Abstract
Background
Neonatal sepsis is the third leading cause of mortality during the neonatal period, with manifestations atypical and obscure. But the gold standard-blood culture test, requiring 3–5 days, makes it difficult to unveil the final pathogen and leads to the increasing ratio of false-negative results. The empirical method is consulting traditional biomarkers, such as procalcitonin (PCT), C-reactive protein (CRP), and white blood cell count. However, they are not specific for neonate in diagnostic capacity, especially for infants within three days after delivery, so more novel biomarkers are urgently needed to assist diagnosing neonatal sepsis. microRNAs (miRNAs) have been widely studied in recent years for their diagnostic and prognostic values in different diseases and we conducted a meta-analysis of miRNAs on the topic that whether they are potentially novel biomarkers in early detection of neonatal sepsis.
Objectives
The purpose of the study was to assess whether circulating miRNAs could be used as potential biomarkers for neonatal sepsis, including early and late-onset neonatal sepsis, then calculate their overall accuracy (OA) via meta-analysis.
Methods
PubMed, Cochrane Library, Embase, Web of Science, Scopus, and Ovid databases were retrieved; data cutoff for this analysis was 15 January 2023. Methodological quality assessment of included studies was performed through the Quality in Prognostic Studies tool. Corresponding 95% confidence interval (95%CI) was calculated to present miRNAs’ diagnostic value including the pooled sensitivity (Sen), specificity (Spe), positive or negative likelihood ratios (PLR or NLR), diagnostic odds ratio (DOR), and area under the curve (AUC). Differences in OA between the septic group and non-septic group were compared using Chi-square test.
Results
After identification, 16 records out of 11 selected articles were eligible for systematic review of miRNAs and four records for PCT; the case group for miRNAs included 945 neonatal sepsis cases; contrast group included 190 respiratory tract infections or pneumonia cases, 60 systemic inflammatory response syndrome (SIRS) cases and 559 healthy neonates. The pooled Sen, Spe, and DOR of miRNAs were 0.87 (95%CI 0.81–0.91), 0.79 (95%CI 0.71–0.85), and 24 (95%CI 12–50), respectively. The pooled Sen, Spe, and DOR of PCT were 0.92 (95%CI 0.83–0.96), 0.64 (95%CI 0.56–0.70), and 20 (95%CI, 7–56), respectively. The OA value of miRNAs was 80.38% and that of PCT was 77.36%, which were not statistically significant difference (p = .13) after the Chi-square test. In addition, no significant publication bias was indicated (p = .92).
Conclusions
Circulating miRNA levels could be applied as diagnostic biomarkers in neonatal sepsis.
Introduction
Neonatal sepsis, a complex and heterogeneous condition produced as response to invasive blood infection mostly caused by bacteria, classified as early or late-onset, is one of the leading causes of mortality and morbidity during the neonatal period. Early onset sepsis (EOS) is defined as the presence of bacteremia or meningitis in the first three days of life. Late-onset sepsis (LOS) is defined as a systemic infection in neonates older than three days (4–28 days). The pathogens mainly include group B streptococcus (GBS), Escherichia coli, coagulase-negative Staphylococcus, Haemophilus influenzae, and Listeria monocytogenes [Citation1]. A systematic analysis pointed out that neonatal sepsis continues to be the third leading cause of morbidity and mortality in infants, following preterm and pregnancy events [Citation2]. Despite antibiotic administration before delivery, there are 0.5 million infants who die from sepsis [Citation3], for there are always no obvious manifestations in early stages of sepsis. If evident symptoms were observed, multiple organ damage may have occurred, such as kidney injury and heart failure, which could even occur simultaneously. Atypical symptoms, like apnea, poor reflexes, and lethargy indicate nervous system impairment, while abdominal distension and bloody diarrhea indicate necrotizing enterocolitis or intestinal perforation, and respiratory distress and apnea always indicate pneumonia [Citation1].
According to clinical features of neonatal sepsis above, early diagnosis and treatment are crucial for improving prognosis and reducing mortality. Hemoculture is the “gold standard” in detecting pathogens for diagnosis, however, that requires 2–5 mL blood volume from neonates and 3–5 days to maximize sensitivity (Sen) [Citation4]. Other biomarkers, such as white blood cell count, C-reactive protein (CRP), procalcitonin (PCT), and interleukins (ILs), have been reported limited in diagnostic specificity (Spe), especially for CRP and ILs [Citation5].
microRNAs (miRNAs) are a class of non-coding RNA molecules with an extension of 18–23 nucleotides that bind target mRNA and induce mRNA degradation or repression of translation, which is essential for development and survival [Citation6]. miRNAs have been reported to be involved in the regulation of exacerbated inflammatory responses, impairment of endothelial function and activation of the coagulation cascade [Citation6]. Dysregulation of several miRNAs, such as miRNA-15b, miRNA-378a, miR-211-5p, and miR-1184, was found to be differentially expressed in the serum of septic neonates [Citation7–9].
Among the neonate populations reported by recent studies, the expression level of circulating miRNAs is inclined to show differences in demographic variation, samples, disease stages, and methodologies. Accordingly, a systematic meta-analysis of the current study could promote comprehension of the diagnostic capacity of miRNAs in neonatal sepsis.
Methods
Selection criteria
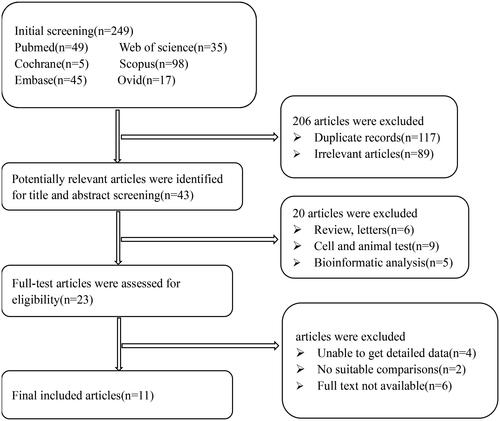
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation10] and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines, we performed a meta-analysis of miRNAs in the diagnostic value of neonatal sepsis. PubMed, Cochrane Library, Embase, Web of Science, Scopus, and Ovid were screened before 15 January 2023. In lexical retrieval process, words like “Neonatal Sepsis”, “Neonatal Sepses”, “Sepses”, “Neonatal Late-Onset Sepsis”, “microRNA”, “microRNAs”, and “miRNA” were included. The inclusion criteria for the literature were as follows: (1) peripheral miRNAs were confirmed to be differentially expressed in EOS or LOS; (2) the data were publicly available, such as true positive (Tp), true negative (Tn), false positive (Fp), and false negative (Fn). The exclusion conditions were as follows: (1) reviews, letters, correspondence, expert-opinions, and editorial; (2) bioinformatic analysis, animal and cellular studies; (3) duplicated articles; (4) lacking sufficient data to obtain accurate diagnosis value; (5) the collected samples were not from blood.
Data collection
The titles and abstracts of the retrieved literature from the above databases were first screened and the full-text literature that met all the above screening conditions were selected for further analysis. Data extraction was performed including author, year of publication, region, miRNAs, specimen, regulation mode, sample size, and diagnostic power including Sen, Spe, and area under the curve (AUC).
Methodologic quality
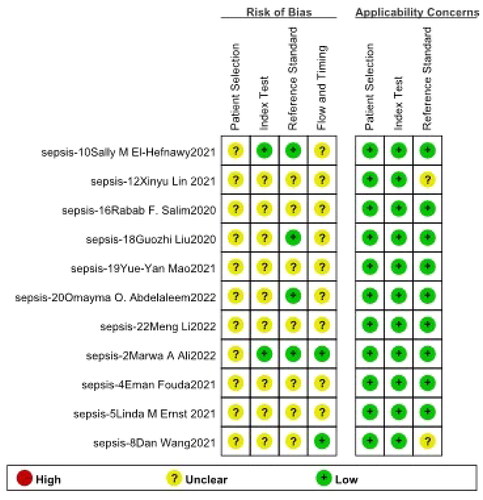
For the methodological quality evaluation of systematic review, Quality Diagnostic Accuracy Studies-2 (QUADAS-2) was applied.
Statistical analysis
After the threshold effect evaluation, the diagnostic index, which included Sen, Spe, negative likelihood ratio (NLR), positive likelihood ratio (PLR), summary receiver operating characteristic (SROC) curve, AUC, and diagnostic odds ratio (DOR) were quantified with 95% confidence interval (95%CI). A bivariate random-effects model was used for pooled analysis of diagnostic indices; meta-regression models were used to estimate heterogeneity and publication bias was assessed by Deek’s funnel plots. All statistics were analyzed with STATA version 17 (StataCorp, College Station, TX). Using SPSS Statistics 25 (SPSS Inc., Chicago, IL), the Chi-square test was used to compare the overall accuracy (OA) value of miRNAs and PCT.
Results
Search results and methodological qualities analysis
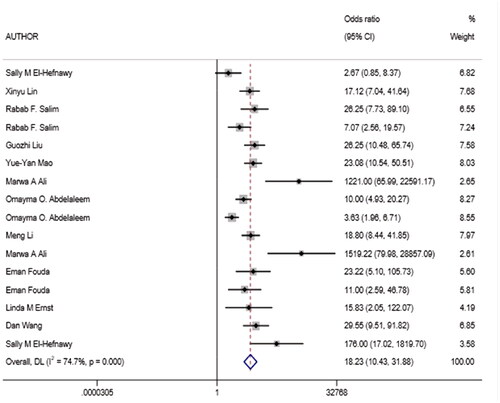
This study found 249 original articles by retrieving six medical databases, 238 duplicates were eliminated, then bioinformatic analysis, animal or cellular studies, irrelevant articles were removed, eventually 11 full-text studies [Citation7–9,Citation11–18] were screened in total, which contained 16 records of miRNAs, four records of PCT. The flow of studies identified to be included in the review is displayed in and methodological qualities assessment is presented in .
Characteristics of included studies
There were 16 records of miRNAs, the control groups include two records of systemic inflammatory response syndrome (SIRS), four records of respiratory infection or pneumonia and 10 records of healthy contrast group and the septic neonates were included for suspected or proven sepsis. The details of above records are displayed in . Among the 16 records, 10 records were published in Egypt, five records were published in China, and one record in America. In addition, 15 records were obtained from peripheral blood and one from umbilical cord blood. There were four records of PCT, including three records of respiratory infection and one record of SIRS.
Table 1. Characteristics of the included studies and participants.
Threshold effect
The Spearman correlation coefficients and p values for miRNAs were obtained by the rank correlation test, the coefficient value was −0.412 (p = .113), indicating that the threshold effect did not result in heterogeneity among the included studies.
Diagnostic accuracy of miRNAs and PCT
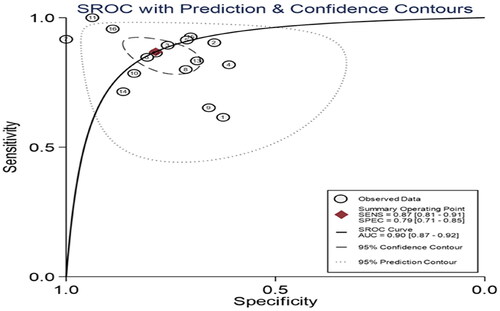
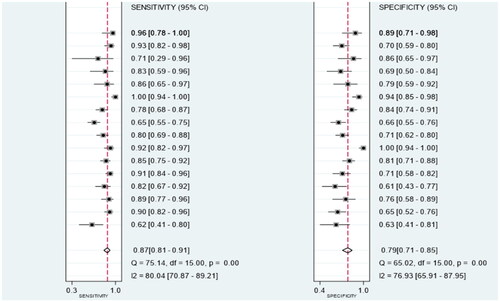
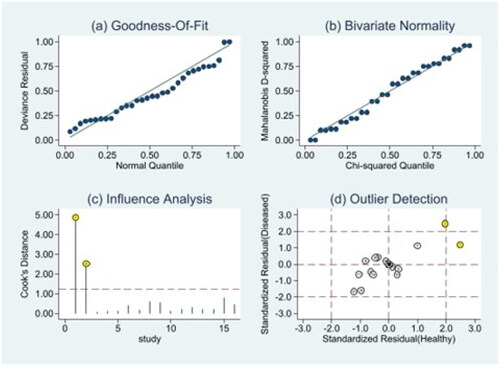
Owing to significant heterogeneity, we selected a random-effects model to perform statistical analysis and generate data among the 16 records (I2 = 80.04% for Sen, I2 = 76.93% for Spe, and I2 = 74.7% for DOR), as shown in and . The yellow spots, which fell outside the 95%CI, were the major sources of heterogeneity in and both were from the same article [Citation11]. The pooled Sen and Spe of the miRNAs were 0.87 (95%CI 0.81–0.91) and 0.79 (95%CI 0.71–0.85), respectively. The PLR and NLR were 4.1 (95%CI 2.9–5.7) and 0.17 (95%CI 0.11–0.26), respectively. The DOR of pooled studies was 24 (95%CI 12–50), the AUC for SROC was 0.9 (95%CI 0.87–0.92) as shown in and . Among those records, four records were used to evaluate diagnostic accuracy of PCT, the pooled Sen and Spe were 0.92 (95%CI 0.83–0.96) and 0.64 (95%CI 0.56–0.70), respectively. The PLR and NLR were 2.5 (95%CI 2.0–3.2) and 0.12 (95%CI 0.05–0.29), respectively. The DOR of pooled studies was 20 (95%CI 7–56) and the AUC for SROC was 0.74 (95%CI 0.70–0.78) as shown in . Finally, the OA value of miRNAs was 77.36% and that of PCT was 80.38%, indicating that their overall diagnostic accuracy scores of them were moderate and not statistically significant difference (p = .13).
Figure 5. Heterogeneity analysis. (a) Goodness-of-fit; (b) bivariate normality; (c) influence analysis; (d) outlier detection.
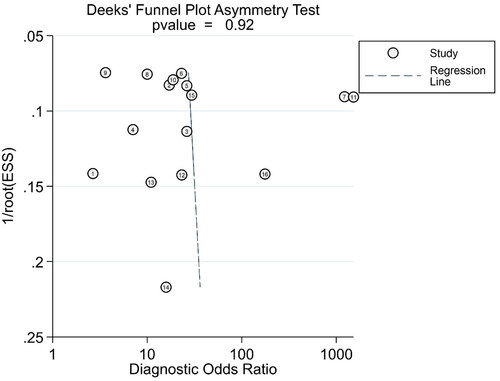
Table 2. Diagnostic value of circulating miRNAs and PCT.
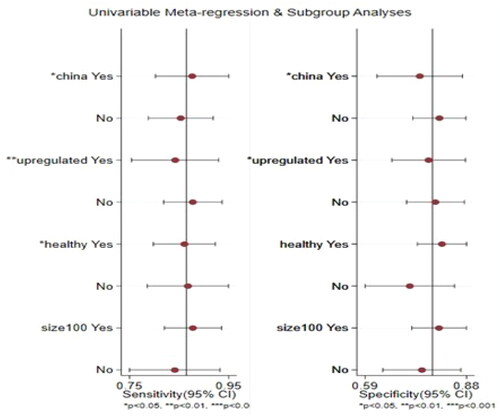
Meta-regression and subgroup analysis
Multiple univariate bivariate meta-regression models were used to explore the heterogeneity of included studies. The effects of each covariate on Sen and Spe were estimated as shown in and . The nation and regulation mode were expected to contribute significant differences in Sen and Spe (p < .05), the physical state did not affect Spe but caused difference in Sen, additionally, there were no significant differences for sample size in Sen and Spe.
Table 3. Summary diagnostic power based on subgroup analysis.
Discussion
Neonatal sepsis is a systemic condition in response to invasive infection of pathogens, such as bacterial, viral, or fungal. The irreversible hemodynamic change and multiple organ impairments could result in substantial morbidity and mortality, so early detection and timely treatment are urgent to improve prognosis in the early or late periods. However, it is difficult to distinguish SIRS from sepsis [Citation19], or local infection with systemic infection by traditional indexes, such as white blood cell count, CRP, PCT, and IL-6, which can vague disease stage and mistake therapeutic strategy. In recent years, increasing number of studies have focused on the disease prediction and prognosis value of miRNAs and analyzed the potential mechanisms by cellular tests, animal tests, bioinformatic database mining, etc. Similarly, more studies are focusing on neonatal sepsis, which is one of the major causes of mortality in neonatal period. In order to evaluate the diagnostic value of miRNAs, it is necessary to summarize recent results by systematic review and meta-analysis in neonatal sepsis.
Based on this systematic meta-analysis, the following conclusions can be obtained that the pooled Spe and DOR of circulating miRNAs were higher than those of PCT in diagnosing sepsis, but Sen was lower. The OA values of miRNAs or PCT were obviously greater than that of CRP, but there were not statistically significant difference between miRNAs and PCT. Furthermore, subgroup analysis indicated that different nations, regulation modes and health state can lead to differences in diagnosis values (p < .05).
Although there are several screening tools for inflammatory markers, such as white cell count, IL-6, CRP, and PCT, which have been applied for early diagnosis of sepsis, disease progression monitoring, and treatment efficacy determining, but none of them have been confirmed specific. For example, recent reports have pointed out that CRP had limited Spe in diagnosing sepsis, especially under conditions like neonatal asphyxia, respiratory distress, and stress response [Citation20]. The level of PCT is a useful index in diagnosing sepsis, monitoring response to antibiotic treatment and progression phases, while within 48 h after delivery, PCT and white blood cell count can increase physiologically in neonates [Citation21], so their Sen and Spe are comparatively lower and lack accurate cutoff values for the diagnosis of early onset neonatal sepsis [Citation22].
Current studies have analyzed the diagnostic value of circulating miRNAs in peripheral blood. In all 16 miRNAs, miR-181a, the most studied one, which had been researched in septic groups with acute kidney and lung injury [Citation23,Citation24], both Liu and Chen pointed out that it was differentially downregulated in the serum of septic neonates compared with non-septic ones [Citation15,Citation25]. Similarly, a meta-analysis in adult group showed that overall Sen of miRNAs was 0.77, overall Spe was 0.91, AUC value was 0.87, and the DOR value was 33, indicating the potential diagnostic value of sepsis in adults [Citation26].
Our results showed that the AUC value of miRNAs was 0.9 (95%CI 0.87, 0.92) higher than that of PTC which was 0.74 (95%CI 0.70, 0.78). However, there was no statistically significant difference in OA values between them. Although miRNAs are promising diagnostic biomarkers for sepsis, certain limitations were observed in this meta-analysis. First, heterogeneity was observed. Second, we could not control the statistical methods among the included studies, which may cause differences in the outcomes.
Conclusions
Circulating miRNAs may be potential biomarkers for distinguishing sepsis from respiratory infections or pneumonia, SIRS and healthy controls.
Author contributions
Yihong Zhao: conceptualization, methodology, software, investigation, formal analysis, and writing – original draft. Ruqin Zhu: data curation. Xiaoyan Hu: conceptualization, supervision, and writing – review and editing.
Ethics statement
An ethics statement is not applicable because this study was based exclusively on published literature.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780. doi: 10.1016/s0140-6736(17)31002-4.
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi: 10.1016/s0140-6736(14)61698-6.
- Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–230. doi: 10.1016/s2213-2600(18)30063-8.
- Tat Trung N, Van Tong H, Lien TT, et al. Clinical utility of an optimised multiplex real-time PCR assay for the identification of pathogens causing sepsis in Vietnamese patients. Int J Infect Dis. 2018;67:122–128. doi: 10.1016/j.ijid.2017.12.015.
- Celik IH, Hanna M, Canpolat FE, et al. Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res 2022;91(2):337–350. doi: 10.1038/s41390-021-01696-z.
- Kingsley SMK, Bhat BV. Role of microRNAs in sepsis. Inflamm Res. 2017;66(7):553–569. doi: 10.1007/s00011-017-1031-9.
- Fouda E, Elrazek Midan DA, Ellaban R, et al. The diagnostic and prognostic role of miRNA 15b and miRNA 378a in neonatal sepsis. Biochem Biophys Rep. 2021;26:100988. doi: 10.1016/j.bbrep.2021.100988.
- Ernst LM, Mithal LB, Mestan K, et al. Umbilical cord miRNAs to predict neonatal early onset sepsis. PLOS One. 2021;16(5):e0249548. doi: 10.1371/journal.pone.0249548.
- Wang D, Han L. Downregulation of miR-1184 serves as a diagnostic biomarker in neonatal sepsis and regulates LPS-induced inflammatory response by inhibiting IL-16 in monocytes. Exp Ther Med. 2021;21(4):350. doi: 10.3892/etm.2021.9781.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open medicine: a peer-reviewed, independent. Open Med. 2009;3(3):e123–e130.
- Ali MA, Khamis Hussein S, Ali Mohamed E, et al. Diagnostic and prognostic values of miR181b-5p and miR21-5p for neonatal sepsis risk and their link to SNAP II score and disease mortality. Noncoding RNA Res. 2023;8(1):115–125. doi: 10.1016/j.ncrna.2022.11.001.
- El-Hefnawy SM, Mostafa RG, El Zayat RS, et al. Biochemical and molecular study on serum miRNA-16a and miRNA-451 as neonatal sepsis biomarkers. Biochem Biophys Rep. 2021;25:100915. doi: 10.1016/j.bbrep.2021.100915.
- Lin X, Wang Y. miR-141 is negatively correlated with TLR4 in neonatal sepsis and regulates LPS-induced inflammatory responses in monocytes. Braz J Med Biol Res. 2021;54(7):e10603. doi: 10.1590/1414-431x2020e10603.
- Salim RF, Sobeih AA, Kareem HMAE. Evaluation of the clinical value of circulating miR-101, miR-187 and miR-21 in neonatal sepsis diagnosis and prognosis. Egypt J Med Hum Genet. 2020;21(1):12. doi: 10.1186/s43042-020-00052-w.
- Liu G, Liu W, Guo J. Clinical significance of miR-181a in patients with neonatal sepsis and its regulatory role in the lipopolysaccharide-induced inflammatory response. Exp Ther Med. 2020;19(3):1977–1983. doi: 10.3892/etm.2020.8408.
- Mao Y-Y, Su C, Fang C-C, et al. Clinical significance of the serum miR-455-5p expression in patients with neonatal sepsis. Bioengineered. 2021;12(1):4174–4182. doi: 10.1080/21655979.2021.1955580.
- Abdelaleem OO, Mohammed SR, El Sayed HS, et al. Serum miR-34a-5p and miR-199a-3p as new biomarkers of neonatal sepsis. PLOS One. 2022;17(1):e0262339. doi: 10.1371/journal.pone.0262339.
- Li M, Huang X, Zhuo Q, et al. Clinical significance of miR-129-5p in patients with neonatal sepsis and its regulatory role in the LPS-induced inflammatory response. Bosnian J Basic Med Sci. 2021;22(2):185–190. doi: 10.17305/bjbms2020.5184.
- Balk RA. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence. 2014;5(1):20–26. doi: 10.4161/viru.27135.
- Sankar V, Webster NR. Clinical application of sepsis biomarkers. J Anesth. 2013;27(2):269–283. doi: 10.1007/s00540-012-1502-7.
- Stocker M, van Herk W, el Helou S, et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns). Lancet. 2017;390(10097):871–881. doi: 10.1016/s0140-6736(17)31444-7.
- Chiesa C, Pacifico L, Osborn JF, et al. Early-onset neonatal sepsis: still room for improvement in procalcitonin diagnostic accuracy studies. Medicine. 2015;94(30):e1230. doi: 10.1097/MD.0000000000001230.
- Ahmad S, Ahmed MM, Hasan PMZ, et al. Identification and validation of potential miRNAs, as biomarkers for sepsis and associated lung injury: a network-based approach. Genes. 2020;11(11):1327. doi: 10.3390/genes11111327.
- Wang J, Song J, Li Y, et al. Down-regulation of LncRNA CRNDE aggravates kidney injury via increasing MiR-181a-5p in sepsis. Int Immunopharmacol. 2020;79:105933. doi: 10.1016/j.intimp.2019.105933.
- Chen J, Jiang S, Cao Y, et al. Altered miRNAs expression profiles and modulation of immune response genes and proteins during neonatal sepsis. J Clin Immunol. 2014;34(3):340–348. doi: 10.1007/s10875-014-0004-9.
- Shen X, Zhang J, Huang Y, et al. Accuracy of circulating microRNAs in diagnosis of sepsis: a systematic review and meta-analysis. J Intensive Care. 2020;8(1):84. doi: 10.1186/s40560-020-00497-6.