Abstract
Objective
We aimed to investigate the relationship between preeclampsia and maternal serum apelin-13 and apelin-36 concentrations.
Methods
This cross-sectional study was carried out in the Gynecology and Obstetrics Clinic of Umraniye Training and Research Hospital. The preeclampsia group consisted of 40 pregnant women diagnosed with preeclampsia, and the control group consisted of 40 healthy pregnant women matched with the preeclampsia group in terms of age and body mass index. The two groups were compared in terms of maternal serum apelin-13 and apelin-36 concentrations.
Results
Both groups were similar in terms of demographic characteristics and the gestational week at blood sampling. Maternal serum apelin-13 and apelin-36 concentrations were significantly lower in the preeclampsia group than in the control group (p = 0.005, p = 0.001, respectively). The optimal cutoff value for the prediction of preeclampsia in receiver operator curve analysis for apelin-13 was determined as 1781.67 pg/ml with 60% sensitivity and 60% specificity, and 885.5 pg/ml for apelin-36 with 67% sensitivity and 65% specificity. We divided the preeclampsia group into two groups mild and severe and compared the three groups in terms of maternal serum apelin-13 and apelin-36 concentrations. The lowest apelin-13 concentration was detected in the severe preeclampsia group, while the lowest apelin-36 concentration was detected in the mild preeclampsia group (p = 0.020, p = 0.003, respectively). Considering the onset of the disease, we divided the preeclampsia group into two groups early and late-onset, then compared the three groups in terms of maternal serum apelin-13 and apelin-36 concentrations. The lowest maternal serum apelin-13 and apelin-36 concentrations were detected in the early-onset preeclampsia group (p = 0.016, p = 0.001, respectively).
Conclusion
It was determined that serum apelin-13 and apelin-36 concentrations were significantly lower in preeclamptic pregnant women, this decrease was more significant in early-onset preeclampsia, and low maternal serum apelin-13 concentration was more associated with the severity of preeclampsia.
Introduction
Preeclampsia, which affects 3–5% of pregnancies, is traditionally known as a pregnancy-specific disease in which increased blood pressure is accompanied by proteinuria [Citation1]. Although the etiopathogenesis of preeclampsia has not been clarified yet, it is thought that up-regulated vasoactive molecules secondary to insufficient placentation resulting from maternal-fetal immune maladaptation are responsible for the systemic findings of the disease [Citation2]. In addition to vasoactive molecules, increased oxidative stress or low antioxidant capacity are also thought to play a role in the pathophysiology of preeclampsia [Citation3].
In 1993, O’Dowd et al. discovered the APJ (angiotensin 2 receptor-like 1) receptor, which is very similar in structure to the angiotensin 2 receptor [Citation4]. At that time, APJ, which did not interact with any endogenous molecule, including angiotensin 2, remained an orphan receptor for 5 years until its endogenous ligand was discovered. In 1998, Tatemoto et al. discovered that the peptide they isolated from the bovine stomach was the ligand of the previously discovered APJ receptor and named it “apelin” [Citation5].
In a study published in 2007, it was determined that the apelin molecule originates from the precursor peptide consisting of 77 amino acids, which is broken down in certain regions and turned into active parts such as apelin-10, apelin-11, apelin-12, apelin-13, apelin-15, apelin-17, apelin-19, and apelin-36 [Citation6]. Tatemoto et al. who discovered the apelin molecule for the first time, showed that the apelin, which is expressed in the endothelium of small arteries in different tissues in rats, reduces blood pressure via a nitric oxide-dependent mechanism [Citation7].
In light of this information presented in the literature, we aimed to investigate the concentrations of apelin-13 and apelin-36 in the serum of pregnant women diagnosed with preeclampsia, assuming that apelin molecules play a role in the pathophysiology of preeclampsia.
Materials and methods
This prospective case-control study was conducted with 80 pregnant women who applied to Umraniye Training and Research Hospital, Gynecology and Obstetrics Clinic, Istanbul, Turkey, between April 2022 and September 2022. The preeclampsia group consisted of 40 preeclamptic pregnant women aged 18-39 who had pregnancy follow-up and gave birth in our hospital. The control group consisted of 40 healthy pregnant women who were followed up and delivered in our hospital and matched with the preeclampsia group in terms of age and body mass index (BMI).
Multiple pregnancies, those who conceived by in vitro fertilization method, those with a history of any systemic disease, those with known vascular disease, thrombophilia, autoimmune disease, and smokers were excluded from the study. Pregnant women who had COVID-19 or another infectious disease during pregnancy were also not included in the study.
Participants’ age, BMI, laboratory findings, and perinatal outcomes were recorded. Fetal biometric measurements were performed by the same clinician with the Esaote MylabX6 model ultrasound device, in line with the ISUOG guideline recommendations [Citation8].
Studies have shown that apelin is also intensely expressed in adipocytes [Citation9]. For this reason, the thickness of the subcutaneous fat tissue in the umbilical and epigastric regions of the participants on the day of blood collection for apelin was measured with the Esaote MylabX6 model ultrasound device. Maternal periumbilical subcutaneous adipose tissue thickness measurement was made from a 2 cm lateral part of the umbilicus using a thin flat-tipped ultrasound probe without including cutaneous tissue. Maternal epigastric subcutaneous adipose tissue thickness measurement was performed by positioning the flat-tip ultrasound probe with one end at the lateral level of the maternal xiphoid bone, without including the cutaneous tissue.
The diagnosis of preeclampsia was made according to the criteria of the American College of Obstetricians and Gynecologists [Citation10]. Before receiving any treatment, approximately 5 ml of blood samples were taken from the participants in laboratory tubes without anticoagulant at the time of initial diagnosis. After the samples were kept at room temperature for 2 h, they were centrifuged at 1000 rpm for 20 min. After centrifugation, the supernatant was separated from the residue and stored at −80 °C.
Apelin-13 concentrations in blood samples from participants were studied with the Fine Test Human Apelin-13 Kit (Wuhan Fine Biotech Co., Ltd., Wuhan, Hubei, China, Catalogue No: EH2649) using the Enzyme-Linked Immunosorbent Assay (ELISA) method. For the Human Apelin 13 Kit used in the study, a measurement value between 125 – 8000 pg/ml and a sensitivity of 75 pg/ml was determined. For the Apelin-13 Kit, the intra-assay coefficient of variation was < 8% and the inter-assay coefficient of variation was < 10%. Apelin-36 concentrations in blood samples from participants were studied with the Fine Test Human Apelin-36 Kit (Wuhan Fine Biotech Co., Ltd., Wuhan, Hubei, China, Catalogue No: EH1971) using the ELISA method. For the Human Apelin-36 Kit used in the study, a measurement value between 46.875 – 3000 pg/ml and a sensitivity of 28.125 pg/ml was determined. For the Apelin-36 Kit, the intra-assay coefficient of variation was < 8% and the inter-assay coefficient of variation was < 10%.
The Local Ethics Committee of Umraniye Training and Research Hospital, Istanbul, Turkey has approved this study (Ethics Committee Approval No: B.10.1.TKH.4.34.H.GP.0.01/59, Date: 11/02/2022). Informed consent was obtained from all the participants.
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) version 25.0. The Kolmogorov-Smirnov test was used to determine whether the data were distributed normally or not. Descriptive statistical methods (mean, standard deviation, median, IQR, frequency, ratio) were used when evaluating the study data. Independent t-test was used for comparison of two groups showing parametric distribution, One Way ANOVA was used for comparisons of more than two groups. Mann Whitney U test was used for the comparison of two groups showing non-parametric distribution, and the Kruskal Wallis test was used for comparisons of more than two groups. Significant differences as a result of comparisons of more than two groups were examined by the Tamhane and Tukey tests. The Chi-square test was used in the comparison of the groups in terms of categorical data. The direction and level of the relationship between numerical variables were determined by Pearson correlation. The receiver operating curve (ROC) was used to determine the significant threshold of apelin-13 and apelin-36 in predicting preeclampsia. Statistical significance was accepted at p < 0.05 for all values.
Results
Preeclampsia and control groups were similar in terms of age, BMI, weight gain during pregnancy, and parity (p > 0.05, for each) ().
Table 1. Demographic characteristics of control and preeclampsia groups.
Systolic and diastolic blood pressure and mean arterial pressure were significantly higher in the preeclampsia group than in the control group (p = 0.000, for each). The protein/creatinine ratio in a spot urine sample was significantly higher in the preeclampsia group, but AST and ALT levels and platelet count were similar in both groups (p = 0.000, p = 0.075, p = 0.069, p = 0.130, respectively). In the preeclampsia group, maternal epigastric and periumbilical subcutaneous adipose tissue thickness was significantly higher than in the control group (p = 0.007, p = 0.011, respectively). The umbilical artery pulsatility index (PI), resistance index (RI), and systole/diastole (S/D) ratio were significantly higher in the preeclampsia group compared to the control group (p = 0.000, for each). In the preeclampsia group, 20 patients had a unilateral or bilateral notch in the uterine artery Doppler, and intrauterine growth restriction (IUGR) developed in 4 patients ().
Table 2. Comparison of control and preeclampsia groups in terms of laboratory and ultrasound findings.
Preterm delivery, number of cesarean sections, and neonatal intensive care unit admission (NICU) were significantly higher in the preeclampsia group (p = 0.000, for each). Gestational age at birth, birth weight, and 1st, and 5th minute Apgar scores were significantly lower in the preeclampsia group than in the control group (p = 0.000, p = 0.000, p = 0.003, p = 0.000, respectively) ().
Table 3. Comparison of control and preeclampsia groups in terms of perinatal outcomes.
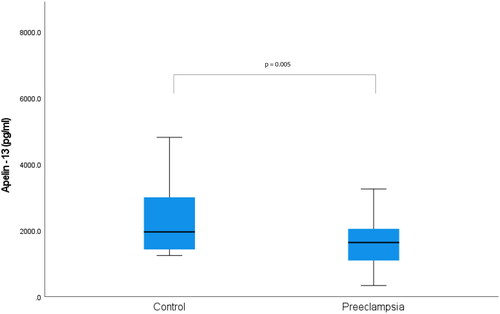
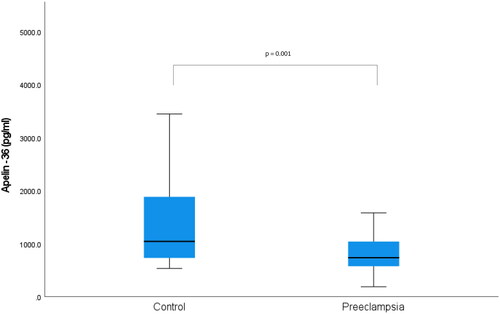
Gestational week at blood sampling for apelin-13 and apelin-36 was similar for the two groups (p = 0.077). The median maternal serum apelin-13 concentration was found to be 1635 pg/ml in the preeclampsia group, while it was determined as 1954 pg/ml in the control group (p = 0.005) (). While the median maternal serum apelin-36 concentration was 735.7 pg/ml in the preeclampsia group, it was 1042.8 pg/ml in the control group (p = 0.001) () ().
Table 4. Comparison of control and preeclampsia groups in terms of maternal serum apelin-13 and apelin-36 concentrations.
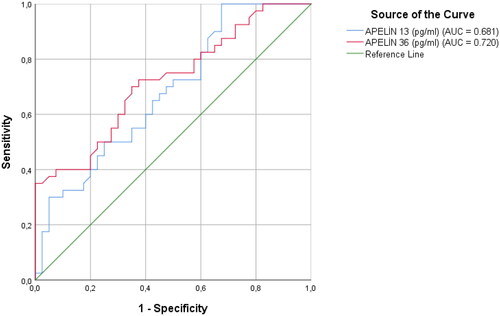
Receiver operator curve (ROC) analysis was performed to determine the value of maternal serum apelin-13 and apelin-36 concentrations in terms of predicting preeclampsia. AUC analysis of maternal serum apelin-13 for preeclampsia estimation was 0.681 (p = 0.005, 95% CI = 0.56 − 0.79). The optimal cutoff value for maternal serum apelin-13 concentration was determined as 1781.67 pg/ml with 60% sensitivity and 60% specificity. AUC analysis of maternal serum apelin-36 for preeclampsia estimation was 0.720 (p = 0.001, 95% CI = 0.61 − 0.83). The optimal cutoff value for maternal serum apelin-36 concentration was determined as 885.5 pg/ml with 67% sensitivity and 65% specificity ().
Figure 3. ROC analysis for the sensitivity, specificity, and positive and negative predictive value of apelin-13 and apelin-36 in preeclampsia.
We divided the preeclampsia group into two groups, mild and severe, and compared them with the control group in terms of maternal serum apelin-13 and apelin-36 concentrations. The median apelin-13 concentration was found to be 1635.3 pg/ml in the mild preeclampsia group and 1514.9 pg/ml in the severe preeclampsia group. The median apelin-36 concentration was found to be 691.9 pg/ml in the mild preeclampsia group and 762.3 pg/ml in the severe preeclampsia group. Among the three groups, the lowest apelin-13 concentration was detected in the severe preeclampsia group, while the lowest apelin-36 concentration was detected in the mild preeclampsia group (p = 0.020, p = 0.003, respectively) ().
Table 5. Comparison of control, mild preeclampsia, and severe preeclampsia groups in terms of maternal serum apelin-13 and apelin-36 concentrations.
Considering the onset of the disease, we divided the preeclampsia group into two groups, early and late-onset, and compared them with the control group in terms of maternal serum apelin-13 and apelin-36 concentrations. The median apelin-13 concentration was found to be 1511 pg/ml in the early-onset preeclampsia group and 1766.6 pg/ml in the late-onset preeclampsia group. The median apelin-36 concentration was found to be 688.1 pg/ml in the early-onset preeclampsia group and 1013.7 pg/ml in the late-onset preeclampsia group. Among the three groups, the lowest maternal serum apelin-13 and apelin-36 concentrations were detected in the early-onset preeclampsia group (p = 0.016, p = 0.001, respectively) ().
Table 6. Comparison of control, early-onset preeclampsia, and late-onset preeclampsia groups in terms of maternal serum apelin-13 and apelin-36 concentrations.
Pearson correlation analysis was performed to investigate the relationship between preeclampsia-related parameters and serum apelin-13 and apelin-36 concentrations. There was no significant relationship between age, BMI, parity, weight gained during pregnancy, epigastric subcutaneous and periumbilical subcutaneous adipose tissue thickness, umbilical artery PI, RI, and S/D, and serum apelin-13 or apelin-36 ().
Table 7. Correlation between maternal serum apelin-13 and apelin-36 concentrations and preeclampsia related parameters.
Discussion
In an animal study conducted in 2001, it was demonstrated that apelin, which is expressed in the endothelium of small arteries in different tissues in rats, plays a role in reducing blood pressure through nitric oxide [Citation7]. In support of this finding, Cheng et al. showed that intravenous injection of apelin caused dilatation of arteries and veins, a dose-dependent decrease in blood pressure, and increased heart rate in rats [Citation11].
In 2007, Cobellis et al. published an article on the distribution of the Apelin/APJ receptor complex in the placenta of normotensive and preeclamptic pregnant women. They stated that while apelin expression decreased in the placental tissue from the first trimester to the third trimester of pregnancy in normotensive pregnant women, the expression of APJ increased. In contrast, they found that the expression of both apelin and APJ receptors was strongly increased in the placental tissue in pregnancies complicated with preeclampsia [Citation12]. In another study published in 2017, it was stated that apelin expression in the placenta was higher in the preeclamptic pregnant women, compared to the normotensive controls, and this increase in apelin expression was associated with the severity of preeclampsia [Citation13]. Contrary to the two studies mentioned above, Liao et al. showed that the expression of apelin-36 in the placenta was decreased in preeclamptic pregnant women compared to the normotensive control. They emphasized that this decrease in apelin-36 expression is also related to the severity of preeclampsia [Citation14]. Similarly, Yamaleyeva et al. showed that placental apelin expression was lower in women whose pregnancy was complicated by preeclampsia compared to normotensive controls [Citation15].
Similar to the contradictory results reported in studies evaluating the expression of apelin in the placenta in preeclampsia, the results of studies on serum apelin concentrations of pregnant women whose pregnancy was complicated by preeclampsia are also contradictory. In a study published in 2012, preeclamptic and normal healthy pregnant women were compared in terms of maternal serum apelin concentrations. This study reported that maternal serum apelin concentration was higher in pregnant women whose pregnancy was complicated by preeclampsia compared to normal controls [Citation16]. In another study by Kucur et al. it was reported that maternal serum apelin levels were significantly higher in pregnant women whose pregnancy was complicated by preeclampsia compared to the normotensive control group [Citation17]. Contrary to our study, we think that the results reported in these studies are related to the evaluation of serum total apelin concentration instead of active apelin fragments such as apelin-13 or apelin-36, which we evaluated in our study.
In two different studies published in 2012 and 2019, it was stated that serum apelin concentrations of pregnant women with preeclampsia were significantly lower than those of healthy pregnant women [Citation18,Citation19]. Although active apelin fragments were not evaluated in these two studies, the reported results are consistent with the results we obtained in this study.
In a study published in 2019, pregnant women diagnosed with preeclampsia and normotensive pregnant women were compared in terms of serum apelin-13 and apelin-36 concentrations. Similar to the results in our study, serum apelin-13 and apelin-36 concentrations in the preeclamptic group were found to be significantly lower than the control group [Citation20]. Different from this study, we measured maternal epigastric and umbilical subcutaneous adipose tissue thickness as an indicator of the current adipose tissue in pregnant women. In our study, although BMI at the time of blood sampling and total weight gained during pregnancy were similar in both groups, maternal epigastric and periumbilical subcutaneous adipose tissue thickness was significantly higher in the preeclampsia group. Studies in the literature have shown that apelin is secreted from mature adipocytes in addition to vascular endothelial cells, so adipose tissue may also contribute significantly to serum apelin concentration [Citation9,Citation21]. Contrary to this information, we found that maternal serum apelin-13 and apelin-36 concentrations were lower in the preeclampsia group with significantly thicker subcutaneous adipose tissue compared to the control group.
We think that the pathophysiology in preeclampsia in which multiple organ systems are involved due to the deterioration of the internal dynamics of the placenta is not as simple as a decrease in the secretion of apelin fragments. In addition to the expression of the apelin/APJ system and changes in the concentrations of apelin fragments in the serum, we think there are many other points worth investigating, such as their interaction with other vasoactive molecules and pathways.
The small number of participants and the lack of evaluation of apelin-13, apelin-36, and APJ receptor expression in placental tissue are the most important limitations of this study. In addition, serum apelin-13 and apelin-36 concentrations in the participants were evaluated only once at the time of initial diagnosis. Therefore, the fact that maternal serum apelin-13 and apelin-36 concentrations were not evaluated before the onset of preeclampsia and changes in serum concentrations were not followed up until delivery are other limiting factors in our study.
In conclusion, we found that serum apelin-13 and apelin-36 concentrations were significantly lower in women whose pregnancy was complicated with preeclampsia compared to healthy controls, this decrease was more significant in early-onset preeclampsia, and low maternal serum apelin-13 concentration seemed to be more associated with the severity of preeclampsia.
Acknowledgments
We thank all participants who voluntarily participated in this study.
Disclosure statement
The authors received no funding or grants and report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Data availability statement
Data supporting the findings of this study is available via the OSFHOME data repository with the 10.17605/OSF.IO/4CG8R DOI identifier.
Additional information
Funding
References
- Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet. 2016;387(10022):1–8. doi:10.1016/S0140-6736(15)00070-7.
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. doi:10.1016/S0140-6736(05)17987-2.
- Tossetta G, Fantone S, Piani F, et al. Modulation of NRF2/KEAP1 signaling in preeclampsia. Cells. 2023;12(11):1545. doi:10.3390/cells12111545.
- O’Dowd BF, Heiber M, Chan A, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136(1–2):355–360. doi:10.1016/0378-1119(93)90495-o.
- Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–476. doi:10.1006/bbrc.1998.9489.
- Carpéné C, Dray C, Attané C, et al. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63(4):358–373. doi:10.1007/BF03165767.
- Tatemoto K, Takayama K, Zou M-X, et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99(2–3):87–92. doi:10.1016/s0167-0115(01)00236-1.
- ISUOG practice guidelines: use of doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol. 2013;41:233–239.
- Castan-Laurell I, Dray C, Attané C, et al. Apelin, diabetes, and obesity. Endocrine. 2011;40(1):1–9. doi:10.1007/s12020-011-9507-9.
- ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:1.
- Cheng X, Cheng XS, Pang CCY. Venous dilator effect of apelin, an endogenous peptide ligand for the orphan APJ receptor, in conscious rats. Eur J Pharmacol. 2003;470(3):171–175. doi:10.1016/s0014-2999(03)01821-1.
- Cobellis L, De Falco M, Mastrogiacomo A, et al. Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol Histopathol. 2007;22(1):1–8. doi:10.14670/HH-22.1.
- Colcimen N, Bulut G, Ergul Erkec O, et al. Investigation of role of vascular endothelial growth factor, annexin A5 and apelin by immunohistochemistry method in placenta of preeclampsia patients. Cell Mol Biol (Noisy-le-grand). 2017;63(11):42–45. doi:10.14715/cmb/2017.63.11.8.
- Liao Y-M, Qiao F-Y. Expression of Apelin in placentas of patients with hypertensive disorders complicating pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2007;42(6):382–385.
- Yamaleyeva LM, Chappell MC, Brosnihan KB, et al. Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2015;309(10):E852–E860. doi:10.1152/ajpendo.00272.2015.
- Simsek Y, Celik O, Yilmaz E, et al. Serum levels of apelin, salusin-alpha and salusin-beta in normal pregnancy and preeclampsia. J Matern Fetal Neonatal Med. 2012;25(9):1705–1708. doi:10.3109/14767058.2012.660221.
- Kucur M, Tuten A, Oncul M, et al. Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens Pregnancy. 2014;33(4):467–475. doi:10.3109/10641955.2014.944709.
- Bortoff KD, Qiu C, Runyon S, et al. Decreased maternal plasma apelin concentrations in preeclampsia. Hypertens Pregnancy. 2012;31(4):398–404. doi:10.3109/10641955.2012.690054.
- Deniz R, Baykus Y, Ustebay S, et al. Evaluation of elabela, apelin and nitric oxide findings in maternal blood of normal pregnant women, pregnant women with pre-eclampsia, severe pre-eclampsia and umbilical arteries and venules of newborns. J Obstet Gynaecol. 2019;39(7):907–912. doi:10.1080/01443615.2019.1572727.
- Gürlek B, Yılmaz A, Durakoğlugil ME, et al. Evaluation of serum apelin-13 and apelin-36 concentrations in preeclamptic pregnancies. J Obstet Gynaecol Res. 2020;46(1):58–65. doi:10.1111/jog.14137.
- Boucher J, Masri B, Daviaud D, et al. Apelin, a newly ıdentified adipokine Up-Regulated by ınsulin and obesity. Endocrinology. 2005;146(4):1764–1771. doi:10.1210/en.2004-1427.