Abstract
Introduction
Intraamniotic infection (IAI) and subsequent early-onset neonatal sepsis (EONS) are among the main complications associated with preterm prelabor rupture of membranes (PPROM). Currently used diagnostic tools have been shown to have poor diagnostic performance for IAI. This study aimed to investigate whether the exposure to IAI before delivery is associated with short-term variation of the fetal heart rate in pregnancies with PPROM.
Methods
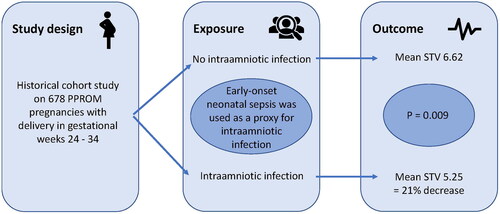
Observational cohort study of 678 pregnancies with PPROM, delivering between 24 + 0 and 33 + 6 gestational weeks from 2012 to 2019 in five labor units in Stockholm County, Sweden. Electronic medical records were examined to obtain background and exposure data. For the exposure IAI, we used the later diagnosis of EONS in the offspring as a proxy. EONS is strongly associated to IAI and was considered a better proxy for IAI than the histological diagnosis of acute chorioamnionitis, since acute chorioamnionitis can be observed in the absence of both positive microbiology and biochemical markers for inflammation. Cardiotocography traces were analyzed by a computerized algorithm for short-term variation of the fetal heart rate, which was the main outcome measure.
Results
Twenty-seven pregnancies were categorized as having an IAI, based on the proxy diagnosis of EONS after birth. Fetuses exposed to IAI had significantly lower short-term variation values in the last cardiotocography trace before birth than fetuses who were not exposed (5.25 vs 6.62 ms; unadjusted difference: −1.37, p = 0.009). After adjustment for smoking and diabetes, this difference remained significant. IAI with a later positive blood culture in the neonate (n = 12) showed an even larger absolute difference in STV (−1.65; p = 0.034), with a relative decrease of 23.5%.
Conclusion
In pregnancies with PPROM, fetuses exposed to IAI with EONS as a proxy have lower short-term variation of the fetal heart rate than fetuses who are not exposed. Short-term variation might be useful as adjunct surveillance in pregnancies with PPROM.
Introduction
Preterm prelabor rupture of membranes (PPROM) occurs in 1%–3% of pregnancies and causes approximately one third of preterm births [Citation1,Citation2]. One of the main complications associated with PPROM is intraamniotic infection (IAI), a microbial infection of the amniotic fluid, fetus, placenta, fetal membranes and/or the decidua [Citation3,Citation4]. IAI increases the risk of severe maternal and neonatal complications such as early-onset neonatal sepsis (EONS) [Citation3,Citation5–8].
Women with PPROM are usually monitored for signs of IAI by combining clinical assessment, blood tests and fetal monitoring. Unfortunately, these tools have shown poor diagnostic and prognostic performance regarding IAI and EONS [Citation9–13]. The delicate decision of iatrogenic delivery, weighing risks of IAI against risks of prematurity, would benefit from diagnostic tools with a better performance. Antenatal cardiotocography (CTG) has been suggested as a supplementary tool in monitoring for signs of IAI. CTG is a noninvasive method used in daily obstetrical practice, and is thus an optimal instrument for further development.
The short-term variation (STV) of the fetal heart rate is the change in the time interval between consecutive beats, analyzed with computerized cardiotocography. STV is known to be affected by metabolic acidemia, gestational age, fetal sleep, fetal tachycardia, maternal diabetes, maternal smoking and medications such as corticosteroids [Citation14–19]. The effect of IAI on STV has only been scarcely studied but in a small cohort study, Vandenbroucke et al. found a lower STV of the fetal heart rate in women with acute histologic chorioamnionitis (HCA) than in women without HCA [Citation20]. This could imply a possible effect of IAI on STV.
The aim of our study was to investigate the association between the exposure to IAI and the outcome of short-term variation of the fetal heart rate.
Material and methods
We performed a historical cohort study. The inclusion criteria were PPROM in a singleton pregnancy and a live-born offspring delivered from 24 + 0 to 33 + 6 gestational weeks in Stockholm County, Sweden, between 2012 and 2019. We collected data from medical records and performed computerized analyses of electronically stored CTG traces. In Stockholm County, there are five delivery units with approximately 30 000 births each year. All women are offered free antenatal and peripartum care including an ultrasound in the second trimester with an estimation of the gestational age and to detect fetal abnormalities and multiple pregnancies.
We excluded women with < 12 h between PPROM and birth to exclude rupture of the membranes as part of impending labor. Women with insufficient data and intrauterine fetal death were excluded, as were women who delivered a neonate with any kind of cerebral malformation because STV is mainly controlled by the autonomic nervous system.
The main exposure of the study was IAI. As no easily available method exists for the confirmation of IAI antepartum, a proxy for IAI is commonly used. HCA has often been used as such, but HCA can be observed in the absence of positive microbiology and biochemical markers for inflammation [Citation21–23]. Its prevalence at the time of delivery is high in women with PPROM and the association with neonatal outcome unclear [Citation24,Citation25]. It may therefore not be an ideal proxy for the exposure IAI. We consider the development of EONS, defined as occurring within 72 h after birth, to be a better proxy. EONS is considered to be caused by vertically transmitted pathogens from the mother to the infant and is strongly associated with IAI. Most preterm infants with EONS are born to mothers with IAI [Citation8,Citation26,Citation27]. We have therefore chosen EONS as a proxy for IAI when studying the association between the exposure IAI and the outcome STV. EONS was defined as sepsis within 72 h from birth with three or more clinical symptoms of sepsis (respiratory, circulatory, neurological, gastrointestinal or hematological), at least one laboratory test indicating infection (white blood cell count < 5 × 109/L, neutrophil count < 1.5 × 109/L, platelet count < 100 × 109/L or C-reactive protein concentrations >20 mg/L) and treatment with antibiotics for at least 5 consecutive days [Citation28]. We divided the neonates with EONS into those with or without positive blood cultures (confirmed and suspected EONS, respectively). We also performed two secondary analyses, with HCA and a fetal inflammatory response in the placenta as proxies for the exposure IAI instead of EONS. These were diagnosed by a perinatal pathologist in accordance with the Amsterdam Placental Workshop Group Consensus Statement defining HCA as leukocyte infiltration in the chorion and the amnion upon microscopic examination, and fetal inflammatory response as leukocyte infiltration in the fetal blood vessels of the chorion and/or the umbilical cord [Citation29].
The main outcome of the study was STV, calculated according to the Dawes/Redman algorithm [Citation30]. In this algorithm, each minute of a CTG trace is divided into 16 segments of 3.75 s. The average pulse interval of each segment is calculated and expressed in ms, and the average difference between consecutive segments within each minute is the STV. Our research group has developed an algorithm for extracting the mean baseline frequency and the mean STV values from existing CTG traces. Our algorithm is described in details in previous publications [Citation31,Citation32]. Using this algorithm, the mean STV in the last 30-min CTG trace before the start of labor was calculated. Traces registered during labor were excluded to rule out labor-related effects on STV. CTG traces exceeding 30 min were divided into 30-min segments, and only the last 30-min segment was used. Traces with more than 10-min continuous signal loss were excluded. Decelerations were excluded from the STV analysis. The fetal heart rate was recorded with one of the following monitors: SonicaidTM (Huntleigh, United Kingdom), AvalonTM (Philips, Netherlands) or EDANTM (EDAN Instruments, China). The STV analysis was carried out utilizing the CTG analysis scripts implemented in MATLAB 2022a (MathWorks Inc., Natick, MA, USA).
PPROM was diagnosed by clinical presentation and an examination. A sterile vaginal speculum examination was performed for leakage of amniotic fluid and in cases of uncertainty, some of the delivery units used a test for placental alpha microglobulin-1 protein (Amnisure®; AGHealth Ltd., London, England). Women with confirmed PPROM were hospitalized and treated with a course of corticosteroids (12 mg betamethasone, two doses 24 h apart) and antibiotics (oral erythromycin or clarithromycin for 10 days). Expectant management was applied after this initial therapy, during which the women were monitored for signs of infection. Expectant management was applied until 34–37 gestational weeks in the absence of indications for earlier delivery. We only included women who delivered before 34 gestational weeks because the timing of induction of labor after PPROM varies with some obstetric units inducing birth already after 34 gestational weeks. During preterm labor, all women received intravenous penicillin as a prophylaxis against Group B Streptococcus.
We collected information on maternal age at delivery, body mass index, smoking status, parity, diabetes, hypertension and preeclampsia. We documented the timepoints of PPROM, administration of corticosteroids, start of labor and birth. The birthweight, sex, Apgar scores and umbilical artery gas measures were recorded. Small for gestational age (defined as weight below two standard deviations from the mean birth weight of the current gestational age) [Citation33] was also determined. Women with no smoking information were assigned a separate level in the smoking category. There was no missing information for other covariates.
Statistical analyses
Continuous data of baseline characteristics in the exposed IAI group and the non-exposed group without IAI, respectively, are presented as the median and interquartile range, and the groups were compared with the Mann–Whitney U test. Categorical data are presented as percentages, and the groups were compared with Pearson’s chi-square test.
Results are displayed as the absolute and relative differences between groups, using linear regression for the absolute difference, and log linear regression for the relative difference, together with 95% confidence intervals and t-test p values. The STV values were moderately positive skew and had a median about 0.5 less than the mean. In the analysis of absolute difference in STV we did not transform the original STV, our interest was in the group means and we anticipated the standard errors to be slightly larger, and the confidence intervals slightly wider because of the deviation from normality in the STV values. However, when log transforming the STV values for the analysis of relative difference, the residuals in the model were normally distributed.
Based on previous knowledge, a directed acyclic graph was created (), seeking to identify potential confounders and mediators. A mediator is a variable that lies in the causal pathway between the exposure and outcome, while a confounder affects both exposure and outcome. This led to a minimally adjusted regression model including the known confounders smoking and diabetes.
Figure 1. Directed acyclic graph showing the relationship between exposure, outcome, and covariates. Green arrows: causal pathways; pink arrows: biasing pathways; black pathways: neither causal nor biasing pathways, but adjusting for baseline frequency makes the pathway through fetal sex a causal pathway; pink circles: confounders (common causes of exposure and outcome); blue circles: covariates affecting the outcome; green circle: covariate affecting exposure. Abbreviations: BMI, body mass index; CTG, cardiotocography.

We examined additional covariates for collinearity and tested adding them one by one to the minimally adjusted regression model to determine if the results were changed. This resulted in a more comprehensive adjusted model with addition of covariates affecting the results: fetal sex, the duration from PPROM to birth (in days) and the baseline fetal heart rate frequency (in beats/minute). These three covariates are considered mediators in our directed acyclic graph. Adjusting for mediators can introduce bias [Citation34], and therefore the results of this second model were interpreted with awareness of the possibility of overadjustments and bias.
Lag time between corticosteroid treatment and STV value has been shown to affect STV [Citation35]. However, as this lag time does not affect the risk for infection [Citation36] it was not regarded as a confounder. Furthermore, this lag time did not change our estimates and was therefore not added to the adjustment models. The same applies for gestational age; it is also known to affect the STV but since it does not affect the risk for infection it was not regarded as a confounder and not added to the adjustment models.
The 95% confidence intervals are shown for the absolute and relative differences, and for all analyses a p value of < 0.05 was considered statistically significant.
Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethics statement
The study was reviewed and approved by the Swedish Ethical Review Authority on March 8, 2017 (2017/323-31), with the approval of additions on June 16, 2019 (2019-03026) and June 25, 2020 (2020-02197). Requirement for consent was waived by the review authority since this is a historical cohort study where all data is deidentified and the results are only presented in aggregated form.
Results
A total of 922 women fulfilled the inclusion criteria. We excluded 186 women who had PPROM < 12 h before giving birth and 58 women because of other reasons (). We included 678 women in the final study population. The demographic and clinical characteristics of the study population are shown in . There were significantly more smokers and more women with diabetes in the IAI group (16.0% vs 6.0% [p = 0.044] and 14.8% vs 3.7% [p = 0.004], respectively), and neonates exposed to IAI had lower 1-min and 5-min Apgar scores than those not exposed (6 vs 8 [p = 0.003] and 8 vs 9 [p = 0.017], respectively). Information on smoking was missing for 50 (7.4%) women. Placenta pathology analysis was available for 365 women.
Figure 2. Flow chart showing study population selection. Abbreviations: PPROM, preterm prelabor rupture of membranes; CTG, cardiotocography.

Table 1. Maternal and neonatal characteristics, and comparison of neonates exposed and non-exposed to intraamniotic infection, using early-onset neonatal sepsis as a proxy for intraamniotic infection.
Mean STV values, the absolute and the relative difference in STV are shown in . In the entire study population, neonates exposed to IAI had significantly lower STV values in the last CTG trace before birth than those who were not exposed to IAI (5.25 vs 6.62 ms; absolute unadjusted difference: −1.37 [95% CI −2.40 to −0.34]; p = 0.009). After adjusting for the prespecified confounders of smoking and diabetes, this difference remained significant (absolute adjusted difference: −1.34 [95% CI −2.38 to −0.30]; p = 0.012). When we only included IAI with a later positive blood culture in the neonate (confirmed EONS), the absolute unadjusted difference was −1.65 (95% CI −3.19 to −0.12; p = 0.034) with a relative decrease of 23.5% (95% CI −37.9% to −5.7%; p = 0.012), and it remained significant after adjusting for smoking and diabetes.
Table 2. Mean short-term variation (STV) in fetuses non-exposed and exposed to intraamniotic infection (IAI), with early-onset neonatal sepsis (EONS) as a proxy for IAI, with standard deviation (SD).
shows the secondary analyses with comparison between pregnancies with and without IAI, using HCA and fetal inflammatory response as proxies instead of EONS. Fetuses in pregnancies with HCA had significantly lower STV values (absolute unadjusted difference: −0.89 [95% CI −1.35 to −0.43], p < 0.001; relative unadjusted difference: −13.7% [95% CI −19.8% to −7.2%], P = <0.001), as well as fetuses with an inflammatory response (absolute unadjusted difference: −1.04 [95% CI −1.49 to −0.60], p < 0.001; relative unadjusted difference: −15.8% [95% CI −21.5% to −9.6%], p < 0.001), than those without HCA or fetal inflammatory response. These differences remained significant after adjusting for confounders.
Table 3. Mean short-term variation (STV) in fetuses non-exposed and exposed to intraamniotic infection (IAI), with acute histologic chorioamnionitis (HCA) and a fetal inflammatory response (FIR) as proxies for IAI, shown with standard deviation (SD).
Discussion
Among pregnancies complicated by PPROM, fetuses affected by an IAI developed lower STV than fetuses not affected by IAI. This decrease was observed regardless of whether EONS, HCA or fetal inflammatory response was used as a proxy for IAI. The difference remained significant after adjustment for diabetes and smoking.
We believe the shown effect on STV in infants exposed to IAI is not a result of metabolic acidemia, but of the infection itself. This is supported by blood gas analyses from the umbilical cords, as shown in , as well as by studies on neonates with decreased heart rate variation preceding the clinical signs of neonatal sepsis [Citation37]. The effect of infection on heart rate variation is thought to be mediated through vagal nerve activation via the cholinergic anti-inflammatory pathway, direct effect of infection on the electrical activity of the sinoatrial node pacemaker cells and proinflammatory cytokines that can depress heart rate variation [Citation38].
In our study population, the incidence of EONS was 4.0% (confirmed EONS: 1.8%), which is in line with other studies on outcomes after PPROM [Citation39,Citation40]. Unfortunately, there is no international consensus regarding the criteria for the diagnosis of neonatal sepsis [Citation41,Citation42]. The clinical presentation varies with gestational age, and preterm neonates often have subtle and nonspecific clinical signs and symptoms, as well as a limited ability to produce inflammatory markers. The use of antenatal and intrapartum antibiotics may result in false negative blood cultures in the neonate. Taking into consideration the above-mentioned possibilities, some infected neonates might not have been diagnosed with EONS according to the applied criteria, and thereby been falsely grouped as neonates without infection. If this was the case, we might have underestimated the difference in STV between the groups exposed and non-exposed to IAI, when using EONS as a proxy for IAI.
Previous studies on the effect of IAI on STV are scarce with contradicting results. Buhimschi et al. found that non-reassuring CTG (recurrent late decelerations, severe variable deceleration and prolonged deceleration or fetal bradycardia with absent variability) at admission was significantly more common in neonates who later developed EONS than in those who did not (35% vs 5%, p < 0.001) [Citation43]. Salafia et al. found a significant relationship between HCA and decreased fetal heart rate variation among patients in preterm labor, but this relationship was not observed in patients with PPROM [Citation44]. Our results are in line with the results of Vandenbroucke et al. [Citation20] where pregnancies complicated by HCA had lower STV values than those without HCA. Meanwhile, a small case-control study by Day et al. showed no significant difference in the CTGs of newborns that developed EONS compared with those without EONS [Citation45].
The strengths of this study include the large population it was performed on and, to the best of our knowledge, this is the first study on the relationship between STV and IAI using EONS as proxy in women with PPROM. Most previous studies have used HCA as proxy but this is suboptimal due to its low specificity and sensitivity for both IAI and neonatal outcome [Citation21–25]. We instead decided to use EONS as a proxy for IAI and motivated our choice with the strong association between IAI and EONS and the speculated plausibility of a correlation between the degree of severity of an IAI and the development of EONS [Citation8,Citation26]. When managing women with PPROM, it is important not only addressing the risk of IAI, but also considering to what extent the neonate becomes affected.
One limitation of the study could be the historical cohort using variables not registered specifically for this study, but we consider the information bias to be low for several reasons. In Sweden, the medical records from antenatal visits include a standardized list of questions, resulting in few missing covariate data. The variables were registered prospectively at the time of pregnancy which diminishes recall bias. We included the entire Stockholm County, which increased the generalizability of the study. Residual confounding cannot be controlled for in an observational study, which should be taken into consideration when interpreting our results. Another possible limitation is the choice of 30-min CTG tracing segments. Fetuses mature enough to have cyclicity in their sleep–activity periods (after approximately 28 gestational weeks) might have been registered only when active or when in quiet sleep (resulting in a higher/lower STV value than that if the CTG trace also contained a quiet sleep/active period) [Citation46,Citation47]. However, this possible information bias would be non-differential because these two scenarios should occur equally.
This study shows lower STV value in PPROM pregnancies complicated by an IAI leading to EONS. Better diagnostic tools for IAI and predictors for neonatal infection can decrease under- and overdiagnoses, which can improve maternal and neonatal outcomes. Our findings indicate that STV could be an additional tool in the delicate balance between expectancy and intervention. The association between STV and intraamniotic infection needs to be confirmed in prospective studies to establish its usefulness in clinical practice, and to define its diagnostic performance and possible cutoff values.
Acknowledgments
We thank Thomas Andersson for his important statistical input and work. We thank Viveka Nordberg who is a neonatologist, and Nikos Papadogiannakis who is a perinatal pathologist, for helpful input and discussion regarding the validation of data and diagnoses of EONS, HCA and FIR. We thank Ellen Knapp, PhD, from Edanz (https://edanz.com/ac) for editing a draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
Additional information
Funding
References
- Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101(1):1–10. doi:10.1016/s0029-7844(02)02366-9.
- Parry S, Strauss JF.3rd. Premature rupture of the fetal membranes. N Engl J Med. 1998;338(10):663–670. doi:10.1056/NEJM199803053381006.
- Committee Opinion No. 712. Intrapartum management of intraamniotic infection. Obstet Gynecol. 2017;130(2):e95–e101.
- Higgins RD, Saade G, Polin RA, et al. Evaluation and management of women and newborns With a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol. 2016;127(3):426–436. doi:10.1097/AOG.0000000000001246.
- Conde-Agudelo A, Romero R, Jung EJ, et al. Management of clinical chorioamnionitis: an evidence-based approach. Am J Obstet Gynecol. 2020;223(6):848–869. doi:10.1016/j.ajog.2020.09.044.
- García-Muñoz Rodrigo F, Galán Henríquez G, Figueras Aloy J, et al. Outcomes of very-low-birth-weight infants exposed to maternal clinical chorioamnionitis: a multicentre study. Neonatology. 2014;106(3):229–234. doi:10.1159/000363127.
- Jain VG, Willis KA, Jobe A, et al. Chorioamnionitis and neonatal outcomes. Pediatr Res. 2022;91(2):289–296. doi:10.1038/s41390-021-01633-0.
- Villamor-Martinez E, Lubach GA, Rahim OM, et al. Association of histological and clinical chorioamnionitis With neonatal sepsis Among preterm infants: a systematic review, Meta-Analysis, and Meta-Regression. Front Immunol. 2020;11:972. doi:10.3389/fimmu.2020.00972.
- Ajayi SO, Morris J, Aleem S, et al. Association of clinical signs of chorioamnionitis with histological chorioamnionitis and neonatal outcomes. J Matern Fetal Neonatal Med. 2022;35(26):10337–10347.
- Etyang AK, Omuse G, Mukaindo AM, et al. Maternal inflammatory markers for chorioamnionitis in preterm prelabour rupture of membranes: a systematic review and meta-analysis of diagnostic test accuracy studies. Syst Rev. 2020;9(1):141. doi:10.1186/s13643-020-01389-4.
- Oh KJ, Kim SM, Hong J-S, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol. 2017;216(6):604.e1–e11. doi:10.1016/j.ajog.2017.02.035.
- Su H, Chang S-S, Han C-M, et al. Inflammatory markers in cord blood or maternal serum for early detection of neonatal sepsis-a systemic review and meta-analysis. J Perinatol. 2014;34(4):268–274. doi:10.1038/jp.2013.186.
- Sung JH, Choi SJ, Oh SY, et al. Revisiting the diagnostic criteria of clinical chorioamnionitis in preterm birth. BJOG. 2017;124(5):775–783. doi:10.1111/1471-0528.14176.
- Derks JB, Mulder EJ, Visser GH. The effects of maternal betamethasone administration on the fetus. Br J Obstet Gynaecol. 1995;102(1):40–46. doi:10.1111/j.1471-0528.1995.tb09024.x.
- Graça LM, Cardoso CG, Clode N, et al. Acute effects of maternal cigarette smoking on fetal heart rate and fetal body movements felt by the mother. J Perinat Med. 1991;19(5):385–390. doi:10.1515/jpme.1991.19.5.385.
- Kutlu T, Ozkaya E, Sanverdi I, et al. Acute fetal heart rate tracing changes secondary to cigarette smoking in third trimester pregnancies. J Matern Fetal Neonatal Med. 2017;30(12):1407–1409. doi:10.1080/14767058.2016.1214708.
- Murray H. Antenatal foetal heart monitoring. Best Pract Res Clin Obstet Gynaecol. 2017;38:2–11. doi:10.1016/j.bpobgyn.2016.10.008.
- Ruozi-Berretta A, Piazze JJ, Cosmi E, et al. Computerized cardiotocography parameters in pregnant women affected by pregestational diabetes mellitus. J Perinat Med. 2004;32(5):426–429. doi:10.1515/JPM.2004.141.
- Tincello D, White S, Walkinshaw S. Computerised analysis of fetal heart rate recordings in maternal type I diabetes mellitus. BJOG. 2001;108(8):853–857. doi:10.1111/j.1471-0528.2001.00208.x.
- Vandenbroucke L, Doyen M, Le Lous M, et al. Chorioamnionitis following preterm premature rupture of membranes and fetal heart rate variability. PLoS One. 2017;12(9):e0184924. doi:10.1371/journal.pone.0184924.
- Kim CJ, Romero R, Chaemsaithong P, et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–S52. doi:10.1016/j.ajog.2015.08.040.
- Roberts DJ, Celi AC, Riley LE, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS One. 2012;7(3):e31819. doi:10.1371/journal.pone.0031819.
- Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–474. doi:10.1111/aji.12296.
- Lee Y, Kim H-J, Choi S-J, et al. Is there a stepwise increase in neonatal morbidities according to histological stage (or grade) of acute chorioamnionitis and funisitis?: effect of gestational age at delivery. J Perinat Med. 2015;43(2):259–267. doi:10.1515/jpm-2014-0035.
- Torricelli M, Voltolini C, Conti N, et al. Histologic chorioamnionitis at term: implications for the progress of labor and neonatal wellbeing. J Matern Fetal Neonatal Med. 2013;26(2):188–192. doi:10.3109/14767058.2012.722724.
- Puopolo KM, Benitz WE, Zaoutis TE, Committee On F, Newborn, Committee On Infectious D. Management of neonates born at </=34 6/7 weeks’ gestation With suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142(6):e20182896. doi:10.1542/peds.2018-2896.
- Briggs-Steinberg C, Roth P. Early-Onset sepsis in newborns. Pediatr Rev. 2023;44(1):14–22. doi:10.1542/pir.2020-001164.
- Hutzal CE, Boyle EM, Kenyon SL, et al. Use of antibiotics for the treatment of preterm parturition and prevention of neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 2008;199(6):620 e1-8–620.e8. doi:10.1016/j.ajog.2008.07.008.
- Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. 2016;140(7):698–713. doi:10.5858/arpa.2015-0225-CC.
- Dawes GS, Moulden M, Redman CW. System 8000: computerized antenatal FHR analysis. J Perinat Med. 1991;19(1-2):47–51. doi:10.1515/jpme.1991.19.1-2.47.
- Gyllencreutz E, Lu K, Lindecrantz K, et al. Validation of a computerized algorithm to quantify fetal heart rate deceleration area. Acta Obstet Gynecol Scand. 2018;97(9):1137–1147. doi:10.1111/aogs.13370.
- Lu K, Holzmann M, Abtahi F, et al. Fetal heart rate short term variation during labor in relation to scalp blood lactate concentration. Acta Obstet Gynecol Scand. 2018;97(10):1274–1280. doi:10.1111/aogs.13390.
- Marsál K, Persson PH, Larsen T, et al. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843–848. doi:10.1111/j.1651-2227.1996.tb14164.x.
- Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217(2):167–175. doi:10.1016/j.ajog.2017.04.016.
- Knaven O, Ganzevoort W, de Boer M, et al. Fetal heart rate variation after corticosteroids for fetal maturation. Eur J Obstet Gynecol Reprod Biol. 2017;216:38–45. doi:10.1016/j.ejogrb.2017.06.042.
- McGoldrick E, Stewart F, Parker R, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12(12):CD004454.
- Fairchild KD, O’Shea TM. Heart rate characteristics: physiomarkers for detection of late-onset neonatal sepsis. Clin Perinatol. 2010;37(3):581–598. doi:10.1016/j.clp.2010.06.002.
- Fairchild KD, Srinivasan V, Moorman JR, et al. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R330–9. doi:10.1152/ajpregu.00487.2010.
- Lorthe E, Ancel P-Y, Torchin H, et al. Impact of latency duration on the prognosis of preterm infants after preterm premature rupture of membranes at 24 to 32 weeks’ gestation: a national population-Based cohort study. J Pediatr. 2017;182:47–52 e2. doi:10.1016/j.jpeds.2016.11.074.
- van der Ham DP, van der Heyden JL, Opmeer BC, et al. Management of late-preterm premature rupture of membranes: the PPROMEXIL-2 trial. Am J Obstet Gynecol. 2012;207(4):276 e1-10–276.10. doi:10.1016/j.ajog.2012.07.024.
- McGovern M, Giannoni E, Kuester H, et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatr Res. 2020;88(1):14–26. doi:10.1038/s41390-020-0785-x.
- Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780. doi:10.1016/S0140-6736(17)31002-4.
- Buhimschi CS, Abdel-Razeq S, Cackovic M, et al. Fetal heart rate monitoring patterns in women with amniotic fluid proteomic profiles indicative of inflammation. Am J Perinatol. 2008;25(6):359–372. doi:10.1055/s-2008-1078761.
- Salafia CM, Ghidini A, Sherer DM, et al. Abnormalities of the fetal heart rate in preterm deliveries are associated with acute intra-amniotic infection. J Soc Gynecol Investig. 1998;5(4):188–191. doi:10.1016/s1071-5576(98)00010-0.
- Day D, Ugol JH, French JI, et al. Fetal monitoring in perinatal sepsis. Am J Perinatol. 1992;9(1):28–33. doi:10.1055/s-2007-994665.
- Brown R, Patrick J. The nonstress test: how long is enough? Am J Obstet Gynecol. 1981;141(6):646–651. doi:10.1016/s0002-9378(15)33305-6.
- Nijhuis JG, Prechtl HF, Martin CB, Jr., et al. Are there behavioural states in the human fetus? Early Hum Dev. 1982;6(2):177–195. doi:10.1016/0378-3782(82)90106-2.