Abstract
Objective
To evaluate the effectiveness of using hospital-based 40% dextrose gel (DG) in preventing and treating asymptomatic hypoglycemia in infants of diabetic mothers (IDM), large for gestational age (LGA), and macrosomic neonates.
Methods
A medical chart review was conducted to compare data between before (April 2018 to March 2019, epoch 1) and after (September 2020 to November 2021, epoch 2) 40% DG implementation. DG, prepared by the hospital pharmaceutical unit, was applied within 30–45 min after birth, and three additional doses could be repeated during the first 6 h of life in combination with early feeding. The primary outcome was the rate of intravenous dextrose administration. Secondary outcomes were the incidence of hypoglycemia, first capillary blood glucose concentrations, and the length of hospital stay.
Results
Six hundred forty-three at-risk newborns were included (320 before and 323 after implementation of DG). Maternal and neonatal baseline characteristics were not different between the two epochs. The incidence of hypoglycemia was not different (17.8% in before versus 14.6% in after implementation, p = 0.26). The rate of intravenous dextrose administration after DG implementation was significantly lower than that before DG implementation (3.4% versus 10.3%, p < 0.001, risk reduction ratio = 0.33, 95% CI = 0.17–0.64). The length of hospital stay was not different between the two epochs.
Conclusions
Implementing a protocol for administration of hospital-based 40% DG can reduce the need of intravenous dextrose administration among IDM, LGA and macrosomic neonates.
Introduction
Neonatal hypoglycemia is a common metabolic condition in newborns, and it affects 5%–15% of healthy newborns and up to 50% of at-risk newborns [Citation1]. At-risk infants include infants of diabetic mothers (IDM), preterm newborns, and small or large for gestational age (SGA or LGA) newborns [Citation1–2]. In healthy newborns, transient low blood glucose concentrations are common. Most of them are asymptomatic and no treatment is required. At-risk newborns may have abnormal adaptation, leading to symptomatic, prolonged, or severe hypoglycemia, which may result in brain injury and long-term neurological sequelae [Citation2–6].
Guidelines for screening and managing neonatal hypoglycemia differ widely among countries and health organizations. The American Academy of Pediatrics (AAP), the Pediatric Endocrine Society, and the Canadian Practice Society have developed their own guidelines [Citation2,Citation7,Citation8]. The main management for neonatal hypoglycemia includes early milk feeding and intravenous dextrose administration. The administration of intravenous dextrose can lead mother─newborn separation, delayed establishment of breastfeeding, and increased hospital costs. Oral 40% dextrose gel (DG) application is an option that is widely used for preventing and treating neonatal hypoglycemia in at-risk newborns. Using 40% DG is effective, resulting in reduced intravenous dextrose administration, improved breastfeeding, reduced mother–newborn separation and hospital costs [Citation9–22]. DG application is also easy, safe, and inexpensive. The recent systematic review and meta-analysis has shown that using DG in at-risk neonates reduces the risk of hypoglycemia and probably reduces the risk of receipt of treatment for hypoglycemia. However, only two randomized controlled trials in high-income countries are included in this review, therefore, the authors suggest that further studies are required in low-and middle-income countries, preterm neonates, and using another DG preparation [Citation9]. In Thailand, there is no commercial DG available. Therefore, we established a guideline using hospital-based DG combined with early feeding. This study aimed to evaluate the effectiveness of using hospital-based 40% DG for preventing and treating neonatal hypoglycemia in IDM, LGA, and macrosomic neonates.
Methods
Study design and participants
We conducted ambispective chart reviews for comparison of the data between the two epochs: epoch 1, before implementing 40% DG (April 2018 to March 2019) and epoch 2, after implementing 40% DG (September 2020 to November 2021) at Ramathibodi Hospital, Mahidol University, Thailand. This study was approved by Ramathibodi Hospital Ethics Committee (COA. MURA2021/349) on May 2021. Therefore, data collection before the ethic approval date was retrospectively reviewed, and we conducted prospective reviews of the data after the ethic approval date. The Ethics Committee also approved a waiver of the signed informed consent for medical record reviews. Our previous study (unpublished preliminary data, Thai Clinical Trials Registry, number TCTR20190805003), a randomized-controlled study, showed that using 40% DG with early feeding was effective in reducing the admission rate for intravenous dextrose in IDM, LGA (birth weight > 90th centile), and macrosomic neonates (birth weight ≥ 4000 g) from 11.5% in placebo group to 5.2% in DG group (p = 0.024) but in late preterm and SGA neonates were not significant different between the two groups (p = 0.89). Therefore, we established our guideline for preventing and treating at-risk newborns for neonatal hypoglycemia, only in IDM, LGA and macrosomic neonates by using hospital-based 40% DG combined with early feeding. The inclusion criteria of this study were newborns who were born at ≥ 35 weeks gestation and at-risk of hypoglycemia, i.e. IDM (regardless of SGA status), LGA, or macrosomic neonates. We excluded newborns who had major congenital malformations, or those who required special care nursery (SCN) admission after birth such as having respiratory distress, requiring oxygen supplementation or respiratory supports, or need of intravenous fluid, or those who were not applied 40% DG in after implementing 40% DG epoch.
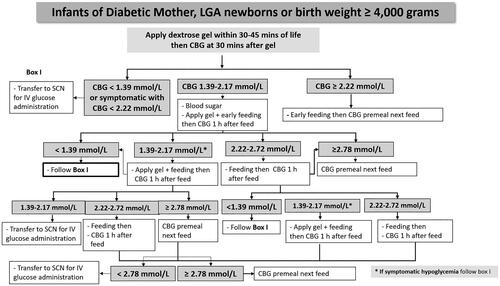
Before implementing 40% DG, our unit used a guideline for managing at-risk newborns for neonatal hypoglycemia with no 40% DG application. The first capillary blood glucose (CBG) concentration was measured 1 h after birth. Neonatal hypoglycemia was defined as CBG concentrations < 2.22 mmol/L. We used the Nova StatStrip glucose meter (Nova Biomedical, Waltham, MA, USA), point-of-care testing (POCT) with the glucose oxidase method, to provide early treatment. If CBG concentrations measured from the POCT were < 2.22 mmol/L, plasma glucose concentrations using the hexokinase/glucose-6-phosphate dehydrogenase method were then measured to confirm diagnosis. Newborns who had hypoglycemic symptoms/signs such as respiratory distress, cyanosis, poor feeding, jitteriness, diaphoresis, high-pitched cry, irritable, coma etc. or CBG concentrations < 1.39 mmol/L were admitted to a SCN for intravenous dextrose administration. Asymptomatic newborns who had first CBG concentrations > 2.22 mmol/L received early feeding with a volume of milk of 60–65 ml/kg/day every 3 h, and CBG concentrations were measured before the next feeding (pre-meal samples) every 3 h for two times. Newborns who had first CBG concentrations of 1.39–2.17 mmol/L were also managed with early feeding, and CBG concentrations were measured 1 h after feeding (post-meal samples), and pre-meal samples were taken every 3 h for two times. The target CBG concentration for pre-meal and post-meal samples was ≥ 2.78 mmol/L. If CBG concentrations did not reach the target, an increase in volume of formula feeding was provided. However, if neonates could not tolerate oral feeding, they developed symptoms of hypoglycemia, or if CBG concentrations did not reach the target for two to three times, they were admitted to the SCN for intravenous dextrose administration.
After implementing 40% DG, we used a guideline as same as before implementing 40% DG but we applied hospital-based 40% DG for prevention and treatment of neonatal hypoglycemia. Gel administration was applied to each side of the buccal mucosa over 30 s within 30–45 min after birth with formula feeding to asymptomatic IDM, LGA, and macrosomic neonates (). If the newborns had suspected signs and symptoms of hypoglycemia, the CBG concentration was measured immediately to determine whether or not they had symptomatic hypoglycemia and then transferred to the SCN for proper management without applying DG. The hospital-based DG was prepared by the pharmaceutical unit at Ramathibodi Hospital, under laminar flow and sterile techniques in a clean room. Gel formulation mainly consisted of dextrose powder and carboxymethycellulose with no added color, flavor, or additive substances added. DG was then filled in a 5-ml syringe with a sterile cap. The prefilled DG syringe labeling contained included name (DG), strength (40% DG), lot number of preparations, and date of expiration. The gel was randomly selected for bacteria culture in each lot of production and kept at a controlled temperature of 2–8 °C for no more than 7 days in the refrigerator. The dose of gel was 200 mg/kg/dose of glucose by using the neonate’s birth weight. The first CBG concentration was measured at 30 min after gel administration. If newborns had a first CBG concentration ≥ 2.22 mmol/L, they received early formula feeding, and pre-meal samples were obtained every 3 h for two times and every 6 h for two times over the first 24 h of life. Asymptomatic newborns who had a first CBG concentration of 1.39–2.17 mmol/L had gel applied again as a second dose with early feeding, and post-meal samples were obtained 1 h later. If post-meal CBG concentrations were 1.39-2.17 mmol/L, they received the next dose of gel and subsequent milk feeding. During the first 6 h of life, up to three doses of gel with formula feeding were applied. The target CBG concentration of pre-meal and post-meal samples was similar to that before DG implementation (≥ 2.78 mmol/L), as well as the criteria for admission for intravenous dextrose. In this study, definition of late hypoglycemia was the onset of hypoglycemia occurring 6 h after birth.
Data collection and analysis
The primary outcome was the rate of intravenous dextrose administration. Secondary outcomes were the incidence of hypoglycemia, first CBG concentrations, and the total length of hospital stay.
Data analysis was performed by using IBM SPSS for windows version 26. Data are shown as percentage, mean (±standard deviation), or median (interquartile). Categorical data were compared between groups by using chi-square test or Fisher’s exact test. Student t test or Mann–Whitney U test was used to compare continuous data. Statistical significance was determined as p < 0.05.
The sample size was calculated using data from our previous study. With approximately 250 in each group, a total of 500 was required to reduce in the rate for intravenous dextrose administration from 12% to 5%, with a power of 80% and a two-sided alpha of 0.05. Because we were unable to predict number of newborns in our study, we wished to collect data approximately 1 to 1.5 years in each epoch to reach an adequate sample size.
Results
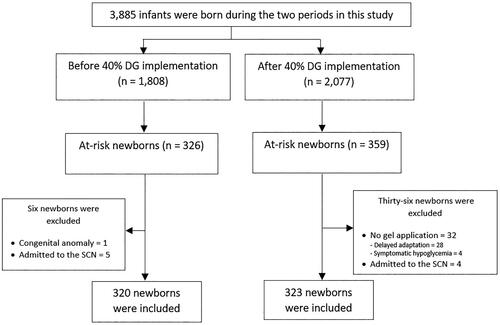
From 1 April 2018 to 31 March 2019 (before 40% DG implementation), 1808 newborns born in our hospital and 320 at-risk newborns were included. We also included 2077 newborns born from 1 September 2020 to 30 November 2021 (after 40% DG implementation) and 323 at-risk newborns (). Thirty-two of these newborns did not receive 40% DG because they had respiratory symptoms and/or desaturation and were transferred to the SCN. Four of them had symptomatic hypoglycemia and the others had delayed adaption. Maternal and neonatal baseline characteristics were not different between the two epochs (). However, the rate of cesarean delivery in mothers was significantly lower before 40% DG implementation than that of after 40% DG implementation (p = 0.002).
Table 1. Maternal and neonatal baseline characteristics.
The primary outcome and secondary outcomes are shown in . The proportion of newborns who required intravenous dextrose administration was significantly lower after 40% DG implementation than before 40% DG implementation (11 [3.4%] versus 33 [10.3%], p < 0.001, risk reduction ratio = 0.33, 95% CI = 0.17–0.64). Although the incidence of neonatal hypoglycemia was not significantly different between the two epochs, newborns were more likely to have asymptomatic hypoglycemia after 40% DG implementation than before 40% DG implementation (p = 0.002). All asymptomatic newborns after 40% DG implementation were successfully treated with the second dose of DG combined with feeding. There was no difference in the mean first CBG concentration between the two epochs. The length of stay of newborns in the SCN for intravenous dextrose administration was significantly shorter after 40% DG implementation than before 40% DG implementation (p = 0.018). However, the total length of hospital stay was not different between the two epochs ().
Table 2. Primary and secondary outcomes.
After 40% DG implementation, 8 of 323 (2.5%) newborns developed late hypoglycemia. In four of them, hypoglycemia occurred within 6–12 h after birth, and in the other newborns, it occurred within 12–24 h after birth. All of them were successfully managed with formula feeding. All newborns who received 40% DG had no adverse events, fluctuation in CBG concentrations, or hyperglycemia.
Discussion
The definition and management of neonatal hypoglycemia remain controversial. The goals of managing neonatal hypoglycemia focus on normalizing blood glucose concentrations and preventing short- and long-term complications, especially neurological sequelae. Early feeding or intravenous dextrose administration is commonly used in managing at-risk newborns with hypoglycemia [Citation1–3]. Early feeding with breast milk in our setting has some limitations. We have a high rate of cesarean delivery in our institution and a limited number of staff to initiate early breastfeeding. Therefore, formula feeding is preferred. Gregory et al. and Sen et al. investigated the effects of DG in different early feeding protocols and found that, after DG application, blood glucose concentrations in formula-fed newborns were significantly higher than those in breastfed newborns. They also found that formula-fed newborns had a lower rate of recurrent of hypoglycemia [Citation23–24]. Therefore, oral DG with formula feeding is a good option in our setting to prevent and manage neonatal hypoglycemia.
Previous studies have shown that oral DG prevents neonatal hypoglycemia, reduces the SCN admission and the length of hospital stay, improves breastfeeding, and reduces mother–newborn separation and hospital costs [Citation9–22]. The mechanism of oral DG in an increased blood glucose concentration can be explained by direct absorption of gel through the systemic circulation via lingual and internal jugular veins. In contrast with given DG via oral-gastric route, the first pass effect of the portal circulation may reduce blood glucose concentration achieved [Citation9]. Our previous study (unpublished data) showed that 40% DG was significantly reduced the SCN admission rate for intravenous dextrose administration only in IDM, LGA, and macrosomic neonates. Therefore, we established a guideline for these newborns by applying hospital-based 40% DG combined with early formula feeding. Some IDM neonates with having SGA or being late preterm were still included in our study because we mainly prioritized IDM to receive 40% DG according to the protocol. In Thailand, commercial DG is not available. Therefore, we prepared our own DG in our pharmaceutical unit. A dose of DG at 200 mg/kg was used on the basis of a study by Hegarty et al. who showed that this dose was the most effective for reducing incidence of neonatal hypoglycemia [Citation12].
This study showed that hospital-based 40% DG significantly reduced the rate of intravenous dextrose administration with a risk reduction ratio of 0.33. In the worst-case scenario, if we added four cases of symptomatic hypoglycemia that were excluded because they were transferred to the SCN before receiving 40% DG, the rate of intravenous dextrose administration still remained significant with a risk reduction ratio of 0.45 (95% CI = 0.25–0.81). This result is supported by previous studies, which showed that DG significantly reduced the rate of intravenous dextrose administration in at-risk newborns [Citation9,Citation13–15,Citation18–22].
In our study, CBG concentrations were monitored for 24 h, while the AAP recommendation suggests that CBG concentrations should be monitored for 12 h in IDM and LGA newborns [Citation2]. One of our concerns regarding DG application is rebound or late hypoglycemia due to insulin stimulation from dextrose administration [Citation2]. These may prolong the duration of management of hypoglycemia. However, Hegarty et al. showed that DG 200 mg/kg did not increase risk of rebound or recurrent hypoglycemia [Citation12]. In our study, we also used a DG dose of 200 mg/kg and found that DG application did not prolong the hospital stay. Only 2.5% of newborns who received DG developed late hypoglycemia, and all of them were successfully treated with oral feeding. In addition, there were no adverse effects related to DG application in accordance with previous studies [Citation9–10].
This study showed no significant difference in the incidence of neonatal hypoglycemia between the two epochs. This finding is supported by a study by Rawat et al. who reported no significant difference in the incidence of neonatal hypoglycemia between DG and non-DG groups [Citation13]. In our study, the proportion of asymptomatic hypoglycemia in newborns after DG implementation was significantly higher than that before DG implementation. All of them were treated successfully with a second dose of DG combined with formula feeding. These findings suggest that DG is effective in preventing symptomatic hypoglycemia and treating neonatal hypoglycemia.
This study also showed that newborns who required SCN admission for intravenous dextrose after 40% DG implementation had a significantly shorter length of stay in the SCN than those before DG implementation. Makker et al. showed a similar finding to that in our study [Citation15]. They found that newborns who were treated with DG had a shorter length of stay in the neonatal intensive care unit for managing hypoglycemia than those who were not treated with DG. Additionally, our finding that the length of hospital stay was not significantly different between the two epochs is supported by study of Ter et al. [Citation14].
There are some limitations in our study to be considered. By nature of chart reviews, some data were possibly missing. However, we did our upmost to complete data collection of all at-risk neonates. We also have limitations on the data of exclusive breastfeeding and separation from the mothers for treatment of hypoglycemia. However, the reduction of intravenous dextrose administration of those at-risk newborns after 40%DG implementation may indirectly improve the breastfeeding rate and reduce the mother-newborn separation. A comparison of hospital costs was not performed to determine the cost effectiveness, and there were no data on the long-term effects of DG application.
Conclusion
Implementing a protocol for administration of hospital-based 40% DG can reduce the need of intravenous dextrose administration among IDM, LGA and macrosomic neonates.
Acknowledgements
We would like to thank Jidapa Chatmapanrangsee, M.Pgarm. for preparing hospital-based dextrose gel used in this study and Ellen Knapp, PhD, Edanz (https://edanz.com/ac) for editing drafts of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author at [email protected] (Dr. Pracha Nuntnarumit).
Additional information
Funding
References
- Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J. Pediatr. 2012;161(5):1–8. doi: 10.1016/j.jpeds.2012.05.022.
- Thompson-Branch A, Havranek T. Neonatal hypoglycemia. Pediatr Rev. 2017;38(4):147–157. doi: 10.1542/pir.2016-0063.
- Adamkin DH,. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics. 2011;127(3):575–579. doi: 10.1542/peds.2010-3851.
- Vain NE, Chiarelli F. Neonatal hypoglycaemia: a never-ending story? Neonatology. 2021;118(5):522–529. doi: 10.1159/000514711.
- McKinlay CJ, Alsweiler JM, Ansell JM, et al. Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med. 2015;373(16):1507–1518. doi: 10.1056/NEJMoa1504909.
- McKinlay CJD, Alsweiler JM, Anstice NS, et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. 2017;171(10):972–983. doi: 10.1001/jamapediatrics.2017.1579.
- Thornton PS, Stanley CA, De Leon DD, et al. Recommendations from the pediatric endocrine society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J. Pediatr. 2015;167(2):238–245. doi: 10.1016/j.jpeds.2015.03.057.
- Canadian Paediatric Society. Screening guidelines for newborns at risk for low blood glucose. Paediatr. Child Health. 2004;9(10):723–729. doi: 10.1093/pch/9.10.723.
- Roberts L, Lin L, Alsweiler J, et al. Oral dextrose gel to prevent hypoglycaemia in at-risk neonates. Cochrane Database Syst Rev. 2023;11(11):CD012152. doi: 10.1002/14651858.CD012152.pub4.
- Edwards T, Liu G, Battin M, et al. Oral dextrose gel for the treatment of hypoglycaemia in newborn infants. Cochrane Database Syst. Rev. 2022;3(3):CD011027. doi: 10.1002/14651858.CD011027.pub3.
- Harris DL, Weston PJ, Signal M, et al. Dextrose gel for neonatal hypoglycaemia (the sugar babies study): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9910):2077–2083. doi: 10.1016/S0140-6736(13)61645-1.
- Hegarty JE, Harding JE, Gamble GD, et al. Prophylactic oral dextrose gel for newborn babies at risk of neonatal hypoglycaemia: a randomised controlled dose-finding trial (the pre-hPOD study). PLOS Med. 2016;13(10):e1002155. doi: 10.1371/journal.pmed.1002155.
- Rawat M, Chandrasekharan P, Turkovich S, et al. Oral dextrose gel reduces the need for intravenous dextrose therapy in neonatal hypoglycemia. Biomed Hub. 2016;1(3):1–9. doi: 10.1159/000448511.
- Ter M, Halibullah I, Leung L, et al. Implementation of dextrose gel in the management of neonatal hypoglycaemia. J Paediatrics Child Health. 2017;53(4):408–411. doi: 10.1111/jpc.13409.
- Makker K, Alissa R, Dudek C, et al. Glucose gel in infants at risk for transitional neonatal hypoglycemia. Am. J. Perinatol. 2018;35(11):1050–1056. doi: 10.1055/s-0038-1639338.
- Glasgow MJ, Harding JE, Edlin R,. Cost analysis of treating neonatal hypoglycemia with dextrose gel. J Pediatr. 2018;198:151–155. e1. doi: 10.1016/j.jpeds.2018.02.036.
- Newnam KM, Bunch M. Glucose gel as a treatment strategy for transient neonatal hypoglycemia. Adv. Neonatal Care. 2017;17(6):470–477. doi: 10.1097/ANC.0000000000000426.
- Meneghin F, Manzalini M, Acunzo M, et al. Management of asymptomatic hypoglycemia with 40% oral dextrose gel in near term at-risk infants to reduce intensive care need and promote breastfeeding. Ital J Pediatr. 2021;47(1):201. doi: 10.1186/s13052-021-01149-7.
- Gibson BL, Carter BM, LeDuff LD, 3rd, et al. 40% Glucose gel for the treatment of asymptomatic neonatal hypoglycemia. Adv. Neonatal Care. 2021;21(5):371–378. doi: 10.1097/ANC.0000000000000823.
- Desai P, Verma S, Bhargava S, et al. Implementation and outcomes of a standard dose dextrose gel protocol for management of transient neonatal hypoglycemia. J Perinatol. 2022;42(8):1097–1102. doi: 10.1038/s41372-021-01284-3.
- Gupta K, Amboiram P, Balakrishnan U, et al. Dextrose gel for neonates at risk with asymptomatic hypoglycemia: a randomized clinical trial. Pediatrics. 2022;149(6):e2021050733. doi: 10.1542/peds.2021-050733.
- Parappil H, Gaffari M, Ahmed J, et al. Oral dextrose gel use in asymptomatic hypoglycemic newborns decreases NICU admissions and parenteral dextrose therapy: a retrospective study. NPM. 2023;16(1):111–117. doi: 10.3233/NPM-221170.
- Gregory K, Turner D, Benjamin CN, et al. Incorporating dextrose gel and feeding in the treatment of neonatal hypoglycaemia. Arch Dis Child Fetal Neonatal Ed. 2020;105(1):45–49. doi: 10.1136/archdischild-2018-316430.
- Sen S, Andrews C, Anderson E, et al. Type of feeding provided with dextrose gel impacts hypoglycemia outcomes: comparing donor milk, formula, and breastfeeding. J Perinatol. 2020;40(11):1705–1711. doi: 10.1038/s41372-020-00776-y.