Abstract
Objective
To determine the effectiveness of oral dydrogesterone in preventing miscarriage in threatened miscarriage.
Methods
A randomized, controlled trial study was conducted among pregnant Thai women at the gestational age of six to less than 20 weeks who visited King Chulalongkorn Memorial Hospital, Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand with threatened miscarriage from August 2021 to August 2022. These pregnant women were randomized to receive oral dydrogesterone 20 mg per day or placebo twice a day until one week after vaginal bleeding stopped or otherwise for a maximum of six weeks.
Results
A total of 100 pregnancies were recruited. Fifty of them were assigned to receive oral dydrogesterone and 50 were assigned to receive placebo. The rate of continuing pregnancy beyond 20 weeks of gestational age was 90.0% (45 out of 50 women) in the dydrogesterone group and 86.0% (43 out of 50 women) in the placebo group (p = 0.538). The incidence of adverse events did not differ significantly between the groups.
Conclusion
Oral dydrogesterone 20 mg/day could not prevent miscarriages in women with threatened miscarriage.
Introduction
Threatened miscarriage is a condition in which a pregnant woman with a gestational age less than 20 weeks presents with vaginal bleeding and a positive viable intrauterine pregnancy is confirmed by an ultrasound [Citation1]. Its prevalence in pregnancy is around 20% [Citation2]. On physical examination, there would be no abdominal tenderness and the cervix would be closed. There is a 12% chance of miscarriage when threatened miscarriage is diagnosed [Citation3].
Other possible obstetric complications that could follow a threatened miscarriage are placental abruption and preterm labor [Citation4]. Bleeding is the most predictive factor for subsequent pregnancy loss. Currently, there is no standard treatment for threatened miscarriage other than observation and follow-up. Acetaminophen could help relieve abdominal pain. However, bed rest does not improve obstetric outcomes, and intercourse is prohibited until bleeding subsides [Citation1,Citation5].
Progesterone is a vital hormone for embryo implantation. It supports the continuation of pregnancy by suppressing the inflammation process of the pregnant woman toward the embryo [Citation6]. It is also a natural relaxant of the uterus. There are two receptors for progesterone, progesterone receptor (PR)-A and PR-B. Nuclear PRs perform their main function as ligand-activated transcription factors. These receptors located in epithelial and stromal/decidual cells in the endometrium, smooth muscle cells in the myometrium, and stromal fibroblasts in the cervix. Nuclear PRs expressed by myometrial cells mediate relaxatory actions during pregnancy. Increased actions of PR-A from altered methylation, thought to inhibit PR-B-mediated uterine relaxation actions. In addition, progesterone inhibits contraction associated proteins (CAP) genes expression in the myometrium resulted in uterine relaxation. Moreover, Progesterone could reduce natural killer (NK)-cell activity, increase HLA-G production in trophoblast cells, increase suppressor-cell levels, inhibit cytotoxic T-cell activity, induce the production of lymphocyte-blocking proteins, and modify the cytokine response from the Th-1 to the pro-pregnancy Th-2 pattern [Citation7,Citation8]. Progesterone is produced from the corpus luteum until the placental-luteal shift at the gestational age of 7-11 weeks. Lutectomy before gestational age of 7 weeks or the use of mifepristone could lead to miscarriage. Therefore, it can be concluded that progesterone is important for the continuation of pregnancy [Citation6].
Dydrogesterone is a synthetic progesterone with a high specificity to progesterone receptors in the human body. No virilization or feminization toward patients and their in-utero fetuses has been reported from its use. The drug has a high bioavailability; therefore, it is effective in lower doses. The oral route is better than the vaginal route in a higher blood concentration of the drug, especially when vaginal bleeding is faced [Citation9]. Dydrogesterone is no longer available in United states, United Kingdom or Australia.
There is a plethora of scientific evidence on the use of oral dydrogesterone in women with threatened miscarriage. Some studies have reported that the oral dydrogesterone group had a higher continuation rate of pregnancy after the gestational age of 20 weeks and a lower rate of miscarriage [Citation10–12]. On the contrary, a study found that the use of oral progestogen in women with threatened miscarriage in the first trimester did not reduce the risk of miscarriage or improve the live birth rate [Citation13]. The other trials consisted of vaginal progestogen compared to conservative treatment or placebo, with concordant results showing that there were no statistically significant differences in terms of preventing miscarriage between the two groups [Citation14–16].
Pregnant women with threatened miscarriage are often concerned about the possibility of adverse pregnancy outcomes, especially miscarriage. Due to the contradictory of the results from previous studies. Some studies found that oral dydrogesterone could reduce the risk of miscarriage [Citation10–12], while one study found that oral dydrogesterone did not reduce the risk of miscarriage [Citation13]. Thus, the objective of this study was to determine the effectiveness of oral dydrogesterone in preventing miscarriage in threatened miscarriage.
Materials and methods
This study was a randomized, double-blind, placebo-controlled trial conducted at the Department of Obstetrics and Gynecology, King Chulalongkorn Memorial Hospital, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. The research protocol was approved by the Institutional Review Board of the Faculty of Medicine of Chulalongkorn University. This clinical trial was registered at ClinicalTrials.gov (Clinical trials registration: NCT04788108). This study followed CONSORT guidelines.
This study was conducted from August 2021 to August 2022. It included 100 pregnant Thai women aged 18 to 45 years who came to King Chulalongkorn Memorial Hospital with threatened miscarriage and gestational age of 6–20 weeks accompanied by confirmation of viable intrauterine pregnancy from transvaginal ultrasound. Exclusion criteria included recurrent miscarriages greater than or equal to three times, cervical polyp, active infection, having an autoimmune disease or cancer as an underlying disease, abnormal coagulation, having an empty gestational sac size of more than 25 millimeters found through transvaginal ultrasound, and being allergic to dydrogesterone. All participants gave their written informed consent.
Participants were randomly assigned to the oral dydrogesterone and placebo group using the block of four technique. A computer was used to generate random numbers. The co-investigator, who had no contact with the participants, generated the allocation sequence before the study began. The nurses enrolled and assigned the participants to their respective groups. The drugs and placebos were prepared prior to the commencement of the study by a pharmacist who was not involved in the study.
Opaque envelopes containing 28 tablets of dydrogesterone or placebo (identical in size, shape, and color) were sequentially labeled. To ensure randomization, each envelope was distributed in a sequential numerical order. In the oral dydrogesterone group, the pregnant women received oral dydrogesterone at a dosage of 20 mg/day twice every day. In the placebo group, participants received placebo drugs orally and at the same time as that of the progestogen group. Both groups received the same instruction to ingest the received tablets until one week after vaginal bleeding had stopped or for a maximum duration of 6 weeks. They were advised against strenuous activity.
The researchers called the participants every week until the vaginal bleeding had stopped for one week or a miscarriage had occurred. Participants were asked about their bleeding symptoms, drug compliance, and adverse effects. They were scheduled to visit the antenatal care clinic two weeks after their first episode of vaginal bleeding as per the hospital protocol. Participants and physicians were blinded throughout the trial.
The trial’s primary outcome was the continuation of pregnancy beyond 20 weeks. In this study, miscarriage was defined as the delivery of the fetus before 20 weeks. The secondary outcomes were live birth rate, obstetric complications (including preterm birth, placenta previa, placental abruption, and fetal growth restriction), neonatal complications (such as respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and early neonatal death), side effects, drug compliance, satisfaction and the duration of drug use until vaginal bleeding stopped. Regarding drug compliance, good compliance was defined as participants who ingested more than 80% of the total amount of the given drugs.
From our pilot study with 20 participants, we observed that the success rate of continuing pregnancy beyond the gestational age of 20 weeks in the dydrogesterone and placebo groups was 60% and 30%, respectively. We further calculated that 40 pregnant women needed to be included in each trial group to provide 80% power to detect the difference between the groups, at a two-sided alpha level of 0.05. We planned to include 100 women in the trial for an expected 25% loss to follow-up.
Statistical analysis was performed using SPSS version 22.0. Categorical variables were presented as frequency and percentages, while continuous variables were presented as mean and standard deviation. The independent t-test was used to compare continuous variables, whereas the Mann-Whitney U test was used to compare nonparametric variables. The Chi-square test and the Fisher exact test were used to compare categorical variables. The relative risk (RR) and the 95% confidence interval (CI) were calculated. A p value < 0.05 was considered statistically significant. The trial was conducted in an intention-to-treat analysis.
Results
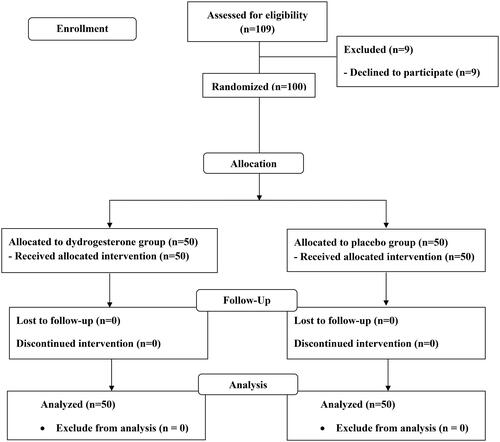
A total of 109 pregnant women were eligible to be included in the study from August 2021 to August 2022. Nine of them refused to participate, and 100 remaining pregnant women were randomly assigned to receive oral dydrogesterone (50 women) or placebo (50 women). The baseline characteristics and demographic data were similar between the two groups (). All participants were included in the analysis due to the intention-to-treat analysis ().
Table 1. Baseline characteristics (n = 100).
The rate of continuation of pregnancy beyond the gestational age of 20 weeks was not significanlyt different between oral dydrogesterone and the placebo group (90.0% and 86.0%, respectively, RR 1.19, 95%CI 0.71–2.02, p = 0.538) (). There was also no significant difference in live birth rate between oral dydrogesterone and the placebo group (90.0% and 86.0%, respectively, RR 1.19, 95%CI 0.71–2.02, p = 0.538).
Table 2. Rate of continuation of pregnancy beyond 20 weeks of gestation and live birth (n = 100).
For obstetric outcomes, the mean gestational age of delivery was 37 weeks for both groups (p = 0.302). The mean birth weights were 2891.0 g and 3,014.1 grams from dydrogesterone and placebo groups, respectively (p = 0.197). The delivery route was not statistically different between the groups, with the cesarean section being the most common (60.0% and 53.5%, respectively). For obstetric complications regarding preterm birth, placenta previa and fetal growth restriction, there were no statistically significant differences between the dydrogesterone and placebo groups (p = 0.704, 1.000 and 1.000, respectively). No placental abruption had occurred in the participants ().
Table 3. Obstetric and neonatal outcomes (n = 88).
For neonatal outcomes, there were no differences in neonatal complications (8.8% vs 4.7%, respectively, p = 0.676). There was an early neonatal death from the oral dydrogesterone group, which was a full-term female newborn with an appropriate birth weight for the gestational age. The fetus was diagnosed with congenital diaphragmatic hernia with persistent pulmonary hypertension and pneumothorax of the left lung, and she died on the day of life 1. No intraventricular hemorrhage or necrotizing enterocolitis was reported. The mean Apgar score at one minute was 8.7 in both groups. Furthermore, the mean Apgar score at five minutes was also not statistically different between the two groups with scores of 9.7 and 9.8, respectively (p = 0.789) ().
Most of the participants had a duration of vaginal bleeding of less than 7 days, resulting in a mean duration of drug use of 10.9 days for both groups (p = 0.951). There were no statistically significant differences between both groups in the aspect of side effects (p = 0.548). Most of the participants had a good drug compliance with more than 80% of tablets regularly ingested without any statistical difference between the groups (90.0% and 94.0%, respectively, p = 0.715). Both groups had good satisfaction with the use of drugs (96% vs. 98%, p = 1.000) ().
Table 4. Duration of bleeding, drug use, side effects, compliance, and satisfaction (n = 100).
Discussion
This randomized, double-blind, placebo-controlled trial found that the continuation of pregnancy beyond gestational age of 20 weeks was not statistically different between the oral dydrogesterone and placebo groups. The mean gestational age at delivery was 37 weeks in both groups. There were no differences in obstetric and neonatal complications, side effects, drug compliance, satisfaction with drug use, and duration of drug use until vaginal bleeding stopped.
This study reported that progesterone in women with threatened miscarriage did not prevent miscarriage or increase the live birth rate. This is corroborated by previous studies [Citation13,Citation17–19]. The result of this study was in contrast to previous studies [Citation10–12] found that treatment of miscarriage with oral progestogens reduced the risk of miscarriage. As there are many possible causes of miscarriage, including chromosomal abnormalities in the fetuses, placental anomalies, increased maternal age and maternal obesity, progestogen might not be able to effectively prevent miscarriage in all cases [Citation20]. The contradictory findings may be due to the different ethnicity and the different cause of threatened miscarriage.
The suggested theory for the results was that progesterone could not prevent miscarriage in all cases of threatened miscarriage. The differences from previous studies [Citation10–12] may be due to the different cause of threatened miscarriage in each population.
Recent large multicenter, randomized, double-blind, placebo-controlled trial in the United Kingdom published in 2019 (PRISM trial) compared vaginal micronized progesterone with placebo in pregnant women with threatened abortion with gestational age less than 12 weeks [Citation19]. Their primary outcome was the birth of a live baby after 34 weeks of gestation. This was 75% and 72% in the progesterone and placebo groups, respectively (RR 1.03, 95%CI 1.00–1.07, p = 0.08). They defined miscarriage as loss of pregnancy before 24 weeks of gestation. They reported no statistical difference in miscarriage rate between the two groups (20% and 22%, respectively, RR 0.91, 95%CI 0.81–1.01). The result of the PRISM trial on the prevention of miscarriage was not different from previous trials comparing vaginal progestogen with placebo or conservative treatment [Citation14–16,Citation19].
The strength of our study was a randomized, double-blind, placebo-controlled trial. Although oral dydrogesterone has a high bioavailability and higher blood concentration than the vaginal route, especially in vaginal bleeding [Citation9], our result still did not establish a difference in terms of preventing miscarriage between oral dydrogesterone and placebo groups.
However, there were some limitations in this trial. The serum progesterone levels were not measured and compared between the two groups. This study included patients in both the first and second trimester which have different characteristics and causes of bleeding. This may be the confounding factor. Furthermore, we did not send conceptus tissue of the miscarriage cases for chromosomal analysis, as there were previous reports on the potential benefit of progestogen for euploid pregnancies, especially with greater numbers of previous miscarriages [Citation21]. Other limitations were that there were small sample size and selection criteria. This study included patients with gestational age less than 20 weeks.
Conclusion
It can be concluded that oral dydrogesterone 20 mg/day was not different from placebo in preventing miscarriage in pregnant women faced with threatened miscarriage. Moreover, oral dydrogesterone also did not differ from placebo in terms of obstetric and neonatal outcomes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, VP. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Sotiriadis A, Papatheodorou S, Makrydimas G. Threatened miscarriage: evaluation and management. BMJ. 2004;329(7458):1–7. doi: 10.1136/bmj.329.7458.152.
- Qureshi NS. Treatment options for threatened miscarriage. Maturitas. 2009;65 Suppl 1:S35–S41. doi: 10.1016/j.maturitas.2009.10.010.
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ. 1997;315(7099):32–34. doi: 10.1136/bmj.315.7099.32.
- Saraswat L, Bhattacharya S, Maheshwari A, et al. Maternal and perinatal outcome in women with threatened miscarriage in the first trimester: a systematic review. BJOG. 2010;117(3):245–257. doi: 10.1111/j.1471-0528.2009.02427.x.
- Aleman A, Althabe F, Belizán J, et al. Bed rest during pregnancy for preventing miscarriage. Cochrane Database Syst Rev. 2005;2005(2):CD003576. doi: 10.1002/14651858.CD003576.pub2.
- Mirza FG, Patki A, Pexman-Fieth C. Dydrogesterone use in early pregnancy. Gynecol Endocrinol. 2016;32(2):97–106. doi: 10.3109/09513590.2015.1121982.
- Patel B, Elguero S, Thakore S, et al. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21(2):155–173. doi: 10.1093/humupd/dmu056.
- Pařízek A, Koucký M, Dušková M. Progesterone, inflammation and preterm labor. J Steroid Biochem Mol Biol. 2014;139:159–165. doi: 10.1016/j.jsbmb.2013.02.008.
- Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2008;61(1-2):171–180. doi: 10.1016/j.maturitas.2008.11.013.
- El-Zibdeh MY, Yousef LT. Dydrogesterone support in threatened miscarriage. Maturitas. 2009;65 Suppl 1: S43–S6. doi: 10.1016/j.maturitas.2009.11.013.
- Omar MH, Mashita MK, Lim PS, et al. Dydrogesterone in threatened abortion: pregnancy outcome. J Steroid Biochem Mol Biol. 2005;97(5):421–425. doi: 10.1016/j.jsbmb.2005.08.013.
- Pandian RU. Dydrogesterone in threatened miscarriage: a Malaysian experience. Maturitas. 2009;65 Suppl 1:S47–S50. doi: 10.1016/j.maturitas.2009.11.016.
- Chan DMK, Cheung KW, Ko JKY, et al. Use of oral progestogen in women with threatened miscarriage in the first trimester: a randomized double-blind controlled trial. Hum Reprod. 2021;36(3):587–595. doi: 10.1093/humrep/deaa327.
- Yassaee F, Shekarriz-Foumani R, Afsari S, et al. The effect of progesterone suppositories on threatened abortion: a randomized clinical trial. J Reprod Infertil. 2014;15(3):147–151.
- Alimohamadi S, Javadian P, Gharedaghi MH, et al. Progesterone and threatened abortion: a randomized clinical trial on endocervical cytokine concentrations. J Reprod Immunol. 2013;98(1–2):52–60. doi: 10.1016/j.jri.2013.01.004.
- Gerhard I, Gwinner B, Eggert-Kruse W, et al. Double-blind controlled trial of progesterone substitution in threatened abortion. Biol Res Pregnancy Perinatol. 1987;8(1 1ST Half):26–34.
- McLindon LA, James G, Beckmann MM, et al. Progesterone for women with threatened miscarriage (STOP trial): a placebo-controlled randomized clinical trial. Hum Reprod. 2023;38(4):560–568. doi: 10.1093/humrep/dead029.
- Coomarasamy A, Harb HM, Devall AJ, et al. Progesterone to prevent miscarriage in women with early pregnancy bleeding: the PRISM RCT. Health Technol Assess. 2020;24(33):1–70. doi: 10.3310/hta24330.
- Coomarasamy A, Devall AJ, Cheed V, et al. A randomized trial of progesterone in women with bleeding in early pregnancy. N Engl J Med. 2019;380(19):1815–1824. doi: 10.1056/NEJMoa1813730.
- Yovich JL, Mariappen U, Hinchliffe PM, et al. MPA given orally during the first trimester for threatened miscarriage carries no specific risk for foetal abnormalities albeit the rate is higher than non-threatened pregnancies. Reprod Biol. 2020;20(3):424–432. doi: 10.1016/j.repbio.2020.03.008.
- Progestogens for preventing miscarriage: ectopic pregnancy and miscarriage: diagnosis and initial management: evidence review C. NICE Evidence Reviews Collection. London2021.