Abstract
Objective
Infants who meet the screening guidelines for retinopathy of prematurity (ROP) based on birth weight and gestational age undergo serial ophthalmological examinations for its detection and treatment. However, <10% of patients require treatment, and less than half develop ROP. Poor postnatal weight gain has been reported to be a strong indicator of ROP development; however, the information regarding this is unclear. Therefore, this study aimed to determine the relationship between postnatal weight gain and ROP development in preterm infants.
Methods
The data of 675 preterm infants with gestational age ≤32 weeks, who were hospitalized in our neonatal intensive care unit, were obtained retrospectively from file records. The infants’ demographic characteristics, clinical findings, and weekly weight gain (g/kg/day) during the first 8 weeks were recorded. The univariate was used to examine the risk factors for ROP followed by multivariate regression.
Results
The incidence of ROP in the infants included in the study was 41% (n = 278) and 13.3% (n = 37) of them required treatment. In the infants of the group that developed ROP, the mean birth weight and gestational age were significantly lower than those in the group that did not develop ROP (973 ± 288 and 1301 ± 349 g, p = 0.001 and 28.48 ± 1.95 and 30.08 ± 1.60 weeks, p = 0.001, respectively). As the gestational week and birth weight decreased, ROP development and the risk of ROP-requiring treatment increased. In the infants of the group that developed ROP, the mean weight gain in the postnatal third week was detected as significantly lower compared to those in the group that did not develop ROP (13.9 ± 8.2 and 15.4 ± 6.8 g, p = 0.034). On multiple logistic regression analysis, birth weight (<750 g) (odds ratio [OR], 8.67; 95% confidence interval [CI], 3.99–18.82, p = 0.001), blood transfusion (OR, 2.39; 95% CI, 1.34–4.24, p = 0.003), necrotizing enterocolitis (OR, 4.79; 95% CI, 1.05–26.85, p = 0.045), bronchopulmonary dysplasia (OR, 2.03; 95% CI, 1.22–3.36, p = 0.006), antenatal steroid therapy (OR, 1.60; 95% CI, 1.05–2.43, p = 0.028), surfactant administration (OR, 2.06; 95% CI, 1.32–3.2, p = 0.001) were independent risk factors for ROP development.
Conclusion
Postnatal weight gain may not be an accurate predictor of ROP development after adjusting for confounding factors. However, the analysis of independent risk factors that influenced the development of ROP revealed a statistically significant effect in cases of low birth weight, blood transfusion, necrotizing enterocolitis, bronchopulmonary dysplasia, and antenatal steroid and surfactant therapies. These findings may help ophthalmologists and neonatologists to pay special attention to this patient group during ROP scanning.
Introduction
Premature retinopathy (ROP) is a condition that develops because of the abnormal proliferation of retinal vessels in low birth weight (BW) and premature infants, the pathogenesis of which is not completely understood [Citation1]. It is a leading cause of preventable blindness in children [Citation2]. Although the chance of survival of premature infants with very small gestational age (GA) and BW has increased owing to developments in newborn care, ROP, which can cause vision problems and blindness, occurs more frequently in both developed and developing countries [Citation3]. Although mild ROP forms can self-correct, if severe cases are not treated, they may cause retinal scars during decollement and blindness [Citation4]. Therefore, the early diagnosis and treatment of ROP are important to prevent disease progression.
Many guidelines consider BW and GA as important risk factors for identifying infants requiring ROP screening. According to the recommendations of the American Pediatric Academy and the American Academy of Ophthalmology, screening is recommended for all infants whose BW is ≤1500 g and/or GA is ≤30 weeks as well as for the infants whose GA is >30 weeks, BW is 1500–2000 g, and who have clinical diseases, and who require cardiopulmonary support [Citation5]. This is a simple risk model that includes two factors, GA and BW, and is very sensitive and covers almost all serious ROP cases. However, it has <100% sensitivity, and because 5% of infants require treatment, it is not very specific [Citation6–8]. ROP examination is a painful process that may lead to acute events in newborns, including decreased oxygen-saturation levels, increased heart rate, and increased apnea frequency. In addition, unnecessary ROP screening may cause additional health expenditure costs [Citation9], and the limited number of ophthalmologists with experience in ROP may present an obstacle to screening [Citation10]. Therefore, over the last 15 years, efforts have been made to develop better screening criteria by adding other risk factors to BW and GA to identify infants with significant ROP development potential requiring treatment and to reduce the number of infant examinations [Citation11]. The most promising predictive factor is poor postnatal weight gain [Citation12].
ROP pathogenesis involves multiple factors, including retinal oxygen levels, local vascular endothelial growth factor (VEGF), and insulin-like growth factor 1 (IGF-1). Clinical studies have shown an association between low postnatal serum IGF-1 levels and increased risk of ROP [Citation13,Citation14]. Serum IGF-1 levels are associated with fetal and postnatal growth, but require blood sampling for measurement and are not routinely used in neonatal intensive care units. In these units, routine weight monitoring and weight gain may be used instead of IGF-1 measurements [Citation12]. Based on this information, Hellstrom et al. first used postnatal weight gain in a predictive model for ROP. In this model, called the Weight and Insulin-Like Growth Factor 1 in Neonatal Retinopathy (WINROP) model, data from 79 Swedish infants were used [Citation15]. While the first algorithm included both postnatal weight measurements and serum IGF-1 levels, later versions included weight gain alone [Citation12]. In another Swedish study, the WINROP model was shown to have 100% sensitivity to the development of severe ROP and reduced the number of examinations by 76% [Citation12]. However, in multiple validity studies, the sensitivity was consistently lower [Citation16,Citation17]. In a recent multicenter study called postnatal growth and ROP (G-ROP), a new BW, GA, and postnatal growth model was proposed to predict the development of ROP. This new model has shown a 30% decrease in the number of infants requiring screening in North America, as well as 100% sensitivity in type-1 and ROP prediction requiring treatment [Citation18]. In a study conducted in Turkey to test the validity of this G-ROP model, sensitivity was determined to be 88.3% and 91.2% for any stage of ROP and ROP-requiring treatment, respectively, and specificity was determined to be 51.7% and 34.1% for any stage of ROP and ROP-requiring treatment, respectively [Citation19]. While current risk models such as WINROP and G-ROP had 100% sensitivity in the first tests, they were shown to have decreased sensitivity when applied to other populations and skipped certain infants with serious diseases when largely applied. Furthermore, many studies assessing the relationship between postnatal weight gain and ROP based on these models have established different outcomes. While in one study, in the fourth and sixth weeks, low weight gain and weight gain rate were predictive of stage 3+ ROP development [Citation20], another study found a relationship between poor weight gain and severe ROP development in the first postnatal 4 weeks, and this relationship was not observed for weight gain in the sixth week. Furthermore, when multiple logistic regression analysis was performed involving all risk factors for ROP, poor weight gain in the fourth postnatal week was not considered an independent risk factor [Citation21].
Although poor postnatal weight gain has been reported to be a strong indicator of ROP development, information regarding this is unclear. Therefore, this study aimed to determine the relationship between postnatal weight gain and ROP development during the first 8 weeks of life and other potential risk factors for ROP in preterm infants.
Materials and methods
Study group
An observational retrospective study was conducted on premature infants ≤ 32 weeks who were admitted to the level three Newborn Intensive Care Unit (NICU) between 2002 and 2011. The preterm infants were excluded from the study when they could not be examined until complete retinal vascularization, they presented congenital anomalies, died before eye examination or had incomplete medical data.
Maternal and neonatal factors that would affect the development of ROP were obtained retrospectively from file records: maternal age, consanguineous marriage, mode of delivery, gestation (single or multiple), antenatal steroid therapy, in vitro fertilization, premature rupture of membranes (PROM), chorioamnionitis, preeclampsia, maternal diabetes mellitus, smoking, gestational age, birth weight, small for GA (SGA), sex, intrauterine growth restriction (IUGR), cardiopulmonary resuscitation, APGAR score at 1 and 5 min, Cord pH, other neonatal factors such as respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH, stage 3–4), hemodynamically significant patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC, ≥2), periventricular leukomalacia, sepsis (clinical or proven sepsis), hyperbilirubinemia requiring phototherapy, apnea, postnatal steroid, caffeine or theophylline therapy, thyroxine (fT4) level, Thyroid Stimulating Hormone (TSH) level, thrombocytopenia (< 100,000/mm3), hyperglycemia (glucose > 180 mg/dL), mechanical ventilation, noninvasive ventilation and supplemental O2 therapy duration (days), parenteral nutrition (PN) duration (days), blood transfusion number, and ROP screening results. The daily measured weights for each infant until the postnatal eighth week was calculated as weekly gain (g/kg/day).
The primary outcome was to assess the effect of postnatal weight gain on the development of ROP during the first 8 weeks. Secondary outcome was to identify the other potential risk factors affecting the development of ROP.
Using the Fenton growth curve, the cases with a birth weight between 10 and 90 percentiles according to the gestational age were called AGA (Appropriate for Gestational Age), and the cases with a birth weight <10 percentile were called SGA (Small for Gestational Age).
ROP diagnosis and screening
In our unit, on the basis of the recommendations of the American Pediatric Academy and the American Pediatric Ophthalmology and Strabismus Association, all the infants whose birth weight was ≤1500 g and/or gestational age ≤ 32 weeks, as well as those infants who were born in after 32 weeks and having birth weight between 1500 and 2000 g and having clinical problems, and those requiring cardiopulmonary support are screened [Citation22]. However, only babies born at ≤ 32 weeks were included in the study. For the infants born within < 27 weeks, the first ROP examination was performed in the postmenstrual thirty-first week. For the infants born within ≥27 weeks, the first ROP examination was performed in the postnatal fourth week. ROP examination was performed with a binocular indirect ophthalmoscope using 20 and 28 diopter lenses by an ophthalmologist who was proficient in ROP after a lid speculum was inserted. One hour before the examination, 1 drop of 2.5% phenylephrine for mydriasis two to three times at 5-min intervals and 0.5% tropicamide were dripped. The ROP monitoring was decided by the ophthalmologist based on the findings of the first examination. In accordance with the follow-up guideline recommended by the American Academy of Pediatrics and the American Academy of Ophthalmology, complete vascularization of the retina, ROP regression, or retinal examination was performed until treatment was required [Citation23].
Statistical analysis
All statistical analyses were performed using Number Cruncher Statistical System 2007 (NCSS, Kaysville, UT). The enrolled infants were divided into two groups: infants who developed ROP or those who did not. In addition, to evaluate the impact of risk factors on the severity of ROP, infants who developed ROP were subsequently divided into subgroups: infants who required treatment or those who did not. To identify risk factors, each one was initially analyzed by univariate analysis. We employed Student’s t-test for intergroup comparisons of normally distributed numerical parameters, and the Mann–Whitney U test for intergroup comparisons of non-normally distributed parameters. Pearson’s chi-square test, Fisher’s exact test, and Yates Continuity Correction test were used to compare qualitative data. Data are presented as the means ± standard deviation (SD), percentages (%), or medians (min-max) as appropriate. Backward stepwise logistic regression analysis using was performed to identify the independent risk factors for ROP development expressed as odds ratios (OR) and 95% confidence intervals (CI). A probability (p) value less than 0.01 and 0.05 were considered statistically significant.
Results
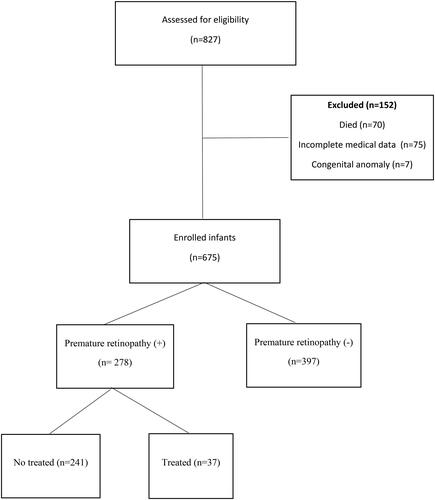
A total of 827 preterm infants with a GA of ≤32 weeks were admitted to neonatal intensive care unit during the study period. A total of 675 infants were included in the study, and 152 were excluded (). The mean GA of all patients included in the study was 29.4 ± 1.9 (23–32) weeks, and the mean BW was 1165 ± 362 (460–2840) g. Of the 675 newborns included in the study, 278 (41%) developed ROP with 19, 253, and 6 in zone-I, -II, and -III diseases, respectively; 115 had stage 1 (41%), 129 had stage 2 (46%), and 32 had stage 3 (11.5%). Aggressive posterior ROP was detected in two infants (0.7%). Of the infants with ROP, 38.5% (n = 111) were <28 weeks of gestational age, and 57.5% (n = 160) had a BW <1000 g. Further, 37 (13.3%) of 278 infants with ROP received treatment. Among the infants who received treatment, 81% (n = 30) were treated with laser photocoagulation, 2.7% (n = 1) with cryotherapy, and 16.2% (n = 6) required both treatments (laser photocoagulation and intravitreal anti-VEGF).
Univariate analysis identified various factors as significant risk factors for ROP development. The demographic characteristics and the clinical results of mothers and infants are presented in . In the infants of the ROP-developing group, the mean BW and GA were significantly lower than those in the group that did not develop ROP (973 ± 288 and 1301 ± 349 g, p = 0.001 and 28.48 ± 1.95 and 30.08 ± 1.60 weeks, p < 0.001, respectively). In the infants of the group that developed ROP, IUGR, cardiopulmonary resuscitation, SGA, and antenatal steroid use rates were significantly higher than those in the group that did not develop ROP (p = 0.012, p = 0.001, p = 0.001, and p = 0.031, respectively). The mean Apgar scores at the first and fifth minutes were significantly lower (p = 0.001). The rates of RDS, surfactant administration, BPD, IVH, PDA, NEC, sepsis, apnea, postnatal steroid therapy, thrombocytopenia, and hyperglycemia were significantly higher in the infants of the group that developed ROP than those in the group that did not (p = 0.001). The mean duration of mechanical ventilation and oxygen therapy, mean duration of total parenteral nutrition, and number of blood transfusions were significantly higher in the infants of the group that developed ROP than those in the group that did not develop ROP (p = 0.001). The free T4 (FT4) and thyroid-stimulating hormone (TSH) levels were significantly lower (p = 0.001) in the infants who developed ROP than those in the infants who did not. There were no significant differences based on maternal age, consanguineous marriage, cesarean delivery, multiple pregnancies, in vitro fertilization, preeclampsia, diabetes, PROM, chorioamnionitis, smoking, sex, cord pH, periventricular leukomalacia, hyperbilirubinemia, caffeine intake, or theophylline therapy for ROP development (p > 0.05; ).
Table 1. Univariate analysis of risk factors for the development of ROP.
In the infants who developed ROP, the mean daily postnatal weight gain at the third, fourth, and fifth postnatal weeks was lower than that in those who did not develop ROP. A statistical significance was observed in the third week (p = 0.034). In the infants of the ROP-developing group, the mean weight gain in the sixth and seventh weeks was significantly higher than that in those of the group that did not develop ROP (p = 0.049 and p = 0.017, respectively), and no significant difference was found between the two groups in terms of mean weight gain in the first, second, and eighth weeks (p > 0.05; ).
Table 2. Comparison of the daily weight gain in non-ROP and ROP groups according to weeks.
All significant risk factors affecting ROP were evaluated using a backward stepwise logistic regression analysis. The analysis was conducted on 160 infants with all risk factors. Taking as reference the birth weight of babies over 1250 g; BW (1000–1249 g) (odd ratio [OR], 2.58; 95% confidence interval [CI], 1.51–4.43, p = 0.001), BW (750–999 g) (OR, 4.91; 95% CI, 2.78–8.66, p = 0.001), BW (<750 g) (OR, 8.67; 95% CI, 3.99–18.82, p = 0.001), blood transfusion (OR, 2.39; 95% CI, 1.34–4.24, p = 0.003), necrotizing enterocolitis (OR, 4.79; 95% CI, 1.05–26.85, p = 0.045), bronchopulmonary dysplasia (OR, 2.03; 95% CI, 1.22–3.36, p = 0.006), antenatal steroid therapy (OR, 1.60; 95% CI, 1.05–2.43, p = 0.028), surfactant administration (OR, 2.06; 95% CI, 1.32–3.2, p = 0.001) were independent risk factors for ROP development ().
Table 3. Independent risk factors for retinopathy of prematurity.
When we evaluated the effect of risk factors on the severity of ROP, the mean BW and GA, apgar scores at the first and fifth minutes and TSH levels were significantly lower in the infants of the ROP-requiring treatment group than those in the ROP group that did not require treatment (p = 0.001). The rates of RDS, surfactant administration, BPD, IVH, NEC, sepsis, postnatal steroids, thrombocytopenia, the mean duration of mechanical ventilation and oxygen therapy, mean duration of total parenteral nutrition, and number of blood transfusions were significantly higher in the infants of the group that ROP-requiring treatment than those in the group that did not (p < 0.01).
There were no significant differences based on maternal age, consanguineous marriage, antenatal steroid, IUGR, cesarean delivery, multiple pregnancies, in vitro fertilization, preeclampsia, maternal diabetes, PROM, chorioamnionitis, smoking, gender, Cord pH, PDA, periventricular leukomalacia, hyperbilirubinemia, apnea, hyperglycemia, thyroxine level and caffeine intake, or theophylline therapy (p > 0.05) (). No difference was found in the comparison of daily weight gain according to weeks in the treated and untreated ROP group (p > 0.05) (Not shown).
Discussion
The results of our study indicate that postnatal weight gain in the third week was significantly lower among infants with ROP; however, when all the risk factors for ROP development in the univariate analysis were included in the logistic regression, poor postnatal weight gain did not arise as an independent risk factor. In our cohort of preterm infants, certain well-defined premature morbidities were more common in infants with ROP. The association between poor postnatal weight gain and ROP may be attributed to common postnatal risk factors that cause them. We consider that poor weight gain lost significance in logistic regression as it was a result of other clinical comorbidities rather than an independent risk factor for ROP.
This is in contrast to the findings of many studies indicating that poor postnatal weight gain is a predictive factor for the development of ROP. Wallace et al. suggested that poor postnatal weight gain (g/day) in the first 6 weeks was an independent risk factor for the development of severe ROP [Citation24]. Similarly, Cabañas Poy et al. demonstrated that GA and poor postnatal weight gain during the first 6 weeks of life were significantly associated with the need for treatment in infants with ROP, and postnatal weight gain at 6 weeks was predictive of the need for ROP treatment [Citation25]. Meanwhile, a multicenter study reported that poor weight gain on postnatal day 28 was an independent risk factor for the development of severe ROP [Citation26].
Contrary to these studies and supporting our findings, Rao et al. reported no relationship between postnatal weight gain in the fourth, fifth, and sixth weeks and severe ROP development in very low BW infants [Citation27]. Similarly, a prospective cohort study of 487 very low BW infants found that weight gain in the first 4 weeks of life was not a significant predictor of ROP [Citation21]. Lundgren et al. showed that after the target oxygen saturation range increased from 88%–92% to 91%–95%, the predictive effect of postnatal weight gain for ROP-requiring treatment decreased [Citation28].
The two predominantly known risk factors for the development of ROP are GA and BW [Citation29]. In our study, BW and GA were significantly lower in patients who developed ROP and ROP-requiring treatment. As GA and BW decreased, the incidence of ROP and ROP-requiring treatment increased. Multiple logistic regression analysis identified low BW as an independent risk factor for ROP development. While having a BW <750 g increases the development of ROP by a factor of 8.67, having a BW 750–999 g increases it by a factor of 4.91, having a BW 1000–1249 g increases it by a factor of 2.58. Similarly, in many studies, BW was reported to be a risk factor for both ROP development and ROP-requiring treatment [Citation30,Citation31]. In a multicenter Cryotherapy for Retinopathy of Prematurity study in which 4099 infants whose BW was <1251 g were included, low BW and GA were found to be related to threshold ROP development [Citation32]. In this study, it was concluded that every 100-g increase in BW decreased the threshold ROP development by 27%, while an increase in GA by every 1 week decreased the threshold ROP development by 19% [Citation32].
Several studies have reported that lower Apgar scores, which are a general indicator of poor neonatal health, may be associated with higher ROP [Citation25]. However, on multivariate regression analysis, the relationship was not significant in most cases, as observed in our study. Furthermore, in our study, neonatal cardiopulmonary resuscitation was associated with increased risks of ROP development and ROP-requiring treatment. In addition to the underlying systemic disease necessitating resuscitation, the possibility of worsening of the patient’s general condition or periods of post-cardiopulmonary resuscitation hypoxia followed by hyperoxia is likely related to abnormal retinal vascular development in these patients.
Antenatal steroid therapy was an independent risk factor for ROP in our study; however, no significant difference was found between ROP patients requiring treatment and those who did not. The effect of antenatal steroid use on ROP development is controversial. In a meta-analysis, antenatal steroid use was reported to reduce ROP development and the risk of progression to severe ROP [Citation33]. However, another study involving 7200 infants conducted in Sweden showed that antenatal steroid treatment increased ROP development by 1.57 times [Citation34]. In other studies, no association was found between antenatal steroid administration and ROP development [Citation35]. Antenatal steroids are administered to pregnant women at risk of premature birth because it accelerates fetal lung maturation and reduces RDS as well as other neonatal morbidities, such as NEC and IVH. In our study, the development of ROP may not be directly related to antenatal steroid use; however, antenatal steroid use is more likely due to the lower gestation weeks of this group of infants.
Many studies have suggested that neonatal respiratory diseases are closely associated with the development of ROP [Citation1]. RDS and apnea are associated with an increased risk of ROP in our study. RDS is caused by a surfactant deficiency; therefore, newborns with RDS may require mechanical ventilation and oxygen therapy. In addition, infants with apnea require mechanical ventilation and oxygen treatment. However, apnea was not associated with ROP-requiring treatment.
Some studies have suggested that surfactant administration is closely associated with ROP [Citation1], which may be interpreted as a higher risk for ROP in infants with RDS-requiring treatment. In this study, we found that surfactant administration was associated with a higher risk of ROP development and ROP-requiring treatment. It was also found to be an independent risk factor on multivariate analysis. Similarly, BPD was associated with an increased risk of ROP development and ROP-requiring treatment. Additionally, BPD was found to be an independent risk factor for the development of ROP. These cases involved patients with chronic respiratory disease, in whom episodes of desaturation and subsequent hyperoxia were frequent during oxygen treatment, which could explain the increased risk. Additionally, postnatal steroid therapy is associated with a higher risk of ROP development and ROP-requiring treatment. Similarly, several studies on preterm infants have reported a significant association between postnatal steroid administration and an increased risk of ROP [Citation36]. As the incidence of BPD is higher in infants who develop ROP, steroid use could be high owing to BPD being a main indication for its use.
In our study, severe IVH was associated with the development of ROP and ROP-requiring treatment. IVH and ROP have similar characteristics. In both cases, incidence increased with decreasing GA and BW. IVH develops because of the fragility of germinal matrix vessels and fluctuations in cerebral blood flow [Citation1]. ROP is caused by immature retinal vascularization and unstable oxygen treatment [Citation1].
In infants with PDA, decreased perfusion due to systemic blood flow bypass may cause retinal hypoxia and predispose them to developing ROP [Citation1]. PDA has been associated with ROP development, but no relationship was found with ROP-requiring treatment in our study. Unlike our study findings, other studies have found that PDA is an independent risk factor for ROP [Citation25].
The pathophysiology of NEC may involve innate immune responses against the gut microbiota, leading to inflammation. Animal models have shown that systemic inflammation affects retinal angiogenesis, suggesting a possible relationship between NEC and ROP [Citation1]. In our study, a significant relationship was found between NEC and the development and treated ROP. NEC has also been identified as an independent risk factor for ROP. Similarly, Fundora et al. reported that infants with surgical NEC, especially early surgical NEC, were at a higher risk of ROP and severe ROP [Citation37].
Many studies have indicated that sepsis was closely related to the appearance of the development of retinopathy [Citation1,Citation25]. In our study, sepsis was a risk factor for the development of ROP and ROP-requiring treatment. Systemic inflammation can disrupt angiogenesis and hypotension, and fluctuations in oxygen saturation can affect retinal perfusion, leading to retinal ischemia [Citation1].
Studies on the effects of thyroid hormones on angiogenesis have shown that thyroid hormones increase angiogenesis, especially by increasing the proangiogenic factor levels. Therefore, it is reasonable to assume a relationship between thyroid hormone levels and ROP [Citation38]. TSH and FT4 levels were significantly lower in infants who developed ROP; however, only TSH levels were significantly lower in infants with ROP-requiring treatment in our study. Coşkun et al. found that TSH measurements were significantly lower in the group with ROP than the measurements in those without ROP. In addition, FT4 levels were significantly lower in those in the treated group than that in those in the non-treated ROP group. However, after multivariate analysis, thyroid hormone levels were not independent risk factors for ROP development [Citation39]. In another study, no relationship was observed between thyroid function and ROP development [Citation40].
Hyperglycemia is associated with increased mortality and morbidity in preterm infants [Citation41]. In our study, hyperglycemia was found to be associated with the development of ROP, but not with ROP-requiring treatment. Similarly, several studies reporting mean blood glucose concentrations in infants with different ROP statuses have suggested a relationship between hyperglycemia and ROP [Citation1]. However, some studies have reported no association between hyperglycemia and ROP [Citation42].
Platelets store, transport, and deliver several key angiogenesis regulators, including VEGF. Therefore, a possible role of platelets in the pathogenesis of ROP can be hypothesized. If platelets provide a mean to sequester accumulating VEGF and temper retinal angiogenesis, thrombocytopenia could permit a greater degree of unregulated retinal neovascularization when IGF-1 levels increase and activate accumulated VEGF [Citation1]. In our study, thrombocytopenia was associated with the development of ROP and ROP-requiring treatment. Similar to our findings, Choręziak et al. also found a relationship between thrombocytopenia and the development of ROP [Citation43]. In addition, Vinekar et al. reported spontaneous resolution of aggressive posterior ROP after correction of thrombocytopenia with platelet transfusion [Citation44]. Considering thrombocytopenia as a potential risk factor for ROP development, the authors proposed platelet transfusion as a new treatment strategy for ROP [Citation44].
The mean duration of oxygen therapy was significantly longer in infants with ROP and those with ROP-requiring treatment. Regarding assisted ventilation, we found that any modality duration (mechanical ventilation or noninvasive ventilation) was related to the development of ROP and ROP-requiring treatment.
This study indicates that blood transfusion has a strong effect on ROP development and is its independent risk factor. Transfusions may increase the retina’s exposure to oxygen due to the low oxygen affinity of adult hemoglobin, catalyze the formation of iron-loaded reactive oxygen species, and may lead to ROP development by accelerating oxidative damage [Citation1]. Although many studies have shown that blood transfusion is a risk factor for ROP development [Citation25], some have reported that a restricted transfusion policy does not reduce ROP development [Citation45].
In our study, the mean duration of parenteral nutrition was significantly higher among infants with ROP and those with ROP-requiring treatment. Similarly, some studies have indicated that prolonged parenteral nutrition is a risk factor for ROP [Citation1]. However, a Turkish randomized controlled study showed that fish oil lipid emulsions were associated with a lower incidence of any-stage ROP [Citation46]. Because parenteral nutrition containing soybean oil was used in our study, a comparison could not be made with lipid emulsions containing fish oil.
Different, supportive, and conflicting conclusions have been reported in clinical studies regarding the risk factors affecting ROP development. The differences in the demographic characteristics of the infants included in the study, such as race, ethnicity, GA, and BW; protocols applied in the neonatal intensive care unit between the hospitals or countries studied; clinical outcomes such as survival rate and premature morbidities; clinical experience of ophthalmologists; diagnostic disputes between ophthalmologists; and changes in ROP screening and treatment guidelines over time may well lead to different outcomes in these clinical trials. Therefore, prospectively designed studies with standardized study groups are needed to identify the risk factors affecting the development of ROP.
The main strength of our study is that it is the most comprehensive study evaluating the risk factors in the development of ROP among the studies conducted till date. However, this study has some limitations. First, this was a retrospective study. Second, we assumed that poor postnatal weight gain was caused by low serum IGF-1 concentration. However, serum IGF-1 concentrations have not yet been measured in clinical practice. In addition, the data are from one center, indicating that a specific population is not adequately reflected. Thus, prospective multi-center cohort studies on relationships between postnatal weight gain and ROP are warranted.
Postnatal weight gain, as predicted in previous studies, may not be an accurate predictor of ROP development after adjusting for confounding factors based on our study. However, the analysis of independent risk factors that influenced the development of ROP revealed a statistically significant effect in cases of low birth weight, blood transfusion, necrotizing enterocolitis, bronchopulmonary dysplasia, and antenatal steroid and surfactant therapies. These findings may help ophthalmologists and neonatologists to pay special attention to this patient group during ROP scanning.
Acknowledgments
Mustafa Yildirim: data collection, study design, interpretation of analysis and manuscript writing; Asuman Coban: study design, critically reviewed and revised the manuscript; Ozgul Bulut: manuscript writing, critically reviewed and revised the manuscript; Nur Kir Mercül: critically reviewed and revised the manuscript; Zeynep Ince: critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, [initials]. The data are not publicly available due to [restrictions e.g. their containing information that could compromise the privacy of research participants].
Additional information
Funding
References
- Kim SJ, Port AD, Swan R, et al. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63(5):1–12. doi:10.1016/j.survophthal.2018.04.002.
- Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi:10.1056/NEJMoa1007374.
- Chan-Ling T, Gole GA, Quinn GE, et al. Pathophysiology, screening and treatment of ROP: a multi-disciplinary perspective. Prog Retin Eye Res. 2018;62:77–119. doi:10.1016/j.preteyeres.2017.09.002.
- Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38(7):397–432. doi:10.1136/bjo.38.7.397.
- Fierson WM, American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology, et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189–195. doi:10.1542/peds.2012-2996.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1.
- Chiang MF, Arons RR, Flynn JT, et al. Incidence of retinopathy of prematurity from 1996 to 2000: analysis of a comprehensive New York state patient database. Ophthalmology. 2004;111(7):1317–1325. doi:10.1016/j.ophtha.2003.10.030.
- Gerull R, Brauer V, Bassler D, et al. Incidence of retinopathy of prematurity (ROP) and ROP treatment in Switzerland 2006–2015: a population-based analysis. Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F337–F342. doi:10.1136/archdischild-2017-313574.
- Mitchell AJ, Green A, Jeffs DA, et al. Physiologic effects of retinopathy of prematurity screening examinations. Adv Neonatal Care. 2011;11(4):291–297. doi:10.1097/ANC.0b013e318225a332.
- Kemper AR, Wallace DK. Neonatologists’ practices and experiences in arranging retinopathy of prematurity screening services. Pediatrics. 2007;120(3):527–531. doi:10.1542/peds.2007-0378.
- Hutchinson AK, Melia M, Yang MB, et al. Clinical models and algorithms for the prediction of retinopathy of prematurity: a report by the American academy of ophthalmology. Ophthalmology. 2016;123(4):804–816. doi:10.1016/j.ophtha.2015.11.003.
- Hellström A, Hård AL, Engström E, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123(4):e638–e645. doi:10.1542/peds.2008-2697.
- Jensen AK, Ying GS, Huang J, et al. Postnatal serum insulin-like growth factor I and retinopathy of prematurity. Retina. 2017;37(5):867–872. doi:10.1097/IAE.0000000000001247.
- Cakir B, Hellström W, Tomita Y, et al. IGF1, serum glucose, and retinopathy of prematurity in extremely preterm infants. JCI Insight. 2020;5(19):e140363. doi:10.1172/jci.insight.140363.
- Löfqvist C, Hansen-Pupp I, Andersson E, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulin-like growth factor I. Arch Ophthalmol. 2009;127(5):622–627. doi:10.1001/archophthalmol.2009.69.
- Wu C, Löfqvist C, Smith LE, et al. Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants: a multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2012;130(8):992–999. doi:10.1001/archophthalmol.2012.243.
- Ko CH, Kuo HK, Chen CC, et al. Using WINROP as an adjuvant screening tool for retinopathy of prematurity in Southern Taiwan. Am J Perinatol. 2015;30(2):149–154. doi:10.1055/s-0034-1376389.
- Binenbaum G, Bell EF, Donohue P, et al. Development of modified screening criteria for retinopathy of prematurity: primary results from the postnatal growth and retinopathy of prematurity study. JAMA Ophthalmol. 2018;136(9):1034–1040. doi:10.1001/jamaophthalmol.2018.2753.
- Yabas Kiziloglu O, Coskun Y, Akman I. Assessment of the G-ROP study criteria for predicting retinopathy of prematurity: results from a tertiary Centre in Turkey. Int Ophthalmol. 2020;40(7):1647–1652. doi:10.1007/s10792-020-01332-5.
- Anuk İnce D, Gülcan H, Hanta D, et al. Poor postnatal weight gain predicts stage 3 + retinopathy of prematurity in very low birth weight infants. Turk J Pediatr. 2013;55:304–308.
- Aydemir O, Sarikabadayi YU, Aydemir C, et al. Adjusted poor weight gain for birth weight and gestational age as a predictor of severe ROP in VLBW infants. Eye (Lond). 2011;25(6):725–729. doi:10.1038/eye.2011.29.
- A Joint Statement of the American Academy of Pediatric, the American Association for Pediatric Ophthalmology and Strabismus, and the American Academy of Ophthalmology. Screening examination of premature infants for retinopathy of prematurity. Ophthalmology. 1997;104:888–889.
- Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics. 2018;142(6):e20183061. Pediatrics. 2019;143:e20183810. doi:10.1542/peds.2018-3810.
- Wallace DK, Kylstra JA, Phillips SJ, et al. Poor postnatal weight gain: a risk factor for severe retinopathy of prematurity. J Aapos. 2000;4(6):343–347. doi:10.1067/mpa.2000.110342.
- Cabañas Poy MJ, Montoro Ronsano JB, Castillo Salinas F, et al. Association between postnatal weight gain and need for treatment in retinopathy of prematurity. J Matern Fetal Neonatal Med. 2022;35(25):8027–8031. doi:10.1080/14767058.2021.1940937.
- Bas AY, Demirel N, Koc E, et al. Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): a prospective, multicentre study in 69 neonatal intensive care units. Br J Ophthalmol. 2018;102(12):1711–1716. doi:10.1136/bjophthalmol-2017-311789.
- Rao KA, Purkayastha J, Hazarika M, et al. Analysis of prenatal and postnatal risk factors of retinopathy of prematurity in a tertiary care hospital in South India. Indian J Ophthalmol. 2013;61(11):640–644. doi:10.4103/0301-4738.119347.
- Lundgren P, Hård AL, Wilde Å, et al. Implementing higher oxygen saturation targets reduced the impact of poor weight gain as a predictor for retinopathy of prematurity. Acta Paediatr. 2018;107(5):767–773. doi:10.1111/apa.14049.
- Sun Y, Hellström A, Smith Lois, E, H. Retinopathy of prematurity. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s neonatal-perinatal medicine-diseases of the fetus and newborn. 10th ed. Philadelphia (PA): Saunders Elsevier. 2015;1767–1774.
- Alajbegovic-Halimic J, Zvizdic D, Alimanovic-Halilovic E, et al. Risk factors for retinopathy of prematurity in premature born children. Med Arch. 2015;69(6):409–413. doi:10.5455/medarh.2015.69.409-413.
- Ali AA, Gomaa NAS, Awadein AR, et al. Retrospective cohort study shows that the risks for retinopathy of prematurity included birth age and weight, medical conditions and treatment. Acta Paediatr. 2017;106(12):1919–1927. doi:10.1111/apa.14019.
- Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1993;100(2):230–237. doi:10.1016/s0161-6420(93)31665-9.
- Yim CL, Tam M, Chan HL, et al. Association of antenatal steroid and risk of retinopathy of prematurity: a systematic review and meta-analysis. Br J Ophthalmol. 2018;102(10):1336–1341. doi:10.1136/bjophthalmol-2017-311576.
- Eriksson L, Haglund B, Ewald U, et al. Short and long-term effects of antenatal corticosteroids assessed in a cohort of 7,827 children born preterm. Acta Obstet Gynecol Scand. 2009;88(8):933–938. doi:10.1080/00016340903111542.
- Maini B, Chellani H, Arya S, et al. Retinopathy of prematurity: risk factors and role of antenatal betamethasone in Indian preterm newborn babies. J Clin Neonatol. 2014;3(1):20–24. doi:10.4103/2249-4847.128724.
- Movsas TZ, Spitzer AR, Gewolb IH. Postnatal corticosteroids and risk of retinopathy of prematurity. J AAPOS. 2016;20(4):348–352. doi:10.1016/j.jaapos.2016.05.008.
- Fundora JB, Binenbaum G, Tomlinson L, et al. Association of surgical necrotizing enterocolitis and its timing with retinopathy of prematurity. Am J Perinatol. 2023;40(11):1178–1184. doi:10.1055/s-0041-1733785.
- Silva JF, Ocarino NM, Vieira AL, et al. Effects of hypo- and hyperthyroidism on proliferation, angiogenesis, apoptosis and expression of COX-2 in the corpus luteum of female rats. Reprod Domest Anim. 2013;48(4):691–698. doi:10.1111/rda.12149.
- Coşkun Y, Kızıloğlu ÖY, Bayram T, et al. Thyroid hormones have any effects on the development of retinopathy of prematurity? Haydarpasa Numune Med J. 2019;60:60–66. doi:10.14744/hnhj27122.
- Korkmaz L, Baştuğ O, Daar G, et al. The effects of thyroid function on retinopathy of prematurity. J Neonatal Perinatal Med. 2016;9(4):349–356. doi:10.3233/NPM-915150.
- Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118(5):1811–1818. doi:10.1542/peds.2006-0628.
- Leung M, Black J, Bloomfield FH, et al. Effects of neonatal hyperglycemia on retinopathy of prematurity and visual outcomes at 7 years of age: a matched cohort study. J Pediatr. 2020;223:42–50.e2. doi:10.1016/j.jpeds.2020.04.059.
- Choręziak A, Szpecht D, Chmielarz-Czarnocińska A, et al. The association of platelet counts with development and treatment for retinopathy of prematurity – is thrombocytopenia a risk factor? Arch Med Sci. 2022;18(2):400–405. doi:10.5114/aoms.2019.85386.
- Vinekar A, Hegde K, Gilbert C, et al. Do platelets have a role in the pathogenesis of aggressive posterior retinopathy of prematurity? Retina. 2010;30(4 Suppl):S20–S23. doi:10.1097/IAE.0b013e3181cafc30.
- Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301–307. doi:10.1016/j.jpeds.2006.05.011.
- Beken S, Dilli D, Fettah ND, et al. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Hum Dev. 2014;90(1):27–31. doi:10.1016/j.earlhumdev.2013.11.002.