorithm are outlined in a range of specified clinical scenarios and settings, including vaginal delivery, cesarean section, and smaller birthing units before transfer to a tertiary care center. The panel agreed that according to available, and new data, as well as real-world experience, there is no evidence that the use of rFVIIa in patients with sPPH increases the risk of thromboembolism. The authors acknowledge that there is still limited clinical effectiveness data, as well as pharmacoeconomic data, on the use of rFVIIa in sPPH, and recommend further clinical trials and efficacy investigation.
Conclusions
This expert panel provides consensus guidance based on recently available data, clinical experience, and expert opinion, augmented by the recent approval of rFVIIa for use in sPPH by the EMA. These consensus statements are intended to support clinical care for sPPH and may help to provide the impetus and a starting point for updates to existing clinical practice guidelines.
Introduction
Despite advancements in treatment, postpartum hemorrhage (PPH) remains the leading cause of maternal mortality worldwide [Citation1] according to the World Health Organization (WHO), accounting for 27% of global maternal deaths [Citation2,Citation3]; of these 8% of deaths occur in high-income countries and 20% in lower-income countries [Citation4]. Indeed, the Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK report found that rate of maternity mortality was doubled in women living in the most deprived areas, compared to the least deprived areas, with PPH accounting for 7% of maternal deaths [Citation5]. Thus, efforts to reduce severe maternal morbidity and mortality caused by PPH are a global health priority, as an estimated 50% of deaths could be potentially prevented with optimal management [Citation5,Citation6]. Appropriate management of PPH relies on prompt recognition and identification of the underlying cause which can be broadly categorized by the four T’s: tone (uterine atony), tissue (retained placenta and/or clots), trauma (lacerations or uterine rupture), and thrombin (clotting factor deficiency) [Citation6]. Medical treatment of severe PPH (sPPH) with recombinant factor VIIa (rFVIIa), when implemented as an adjunctive therapy and alongside other medical and surgical interventions, may reduce the need for invasive interventions [Citation7]. Off-label use of rFVIIa, prior to the European Medicines Agency (EMA) approval in 2022, provided a preliminary indication of efficacy as a treatment for patients with sPPH [Citation8,Citation9].
Despite the recommendations for use of rFVIIa in evidence-based guidelines, adoption of these is not common at the point of care [Citation10,Citation11]. Until recently, there has been a lack of efficacy and safety data from a dedicated clinical trial on the use of rFVIIa in the management of sPPH [Citation12]; this lack of evidence combined with the safety concerns surrounding the treatment side effects has resulted in caution from physicians in administering rFVIIa to these patients. Currently, there is no consensus amongst clinicians on a universally agreed best practice approach for the use of rFVIIa in the PPH management algorithm. However, the recent approval of rFVIIa in the indication of sPPH by the EMA, based on new safety data, reinforces the rationale for earlier use of rFVIIa within the clinical algorithms for management of sPPH.
Until the available guidelines are updated, our consensus recommendations aim to incorporate current clinical experience and expertise with recently available data on the use of rFVIIa and provide guidance for the safe and reasonable use of rFVIIa in the appropriate scenarios. We hope that this compendium of consensus statements informs the treatment decisions of clinicians managing sPPH and facilitates optimal care for women undergoing life-threatening obstetric hemorrhage.
Materials and methods
The consensus statements outlined in this paper were developed by an expert panel including an initial online collaboration, followed by a face-to-face meeting, and finally several rounds of revision. Two additional panel members, one anesthesiologist and one obstetrician, were included during the revision and review rounds, as planned ahead of the consensus conference. The final document represents the opinions and guidance of all panel members.
Pre-meeting initial collaboration
We conducted a global search of the currently available guideline recommendations on the management of sPPH. Within these, we manually reviewed if use of rFVIIa in the management of sPPH was permitted, restricted, should only be used as a last resort, or was not recommended to be used outside of clinical research protocols. Articles that were not published in English were translated using an online translator. A review of the current international published guidelines indicated that, although the EMA only recently granted approval for the indication of rFVIIa for the treatment of sPPH, most of the guidelines (26/29; 90%) already include recommendations for use of rFVIIa ().
Figure 1. Summary of country-specific recommendations and guidelines on the use of rFVIIa in the management of sPPH.
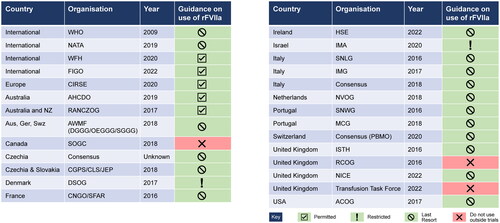
Prior to the face-to-face meeting, and based on the guidelines and recommendations outlined in , 13 key focus questions requiring clarification were developed, pioneered by Professors Di Renzo, Surbek, and Blatný. The face-to-face meeting was held on 21 September 2023 in Florence, Italy, at the PREIS School, and was funded by Novo Nordisk. The consensus group included nine physicians with extensive experience in the clinical management of PPH in their local countries, eight of whom were obstetrician-gynecologists, and one of whom was a hematologist.
Face-to-face meeting
The full-day meeting involved an overview of the current PPH guidelines, new data regarding the use of rFVIIa in the management of sPPH, and management of PPH from a hematologist’s perspective. The previously defined questions were discussed among the experts until the entire panel engaged in the following questions and an overall consensus was reached.
All participants reviewed the final statements offline after the meeting.
Results and discussion
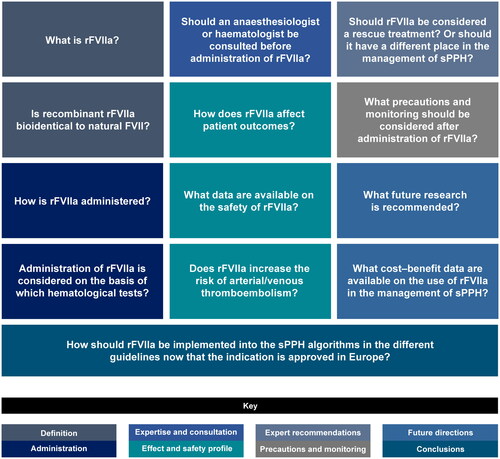
The expert panel met face-to-face and discussed 17 statements related to the use of rFVIIa in the management of sPPH, based on the pre-defined questions (). The following consensus statements are based on their opinions, clinical expertise and knowledge and review of the current literature, and were agreed upon by all members.
Figure 2. Questions discussed by the Steering Committee during the face-to-face meeting. All participants reviewed the final statements offline after the meeting.
Definition of rFVIIa
Eptacog Alfa (activated) is recombinant coagulation factor VIIa with a molecular mass of approximately 50,000 Da produced in baby hamster kidney (BHK) cells by recombinant DNA technology [Citation13].
rFVIIa is the activated form of FVII and shares the same sequence. rFVIIa is administered in pharmacological doses (×200 higher than endogenous concentrations), and as such, is able to bind to activated platelets with relatively low affinity, thus bypassing the tissue factor (TF)-dependent mechanism that endogenous FVIIa functions through [Citation13,Citation14].
Administration of rFVIIa
FVIIa is administered intravenously, and it needs to be diluted, prior to which it has a shelf life of up to 36 months [Citation11]. Once diluted and kept in aseptic conditions, FVIIa is stable at room temperature for up to 24 h, but should be administered as soon as possible. The recommended standard dose range is 60–90 µg/kg body weight, and a second dose can be administered in the case of insufficient hemostatic response no sooner than after 30 min.
The optimal hematological parameters for administration of rFVIIa are: no hypothermia, i.e. a core body temperature >35 °C and no acidosis; pH >7.2; no hypofibrinogenemia or severe thrombocytopenia (fibrinogen >1–2 g/L and platelets >50,000/mm3).
Effect and safety profile of rFVIIa
Treatment with supraphysiologic (pharmacologic) doses of rFVIIa has the potential to improve hemostasis in patients who are experiencing life-threatening hemorrhage, which can lead to a reduced requirement for further invasive procedures, in turn reducing morbidity and preserving fertility.
According to new data in sPPH, there is no evidence to show that rFVIIa increases the risk of venous or arterial thromboembolism, and this is the only safety concern that has been raised [Citation12].
Expertise and consultation in the administration of rFVIIa
Multidisciplinary expertise in the management of sPPH is recommended: an anesthesiologist should be consulted when considering use of rFVIIa. A hematologist should be consulted when setting up local treatment algorithms at individual obstetric units and/or when releasing regional/national guidelines on sPPH. While consultation with a hematologist in an acute setting of sPPH for an individual patient might be desirable, this possibility may not be available in many obstetric units.
Precautions and safety considerations following rFVIIa administration
The patient should continue to be monitored as usual according to local department protocols, and no further specific tests or extra monitoring is required for the management of sPPH following rFVIIa administration. Also, further steps of medical or surgical/invasive treatments should be considered as indicated according to the clinical situation and according to the local treatment algorithm.
Expert recommendations on the timing of rFVIIa administration in patients with sPPH
At initial diagnosis of sPPH, uterotonics and tranexamic acid are the mainstay treatments that are administered as first-line therapy, and the recommendations that follow for treating sPPH are based on the assumption that these initial management steps have been previously implemented.
There are numerous potential scenarios to consider with respect to optimal timing of rFVIIa administration for sPPH; these are summarized in , and detailed below.
Table 1. The recommendations on the timing of rFVIIa use in sPPH during specified clinical scenarios.
Scenario 1: following vaginal delivery
In sPPH after vaginal delivery, consider administration of rFVIIa after use of all first-line noninvasive and invasive measures (uterotonics, tranexamic acid, IV fluid/blood/blood product and fibrinogen administration, laceration suturing, curettage, uterine tamponade) if the patient is still bleeding, before proceeding to laparotomy or uterine artery embolization.
Scenario 2: during a cesarean section (C-section)
In sPPH during a C-section, consider use of rFVIIa after failure of first-line surgical measures (uterine compression sutures, vessel ligation, uterine tamponade), but prior to uterine artery embolization or hysterectomy.
Scenario 3: following a C-section
After a C-section, when the patient is in the ICU or PACU and the laparotomy has been closed but persistent non-severe bleeding is apparent, consider administration of rFVIIa (eventually in combination with uterine artery embolization), while not delaying re-laparotomy. In cases of severe intraabdominal bleeding with significant hemoglobin decrease and/or cardiovascular instability, emergency revision laparotomy should be performed without delay.
Scenario 4: in a peripheral birthing unit resource setting
In the case of sPPH in a patient in a peripheral birthing unit, when transfer to a larger center is considered, rFVIIa can be administered during the transfer set-up to stabilize the patient and to decrease the risk of deterioration of the bleeding during transfer. Transfer should be considered as early as possible, particularly in the case of failure of first-line uterotonics. Administration of rFVIIa before transfer should be considered based on the current clinical situation and the expected timeframe of the transfer until the patient arrives at the center. However, treatment with rFVIIa shall not delay the patient’s transfer to a larger center, nor the implementation of second-line uterotonic treatment (prostaglandins) and intra-uterine tamponade balloon where available.
The above recommendation depends on the local medical resources and organization of care.
Scenario 5: during a hysterectomy
During a postpartum hysterectomy due to sPPH, if continual severe bleeding occurs and hemostasis is difficult, consider administration of rFVIIa, together with other standard measures.
For all the presented cases above, it is of the utmost importance that if a patient is hemodynamically unstable or uncontrolled bleeding continues, further medical treatments or surgical intervention must be considered without delay. In addition, prerequisites for optimal effect of rFVIIa use are sufficient levels of platelets and fibrinogen, which should be achieved by respective measures, if necessary, prior to treatment with rFVIIa.
Future directions
Currently, there are no cost–benefit/effectiveness data available for rFVIIa in the management of sPPH. As data from the randomized clinical trial (RCT) show that invasive procedures can be reduced with the use of rFVIIa [Citation15], and as treatment of a patient with sPPH would already incur significant costs, it is conceivable that rFVIIa use in sPPH may even have a positive cost–benefit ratio. Nevertheless, we recommend collecting these data in future research. Given that sPPH is an emergency situation, minimizing costs should not be the first priority when considering use of rFVIIa.
In addition, more clinical research is needed to provide effectiveness data for the use of rFVIIa in sPPH, specifically regarding the combination with current standard of care (tranexamic acid and fibrinogen); at the time of ongoing severe bleeding after balloon tamponade; evaluation of the optimal timing of administration of rFVIIa in sPPH patients; and in further specific scenarios, such as in the case of placental accreta syndrome (PAS).
Conclusions
We recommend that guidelines should be revised to appropriately incorporate the use of rFVIIa in sPPH management algorithms, taking into account the availability of new and reassuring safety data on rFVIIa, and the approval for the treatment of sPPH in Europe by the EMA and Swissmedic. The novel data did not find an increased thromboembolic risk or any additional safety concerns regarding the use of rFVIIa in patients with sPPH. Furthermore, the timing of rFVIIa administration may be crucial to its effectiveness, i.e. the earlier rFVIIa is administered in sPPH, the more beneficial it is likely to be [Citation16,Citation17].
Author contributions
D. Surbek, J. Blatný, M. Wielgos, N. Acs, H. Edwards, O. Erez, J. L. Bartha, H. Madar, and G. C. Di Renzo were involved in the face-to-face meeting and developed the initial consensus statements included in this study, coordinated by D. Surbek and G. C. Di Renzo. F. J. Mercier and D. Schlembach were included in the expert panel as external advisors to the consensus. All authors were involved in the refinement and finalization of the consensus statements included in this paper, and the consensus statements are based on clinical expertise, the current literature and the opinions of the experts. All authors were involved in writing this manuscript. All authors read and approved the final manuscript.
Disclosure statement
D. Surbek: advisory board and lecture honoraria from Novo Nordisk, CSL Behring, and Vifor (in part in favor of departmental research funding). J. Blatný: speaker and/or consultation fee from Novo Nordisk, Roche, Takeda, Octapharma, and Sobi. N. Acs: advisory board and lecture honoraria from Novo Nordisk. H. Edwards: advisory board for Novo Nordisk. F. J. Mercier: honoraria received by Novo Nordisk as speaker (symposium/webinar) and consultant. D. Schlembach: advisory board rFVIIa Novo Nordisk, Organon Advisory Board PPH, and CSL Behring – lecture fee (on PPH). M. Wielgos, O. Erez, J. L. Bartha, H. Madar, and G. C. Di Renzo have no conflicts of interest to declare.
Data availability statement
No new data were created or analyzed during this study. Data sharing is not applicable to this article.
Additional information
Funding
References
- Park SC, Yeom SR, Han SK, et al. Recombinant activated factor VII as a second line treatment for postpartum hemorrhage. Korean J Crit Care Med. 2017;32(4):1–8. doi: 10.4266/kjccm.2016.00787.
- Chauke L, Bhoora S, Ngene NC, et al. Postpartum hemorrhage – an insurmountable problem? Case Rep Womens Health. 2023;37:e00482. doi: 10.1016/j.crwh.2023.e00482.
- Gallos I, Devall A, Martin J, et al. Randomized trial of early detection and treatment of postpartum hemorrhage. N Engl J Med. 2023;389(1):11–21. doi: 10.1056/NEJMoa2303966.
- Bienstock JL, Eke AC, Hueppchen NA, et al. Postpartum hemorrhage. N Engl J Med. 2021;384(17):1635–1645. doi: 10.1056/NEJMra1513247.
- Knight M, Bunch K, Patel R, et al. Saving lives, improving mothers’ care core report – lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2019–21. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2023.
- Evensen A, Anderson JM, Fontaine P, et al. Postpartum hemorrhage: prevention and treatment. Am Fam Physician. 2017;95(7):442–449.
- Welsh A, McLintock C, Gatt S, et al. Guidelines for the use of recombinant activated factor VII in massive obstetric hemorrhage. Aust N Z J Obstet Gynaecol. 2008;48(1):12–16. doi: 10.1111/j.1479-828X.2007.00823.x.
- Magon N, Babu K. Recombinant factor VIIa in post-partum hemorrhage: a new weapon in obstetrician’s armamentarium. N Am J Med Sci. 2012;4(4):157–162. doi: 10.4103/1947-2714.94938.
- Alfirevic Z, Elbourne D, Pavord S, et al. Use of recombinant activator factor VII in primary postpartum hemorrhage. Obstet Gynecol. 2007;110(6):1270–1278. doi: 10.1097/01.AOG.0000288515.48066.99.
- Surbek D, Vial Y, Girard T, et al. Patient blood management (PBM) in pregnancy and childbirth: literature review and expert opinion. Arch Gynecol Obstet. 2020;301(2):627–641. doi: 10.1007/s00404-019-05374-8.
- Schlemback D, Helmer H, Henrich W, et al. Peripartum haemorrhage, diagnosis and therapy. Guideline of the DGGG and SGGG (S2k Level, AWMF Registry No. 015/063, March 2016). Geburtshilfe Frauenheilkd. 2018;78:382–399.
- Committee for Medicinal Products for Human Use (CHMP). CHMP extension of indication variation assessment report (rFVII®, Eptacog Alfa), European Public Assessment Report (EPAR); 2022 [cited 2022 Apr 22]. Procedure No. EMEA/H/C/000074/II/0116.
- NovoSeven (Eptacog Alfa) Summary of Product Characteristics; 2023; [cited 2023 Nov]. Available from: https://www.ema.europa.eu/en/documents/product-information/novoseven-epar-product-information_en.pdf
- Monroe DM, Hoffman M, Oliver JA, et al. Platelet activity of high-dose factor VIIa is independent of tissue factor. Br J Haematol. 1997;99(3):542–547. doi: 10.1046/j.1365-2141.1997.4463256.x.
- Lavigne-Lissalde G, Aya AG, Mercier FJ, et al. Recombinant human FVIIa for reducing the need for invasive second-line therapies in severe refractory postpartum hemorrhage: a multicenter, randomized, open controlled trial. J Thromb Haemost. 2015;13(4):520–529. doi: 10.1111/jth.12844.
- Sørensen B, Dargaud Y, Kenet G, et al. On-demand treatment of bleeds in haemophilia patients with inhibitors: strategies for securing and maintaining predictable efficacy with recombinant activated factor VII. Haemophilia. 2012;18(2):255–262. doi: 10.1111/j.1365-2516.2011.02612.x.
- Lusher JM. Early treatment with recombinant factor VIIa results in greater efficacy with less product. Eur J Haematol Suppl. 1998;63:7–10. doi: 10.1111/j.1600-0609.1998.tb01103.x.

