ABSTRACT
Introduction
Antimicrobial resistance (AMR) is a major threat to global health requiring continuous development of new antimicrobial agents. Antimicrobial research and development (R&D) should be promoted in the pharmaceutical industry and academia to ensure sustainable patient access to new treatment options and reduce the global AMR burden.
Areas covered
This review describes the historical challenges in novel antimicrobial drug development in Japan, current national efforts to promote the development, and proposals to effectively manage future AMR pandemics. Literature searches were performed in the PubMed database (from inception to January 2022).
Expert opinion
R&D activities in the antimicrobial space in Japan have been insufficient due to multiple factors, including unfavorable cost-profit balance and differences in regulatory requirements between Japan and Western countries. However, the situation is improving with the implementation of the Japanese AMR action plan, drug R&D programs led by the Japan Agency for Medical Research and Development, and efforts of regulatory agencies in the United States, Europe, and Japan in aligning and expediting the clinical development process. Further actions during the interpandemic period will strengthen antimicrobial R&D, including international and interdisciplinary collaboration, continued funding and investment with the national government’s leadership, and fostering of new-generation academic research leaders.
PLAINLANGUAGE SUMMARY
Every year, many people suffer and die of antimicrobial-resistant infections worldwide. New treatment options are required to tackle antimicrobial-resistant infections; however, pharmaceutical companies have not been very active in developing antimicrobial agents in the last two decades. This was mainly due to the difficulty in discovering new and effective compounds and insufficient funds being spent on drug discovery. In addition, differences in drug development requirements between the United States (US), Europe, and Japan have made it difficult for Japanese pharmaceutical companies to develop antimicrobial agents that can be used in all regions in a timely manner. In the last decade, several measures have been taken to re-activate antimicrobial research and development in the pharmaceutical industry, as well as in academia, in Japan. These measures include a national action plan to combat antimicrobial-resistant infections and research support programs led by the Japan Agency for Medical Research and Development. Regulatory authorities in the US, Europe, and Japan have initiated efforts to expedite the development of drugs to treat infections. Moreover, pathways for accelerated regulatory review have been established to reduce the time taken for new drugs to be approved, and this has already been applied to several new anti-infective drugs. To combat the coronavirus disease 2019 (COVID-19) pandemic, the development of novel vaccines and antiviral drugs has been accelerated with unprecedented speed. Additional actions, such as international research collaboration programs and investment in new antimicrobial development, may help promote antimicrobial research and development activities in Japan.
1. Introduction
Antimicrobial resistance (AMR) is a major global health concern that frequently results in prolonged hospital stays [Citation1], higher medical costs [Citation1], and increased mortality rates [Citation2]. The Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) report revealed that in 2019, 4.95 and 1.27 million deaths were associated with and attributable to AMR, respectively [Citation3]. AMR affects both developing and developed nations; however, its burden is disproportionally higher in low- and middle- income countries than in high-income countries. The mortality rate associated with AMR is reportedly 114.8 per 100,000 persons in western sub-Saharan Africa versus 28.0 per 100,000 persons in Australasia [Citation3]. The high-income Asia-Pacific region, including Japan, is ranked ninth among 21 global regions in terms of the number of deaths associated with bacterial AMR (70.7 per 100,000 persons) [Citation3]. According to an estimate made in 2014, if there is no change in current policies, globally, 10 million deaths per year will be attributed to AMR in 2050 [Citation4]. Moreover, the mortality rate attributable to AMR will become disproportionally high in developing countries in Africa and Asia [Citation4]. The rising levels of AMR and lack of effective antimicrobials to treat infections hinder the achievement of many Sustainable Development Goals set for global health improvement, poverty reduction, food security, and economic growth [Citation5].
The collective nature of AMR, i.e. multiple infectious diseases caused by different bacteria or other pathogens, requires varied, renewable, and innovative antimicrobial treatment options; in other words, continuous development of novel treatment options for different types of infections with sufficient response to new and increasing resistance levels is essential [Citation6]. Despite the high global burden of AMR, many pharmaceutical companies have withdrawn from developing new antimicrobial agents for multiple reasons, including scientific and regulatory challenges and unsatisfactory commercial returns [Citation7]. Currently, most discovery and early-phase development activities for antimicrobial agents are conducted by small- and medium-sized enterprises. The research and development (R&D) cost is lower than that incurred by large pharmaceutical companies [Citation8]. However, continuous development of effective antimicrobials requires multifaceted approaches involving cutting-edge science and technology, such as antimicrobial drug discovery platforms and rational drug design to select promising lead compounds [Citation9]. Therefore, to build a sustainable pipeline of antimicrobial agents, the reentry of large pharmaceutical companies into this space is essential because of their advantageous access to expertise across various fields and experience in fundamental exploratory research [Citation10].
This review article aims to summarize the hurdles in the discovery and development of novel antimicrobial agents and regulatory challenges that have led to the withdrawal of pharmaceutical companies from antimicrobial agent development in Japan. We also discuss current efforts to promote the development of novel antimicrobial agents and proposals to overcome the scientific, regulatory, and financial barriers in Japan.
2. Literature search strategy
The information in this article was derived from a literature search performed in the PubMed database (inception to January 2022). Two initial search strings were generated to identify the discussion points essential to the current work: (i) ‘antimicrobial resistance’ AND Japan and (ii) ‘antimicrobial resistance’ AND (‘drug development’ OR ‘drug discovery’ OR ‘antimicrobial development’ OR ‘antimicrobial discovery’ OR ‘discovery and development’ OR ‘antimicrobial R&D’). Original articles, systematic and narrative reviews, mini reviews, and short communications were eligible for further evaluation. Non-English and non-Japanese articles, case reports, editorials, and commentaries were excluded. Additional findings to facilitate the readers’ understanding of the current work were derived from published papers and data shared by key organizations, such as the World Health Organization (WHO), National Institute of Infectious Diseases (NIID), and International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Information related to drug development in Japan was cited from the websites of the Japan Agency for Medical Research and Development (AMED), Pharmaceuticals and Medical Devices Agency (PMDA), and Japan Pharmaceutical Manufacturers Association (JPMA).
3. Commercial and demographic challenges in the development of novel antimicrobial agents
Globally, the development of antimicrobial agents has remained stagnant over the past two decades, with very few novel agents reaching the market. Large pharmaceutical companies have withdrawn from antimicrobial drug development for several reasons, including scientific, regulatory, and commercial challenges [Citation7]. Some reasons for withdrawal are applicable to all high-income regions, whereas some are attributable to the Japan-specific antimicrobial discovery and development landscape. Consequently, there has been a steady decrease in the rate of novel antimicrobial agent approvals. The number of novel antimicrobial agents approved by the United States (US) Food and Drug Administration (FDA) declined by more than 90%, from 16 during 1983–1987 to two during 2008–2012 [Citation11]. A scientific challenge leading to the decline of novel antimicrobial agent approvals is the rapid emergence of drug-resistant bacteria, which makes drugs with novel modes of action less effective. Overall, it does not take more than 10 years for a bacterium to become potentially drug resistant after the discovery of an antimicrobial agent and its introduction into clinical use [Citation11]. According to research conducted in 14 high-income countries, patient access to novel antimicrobial agents was limited in some countries, including Japan. Japan was ranked 13th among the 14 high-income countries evaluated in terms of the number of newly launched antimicrobial agents during 2010–2019; moreover, new antimicrobial agents were launched last in Japan, with a median of more than a 1400-day delay from the world’s first launch date [Citation12]. The limited patient access to novel antimicrobial agents could be attributed to the pharmaceutical companies’ decisions to postpone or discontinue the commercialization of antimicrobial agents because of the low expected profitability [Citation12].
Multiple factors have resulted in the low expected profitability of antimicrobial agents, including short treatment durations of antimicrobial agents, prolonged time to profitability, and a negative predicted net present value (i.e. developmental costs exceeding projected earnings) [Citation13]. According to financial analyses of new drugs launched in 2014–2016, the expected financial profitability for antibiotics was considerably lower than that of drugs for chronic disease areas, such as oncology, respiratory diseases, and dermatology [Citation14].
Moreover, in the traditional funding model, pharmaceutical companies have gained profits from new antimicrobial drugs through patent protection. This model has resulted in high prices of newly developed agents and limited access to novel treatment options in low- and middle-income countries where the burden of AMR is disproportionally high and significant [Citation15].
3.1. Scientific and discovery challenges
The probability of success for new drug discovery is generally low [Citation13]; this is especially true for antimicrobial agents because microorganisms quickly develop drug resistance and make the new antimicrobials irresponsive to infections [Citation11]. Historically, the discovery of novel antimicrobials was mainly driven by natural product screening and chemical modification of existing compounds [Citation13]. R&D-based pharmaceutical companies, however, tended toward rediscovering antimicrobial compounds as compared to uncovering new compounds, possibly because research for agents with a novel mode of action has a high attrition rate [Citation7]. Unfortunately, the traditional discovery models have been insufficient to deliver novel agents against antimicrobial-resistant bacteria in a timely manner [Citation7]. Newly introduced strategies, such as a genomics-derived target-based approach [Citation16], have been unable to introduce new agents to the market; the reasons for this failure include a lack of correlation between in vitro activity of compounds observed in target-based screening platforms and that in living bacterial cells [Citation13].
3.2. Differences in the national initiatives for promotion of antimicrobial agent research and development in the United States, Europe, and Japan
National initiatives to re-activate antimicrobial agent development differed between the US, Europe, and Japan. In the US, to facilitate the discovery and development of novel antimicrobial agents, the Generating Antibiotic Incentives Now (GAIN) Act was established in 2011 and came into effect in 2012 [Citation17], which contributed to the approval of 20 novel agents between 2010 and 2019 [Citation18]. Under the GAIN Act, industries that sponsor the development of antimicrobial drugs for serious or life-threatening infections can petition the FDA for a Qualified Infectious Disease Product designation and receive a fast track and priority review status, as well as an additional 5 years of exclusivity for the approved agent [Citation13]. In Europe, the European Union (EU) and European Federation of Pharmaceutical Industries and Associations (EFPIA) launched the Innovative Medicines Initiative (IMI) in 2008 as a public-private partnership, which has invested considerable resources in the development of novel antimicrobial strategies [Citation19]. Moreover, the Global Antibiotic Research and Development Partnership (GARDP) was established in 2016 as a joint public-private partnership to accelerate the development of novel antibiotics for drug-resistant bacterial infections [Citation20]. In contrast, in Japan, R&D activities in the antimicrobial space have remained low, and there is a significant delay in novel agent approvals. Only six of the 20 antimicrobial agents approved in the US between 2010 and 2019 were approved in Japan, and four of them (fidaxomicin, bedaquiline, tedizolid phosphate, and tazobactam plus ceftolozane) were approved more than 3 years after their approval in the US [Citation18]. Additionally, in Japan, there has previously been little political momentum and no legislation or law issued to promote R&D.
3.3. Differences in clinical and regulatory requirements among the United States, Europe, and Japan
The US FDA, European Medicines Agency (EMA), and Japan PMDA regulatory requirements for antimicrobial agent development are different, which poses additional challenges to align the development of novel agents globally. The US FDA [Citation21], EMA [Citation22], and Japanese Ministry of Health, Labour and Welfare (MHLW)/PMDA [Citation23–25] have issued guidance or guideline documents for the clinical development of antimicrobial agents (). The US FDA guidance for industry [Citation21] and EMA guidelines (addendum) [Citation22] were drafted in 2013 and 2012 and finalized in 2017 and 2014, respectively, to provide recommendations for the development of novel treatment options for serious bacterial infections, including antimicrobial-resistant infections. In contrast, the Japanese guidelines discuss the general principles for non-clinical and clinical evaluations of antimicrobial agents but do not provide specific guidance on agents for serious bacterial infections or antimicrobial-resistant infections [Citation23–25]. In other words, no clinical development guidelines focused on AMR have been established in Japan.
Table 1. Regulatory guidance and guidelines for clinical development of antimicrobial agents.
These comparisons highlight a delay in Japan in the establishment of regulatory guidelines to combat AMR. Actually, there also had been some efforts to improve the regulatory environment in Japan. As part of the regulatory science promotion work of the Japan PMDA, the ‘Report on clinical evaluation of antimicrobial agents for AMR’ was published in 2019 as the summary of a study conducted by the Subcommittee on AMR of the Science Board [Citation26]. In addition, face-to-face, tripartite meetings attended by experts from the EMA, US FDA, and Japan PMDA have been held since 2016 to promote antimicrobial agent development. The most recent meeting held in 2019 reported that ‘significant progress was made in our discussions on recommendations for clinical development of new anti-infective products’ [Citation27]. However, these results have not been applied to the formulation of guidelines in Japan; therefore, these discussions cannot be used for efficient clinical development planning based on the most advanced science.
In addition, several factors made it difficult for the Japanese pharmaceutical industry to realize a globally harmonized development of novel antimicrobial agents. For example, differences in acceptable efficacy endpoints in anti-infective agent clinical trials between regulatory authorities [Citation28] required additional efforts by the pharmaceutical companies in preparing well-designed clinical development plans and clinical data packages that addressed the expectations of each regulatory authority. Moreover, the Japanese regulatory authority performed reevaluations of the contents of approved marketed antimicrobial prescription drugs and standardized the wordings of indications in 2004, which introduced differences in the wordings of indications between Japan and overseas; for example, the ‘bacteremia’ indication in Japan was changed to ‘sepsis (for injection)’ [Citation29].
The ICH efficacy guidelines E5 (Ethnic Factors in the Acceptability of Foreign Clinical Data) and E17 (General Principles for Planning and Design of Multi-Regional Clinical Trials) have evolved over the past two decades in terms of the importance of ethnic and regional factors in multiregional drug development [Citation30]. In Japan, the MHLW/PMDA issued several guidelines on the participation of the pharmaceutical industry in multiregional clinical trials [Citation31–33]. Participation in multiregional clinical trials enabled Japanese pharmaceutical companies to facilitate early development of new drugs and shorten the ‘drug lag’ (i.e. the time lag between the drug approval in the US or Europe and that in Japan). However, local drug development strategies are still preferred by the regulatory agency and Japanese medical experts and are employed in Japan; consequently, there has been an average delay of more than 3 years in the regulatory approval of new drugs compared with multiregional drug development strategies [Citation34].
4. Current national efforts to promote the development of novel antimicrobial agents in Japan
To address the challenges in antimicrobial drug development, the Japanese government and regulatory authorities have implemented initiatives to promote R&D activities, ranging from the establishment of a national action plan for AMR to expedited regulatory pathways for early approval of novel agents ().
Table 2. National and regulatory actions in Japan to promote clinical development of novel anti-infective agents.
4.1. National strategy and action plan for infectious diseases crisis management: the Japanese AMR National Action Plan
The Japanese AMR National Action Plan for 2016–2020 was established in April 2016 [Citation35,Citation36]. This action plan consists of the five strategic goals outlined in the WHO Global Action Plan on AMR: (i) improve public awareness and understanding and promote education and training of professionals; (ii) continuously monitor AMR and the use of antimicrobials and appropriately understand the signs of change and spread of AMR; (iii) prevent the spread of antimicrobial-resistant organisms by implementing appropriate infection prevention and control measures; (iv) promote appropriate use of antimicrobials in the fields of healthcare, livestock production, and aquaculture; and (v) promote research on AMR and foster R&D of the prevention, diagnosis, and treatment modalities of antimicrobial-resistant infections. Moreover, an additional objective – (vi) enhance global multidisciplinary countermeasures against AMR – is set as a Japan-specific goal [Citation35–37]. In terms of R&D of novel antimicrobial agents, key actions listed in the AMR action plan include the promotion of research activities; promotion of cooperation among the pharmaceutical industry, academia, and government; creation of incentive systems; and formulation of internationally harmonized guidelines for clinical evaluation and trials of antimicrobial agents [Citation36]. The initial 5-year action plan was completed in 2020; however, the new Japanese action plan for the next 5 years is yet to be established, owing to the novel coronavirus disease 2019 (COVID-19) pandemic [Citation37].
4.2. Drug discovery and research programs led by a national agency
The AMED was established in 2015 as a national agency dedicated to promoting integrated medicinal R&D from basic research to clinical research [Citation38]. As part of AMED’s initiatives, the Japanese Initiative for Progress of Research on Infectious Disease for global Epidemic (J-PRIDE) was established in 2017 [Citation39] as an open research program on infectious diseases. This program has contributed to infectious disease research through financial support [Citation40–42]. The Cyclic Innovation for Clinical Empowerment (CiCLE) is an AMED research program that aims to accelerate the practical application of pharmaceutical products through medical R&D [Citation43]. It has provided financial support to several medicinal product research projects in Japan [Citation44–46]. The AMED has also established the Research Program on Emerging and Re-emerging Infectious Diseases, which aims to provide support to promote R&D activities for domestic and global infectious diseases [Citation47]. Multiple research projects are taking place under the Research Program on Emerging and reemerging Infectious Diseases [Citation47]. The examples in fiscal year 2020 include projects focusing on highly pathogenic avian H7N9 influenza, antimicrobial-resistant Neisseria gonorrhoeae infection, and tick-borne bacterial diseases. Other initiatives led by the AMED include R&D support for COVID-19–related projects [Citation48] and participation in the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) [Citation49].
4.3. Initiatives for globally aligned and streamlined clinical development
In a joint initiative between the US FDA, EMA, and Japan PMDA, it was agreed that a streamlined and innovative approach to generate clinical evidence is required for developing antimicrobial agents that can address unmet medical needs related to AMR. The examples of such approaches include a well-conducted single trial with the support of pharmacokinetic/pharmacodynamic analyses and use of data from small trials [Citation28]. Moreover, to facilitate the clinical development of antimicrobial agents for AMR in Japan, a report was submitted by the AMR Subcommittee of the PMDA Science Board to the regulatory authority in October 2019 [Citation26]. This report provided recommendations on several key scientific strategies, including the facilitation of global clinical trials and establishment of clinical trial networks ().
Table 3. Key scientific proposals from the PMDA Science Board AMR subcommittee to facilitate clinical development of novel antimicrobial agents.
4.4. Expedited regulatory pathways for early approval of novel agents
The Sakigake (Japanese word meaning ‘pioneer’ or ‘frontrunner’) Designation System was established in Japan in 2015 to facilitate the development and early patient access to innovative drugs that are intended to address unmet medical needs [Citation50,Citation51]. The system is similar to the Breakthrough Therapy Designation in the US and Priority Medicines in the EU; it offers several advantages to granted pharmaceutical products, such as prioritized regulatory consultation, rapid review, premium drug pricing, and an extended data protection period [Citation50]. In addition, the Conditional Early Approval System was initiated in 2017 to enable the early delivery of effective therapeutic options with conditional approvals and specific post-marketing regulatory requirements [Citation51]. The Sakigake Designation System and Conditional Early Approval System were legislated in December 2019 as part of the revised Japanese PMDA [Citation52]. The approval of baloxavir marboxil for the treatment of influenza A or B virus infection is a good example of the application of the Sakigake Designation System in the infectious disease field [Citation51]. In addition, for the approval of COVID-19 treatment drugs, including remdesivir [Citation53], the Special Approval for Emergency System was applied based on Article 14–3 of the revised Japanese PMDA [Citation52]. However, the Special Approval for Emergency System was established primarily as emergency measures to cope with the COVID-19 pandemic. In view of the significant global burden of AMR, additional, long-term regulatory policies for the development of anti-infective drugs, including antimicrobials, should be formulated in Japan.
One of the issues highlighted during the COVID-19 pandemic in Japan was the time required to commercialize new vaccines and therapeutics. Indeed, the approval of the Pfizer and Moderna COVID-19 vaccines in Japan was approximately 2 and 5 months later than that in the US or Europe, respectively. Owing to the existing regulatory approval system, even in the circumstances of Special Approval for Emergency System, additional domestic clinical trials to confirm efficacy in the Japanese population living in Japan were mandated for COVID-19 vaccines, which caused a delay in their regulatory approval. In November 2021, a proposal was put forth to amend the law to establish a new system that allows the emergency approval of vaccines and therapeutics in Japan [Citation54,Citation55]. This new emergency approval system allows early commercialization of drugs and medical devices necessary to protect the lives and health of the public in emergency situations, such as the spread of infectious diseases or bioterrorism. Emergency approval can be applied for when the efficacy of a drug or vaccine can be estimated from available clinical study data and safety can be confirmed at the same level as that of ordinary regulatory pharmaceutical approval [Citation54,Citation55].
However, robust scientific evaluation of clinical trial results and evidence-based decisions for regulatory approval were not skipped or eliminated in these expedited drug approval efforts. The importance of well-designed clinical trials and rigorous scientific evaluation of the efficacy of anti-infective agents prior to approval can be discussed using a case study of the viral RNA polymerase inhibitor, favipiravir [Citation56]. Despite high expectations from the mass media and politicians in Japan, favipiravir is yet to be approved for COVID-19 treatment. Although discussions with the regulatory authority had been held before the initiation of the pivotal phase 3 trial, regulatory review has revealed flaws in the study design and assessments; this trial was designed as a single-blinded study and employed soft endpoints, including symptom relief evaluated in an arbitrary assessment schedule. The regulatory agency has concluded that these flows in the study design could have resulted in a significant bias in the efficacy results between the favipiravir and placebo groups [Citation56].
5. Lessons learned from the coronavirus disease 2019 pandemic
Several original research and review articles have discussed the lessons learned from the COVID-19 pandemic in Japan from a public health point of view [Citation57–60]. In this section, we focus on the lessons learned from the pandemic regarding the R&D of novel antimicrobial agents in Japan.
Concerns about the spread of AMR associated with the global COVID-19 pandemic include the high number of secondary multidrug-resistant bacterial infections and an increase in the use of antimicrobial treatments [Citation61]. In addition, one of the most crucial lessons learned from this global pandemic is the importance of establishing a sustainable environment for developing novel agents during the interpandemic period. The following three factors are considered key to achieving this aim: (i) international collaboration and funding, (ii) continued investment in novel antimicrobial drug development, and (iii) establishment of an independent pandemic command center and risk management plan for infectious disease pandemics ().
Table 4. What do we need to establish during the interpandemic period for the development of novel agents against antimicrobial-resistant bacteria?
5.1. International collaboration and funding
The first sincere attempt against COVID-19 was to conduct a huge number of clinical trials. However, the traditional phase 3 study design required independent patient enrollment in each study to test a single hypothesis. This traditional strategy led to sharing of the limited number of patients among multiple clinical trials and a delay in patient recruitment. Throughout the COVID-19 pandemic, significant progress has been made in expanding research networks and leveraging key innovations in the clinical trial design. Adaptive platform trials, for example, allow researchers to validate multiple interventions for a single disease simultaneously by comparing them with a single common control group [Citation62,Citation63]. Adaptive platform trials also allow modifications and adjustments as the trial progresses by adding or removing treatments and updating the trial design [Citation62,Citation63]. Facilitation of clinical trials through international networks and collaboration and an adaptive platform design is achieved for COVID-19 in the RECOVERY (NCT04381936) [Citation64], WHO SOLIDARITY (ISRCTN83971151; NCT04315948) [Citation65], the Adaptive COVID-19 Treatment Trial (NCT04280705) [Citation66,Citation67], and the Accelerating COVID-19 Therapeutic Interventions and Vaccines initiative announced in April 2020 [Citation68].
A similar approach of an adaptive clinical platform design can be applied to antimicrobial agents. The Randomized, Embedded, Multi-factorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP; NCT02735707) was established to evaluate treatment options for community-acquired pneumonia [Citation69] and contributed to the conduct of a clinical trial of hydrocortisone for patients with severe COVID-19 [Citation70]. Japan has joined the REMAP-CAP community with endorsements from the Japanese academic societies. In addition to the efforts by academic researchers to maximize the value of evidence generated via this international platform, collaboration with the government, regulatory bodies, pharmaceutical industry, and policymakers in Japan is expected [Citation71]. Using adaptive platforms, researchers worldwide could pool their knowledge and resources to add and remove study arms based on tentatively decoded information, real-world data, and insights from treatment settings. Japan’s role in drug development and conducting multinational clinical trials, especially in the Asian region, is significant. In addition, a scientific platform for collaboration among pharmaceutical industry, academia, and governmental bodies, originating in Japan, would be extremely beneficial.
Acceleration of clinical trials conducted during the pandemic is critical in enabling the early delivery of novel and effective antimicrobial treatment to patients in need. However, owing to multiple factors, including lack of clinical trial experience, Japanese infectious disease specialists have encountered difficulties in conducting clinical trials for the timely development of COVID-19 drugs. Japanese medical institutions designated for infectious diseases played a key role in enrolling patients at the early stage of the pandemic; nevertheless, the difficulties in conducting COVID-19 clinical trials revealed that the research function of these institutions was not strong enough. The research function of such designated medical institutions should be enhanced in Japan, and collaboration between academic institutions and the pharmaceutical industry is warranted to achieve this aim. In addition, academic research organizations (AROs) have been established at Japanese universities for interdisciplinary research collaboration and knowledge sharing [Citation72]. The AROs and the ARO Council, which was founded in 2013 with the aim of global data sharing [Citation73], should strengthen their functions and expertise and act as a key contributor for conducting antimicrobial clinical trials.
Funding from international platforms is also crucial for the development of novel antimicrobial agents. The AMR Action Fund was established by multinational pharmaceutical companies in collaboration with the WHO, European Investment Bank, and Wellcome Trust. The fund invested $1 billion in small antibiotic development companies and aims to provide expertise and technical support for the development of novel agents [Citation74,Citation75].
5.2. Continued investment in novel antimicrobial drug development
During the COVID-19 outbreak, several national governments offered financial support to the pharmaceutical industry and academic research teams outside of Japan, which contributed to the accelerated development of novel vaccines [Citation76]. This illustrates the need for investment in antimicrobial research with governmental leadership to facilitate novel antimicrobial drug development during the interpandemic period.
Investment in antimicrobial agents can be incentivized through push strategies, pull strategies, or a combination of the two (hybrid strategies) [Citation10,Citation77] (). Outside Japan, the current push incentive strategies for AMR include the New Drugs for Bad Bugs (ND4BB) led by the IMI [Citation78], Combating Antibiotic-Resistant Bacteria (CARB-X) led by Boston University [Citation79], and Biomedical Advanced Research and Development Authority (BARDA) supported by the US Department of Health and Human Services [Citation80]. The US GAIN Act [Citation17] is an example of a pull strategy applied at the national government level, which includes prolonged exclusivity rights. An example of the hybrid incentive strategy is the Options Market for Antibiotics Model, in which nongovernmental organizations or government agencies purchase options for antibiotics in development [Citation81]. Additional pull-type incentive strategies for antimicrobial development promotion are actively pursued outside of Japan. Such initiatives include the Developing an Innovative Strategy for Antimicrobial Resistant Microorganisms (DISARM) Act and Pioneering Antimicrobial Subscriptions To End Up surging Resistance (PASTEUR) Act in the US. In the United Kingdom, a subscription-based payment model is employed by the National Institute for Health and Care Excellence [Citation82]. In December 2021, the G7 member countries jointly issued a statement on actions to support antibiotic development [Citation83]. In the joint statement, all the member countries committed to expedite the implementation of their AMR action plans and strategies, address market failure, and formulate the right economic environment, including strengthened AMR antibiotic R&D and introducing new drugs to market [Citation83].
Table 5. Push, pull, and hybrid incentive strategies for antimicrobial drug research and development.
In Japan, the JPMA has submitted several recommendations regarding AMR to the national government and regulatory authorities, including the introduction of incentive programs to facilitate R&D of novel antimicrobial agents [Citation84]. Similarly, establishment of incentive models was proposed by the AMR Alliance Japan, a multistakeholder organization advocating for strengthened AMR policies [Citation85]. However, these recommendations are limited to post-marketing, pull-type incentives, and there have been no proposals or discussions in Japan regarding the introduction of push-type incentives to support antimicrobial clinical development. This situation does not mean that push-type incentives are sufficient. Neither pull-type nor push-type incentives are sufficient in Japan. However, since a lack of pull-type incentives is the most critical reason why pharmaceutical industries do not develop products for AMR, the past discussions were focused on pull-type incentives. This topic warrants further discussion among key stakeholders in Japan.
5.3. Establishment of a pandemic command center for infectious disease control and risk management plan for infectious disease pandemics
During the COVID-19 pandemic in Japan, key infection control actions were decided by the Japanese government in consultation with its COVID-19 measures advisory board. Unlike the US Centers for Disease Control and Prevention (CDC), the NIID in Japan is not authorized to perform nationwide infection control or develop national guidelines for managing infectious disease pandemics [Citation86]. The NIID oversees infectious disease surveillance and collection, analysis, and dissemination of the surveillance data [Citation86]. Therefore, an independent entity that can play key roles in the nationwide infection control and establish a risk management plan is warranted in Japan. Moreover, the initiation of national risk management plans to promote new antimicrobial development in Japan during the interpandemic period is eagerly anticipated, similar to the long-term, continuous national strategy for developing vaccines, which was launched in June 2021 [Citation87].
The Japanese government has initiated discussions regarding medical countermeasures (MCMs) to strengthen infectious disease crisis management. The MCMs are intended to prepare for future outbreaks of AMR, known infectious diseases, and new emerging infectious diseases. The MHLW of Japan is currently conducting discussions on how to ensure the availability of effective drugs and therapies in the event of an infectious disease crisis, as well as the designation of ‘priority infectious diseases’ for which these drugs should be made available early [Citation88]. These MCMs will help to save lives, control epidemics, and maintain social activities and aid in defining and promoting crisis measures against infectious diseases in advance [Citation88].
5.4. Preparations during the interpandemic period to manage future AMR pandemics
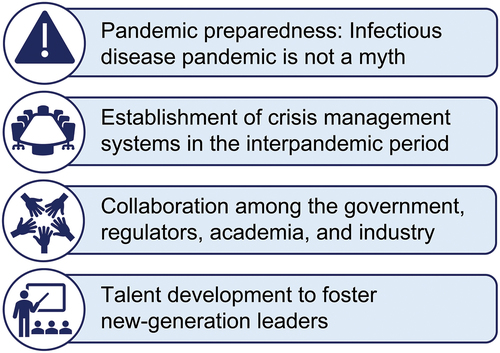
The lessons learned from the global COVID-19 pandemic can be extrapolated to effectively manage future AMR pandemics; essential points to consider are depicted in . First, pandemic preparedness [Citation89] is crucial as the emergence of an infectious disease pandemic in the absence of effective treatment options is a serious threat that could reoccur in the near future. Second, the establishment of crisis management systems is critical for timely patient access to novel and effective antimicrobial treatment options during the pandemic. Such crisis management systems may include, but are not limited to, national strategies and risk management plans, streamlined regulatory pathways, networks for conducting clinical trials, and continuous investment in R&D activities. Third, interdisciplinary collaboration among the government, regulatory bodies, academia, and pharmaceutical industry is crucial for rapid drug development. Lastly, from the academia perspective, talent development will facilitate the rise of new-generation basic research and clinical research leaders capable of dealing with future infectious disease pandemics.
6. Conclusion
Withdrawal of the large pharmaceutical companies from antimicrobial agent development due to scientific, regulatory, and financial challenges has resulted in a significant decline in the number of novel antimicrobial agents. However, AMR pandemics are palpable threats, and facilitation of novel antimicrobial agent R&D is warranted. Owing to the recent joint efforts by the pharmaceutical industry, academia, regulatory bodies, and government, solutions to replenish our arsenal of antimicrobial agents appear to be promising. We hope that this review will help infectious disease specialists to better understand the challenges and current initiatives in antimicrobial drug development and to join the effort in the development of these life-saving drugs.
7. Expert opinion
The GBD assessments of the AMR burden [Citation3] emphasize the need for robust and sustainable access to antimicrobial agents on a global scale. Nevertheless, owing to multiple factors discussed in this article, R&D activities for novel antimicrobial agents have long remained stagnant in Japan. Moreover, relevant social science research has been low [Citation90]. However, the antimicrobial R&D landscape has improved over the last decade, and we anticipate the revival of antimicrobial research activities in the Japanese pharmaceutical industry and academia. This is expected to substantially increase the number of novel antimicrobial agent approvals in Japan, and eventually, reduce the global AMR burden.
One of the reasons for the withdrawal of large pharmaceutical companies from new antimicrobial development is the unfavorable cost-profit balance. To overcome the financial challenges faced by the industry, key stakeholders in Japan have made several recommendations, including the introduction of incentive programs [Citation84,Citation85]. However, to revolutionize this situation, involvement of various stakeholders outside of the pharmaceutical industry, such as the government, regulatory bodies, and healthcare providers – especially, proactive actions by the national government – would be required. From the regulatory perspective, international harmonization of drug development processes and regulatory requirements has long been discussed between the pharmaceutical industry and regulatory agencies of various countries. These discussions have been particularly active in the development of anti-infective therapies, including antimicrobial agents [Citation28]. Global harmonization of regulatory requirements for antimicrobial agents will help the industry to develop novel and effective therapeutic options for AMR in a timely manner and eliminate the ‘drug lags’ [Citation34]. In addition, Japan’s leading role in establishing clinical trial networks in Asia and conducting multinational clinical trials for antimicrobial agents is expected.
Countermeasures to reduce the burden of AMR include both the development of novel antimicrobial agents and the appropriate use of antimicrobial agents (antimicrobial stewardship), which is a part of the Japanese AMR action plan [Citation36]. Recent research findings from Japan suggest that antimicrobial stewardship programs have a positive effect across various clinical settings [Citation91–94]. Further promotion of antimicrobial stewardship and long-term assessment of its impact on Japanese daily clinical practice are needed. As part of global efforts to reduce AMR burden, research that aims to improve patient access to antimicrobial agents in low- and middle-income countries is urgently needed [Citation95]. Moreover, measures should be taken to promote tailored approaches to deliver the most effective antimicrobial agents to each global region [Citation3].
In the next 5 years, the pharmaceutical industry is still expected to be the key contributor to the development of novel antimicrobial agents. In addition, academic infectious disease researchers are also expected to participate in antimicrobial R&D activities more proactively than in the past through multifaceted approaches. Clinical trial conduct, advisory roles in pharmaceutical companies, and patient-enlightenment activities can be the key areas in which academic researcher participation is eagerly anticipated. During the interpandemic period, joint effort among specialists and other key stakeholders will facilitate knowledge sharing and development of new-generation infectious disease academic research leaders. Interdisciplinary and international research collaborations will be strengthened through domestic and multinational scientific platforms. Moreover, critical assessment of the outcomes of the Japanese AMR action plan for 2016–2020 [Citation37] will help define new goals and targets for the next 5 years.
Article highlights
The high global burden of antimicrobial resistance (AMR) underscores the need for reviving antimicrobial research and development (R&D) activities in the pharmaceutical industry and academia to ensure robust patient access to new treatment options.
Although the Japanese pharmaceutical industry has faced difficulties in developing novel antimicrobial agents in a timely and globally harmonized manner, the situation has improved in the last decade owing to differences in the antimicrobial agent R&D landscapes and regulatory requirements across countries or regions.
In Japan, national efforts to promote antimicrobial R&D have been initiated, such as the AMR National Action Plan and research support programs hosted by the Japan Agency for Medical Research and Development.
Robust funding and investment in antimicrobial research through international platforms and the national government’s leadership can facilitate the development of novel antimicrobial agents.
Lessons learned from the unprecedented/accelerated vaccine and antiviral drug development for coronavirus disease 2019 are applicable to novel AMR drug R&D activities among pharmaceutical companies, government, and academia.
To effectively manage future AMR pandemics, pandemic preparedness, establishment of crisis management systems, and interdisciplinary collaboration among the government, regulators, academia, and pharmaceutical industry are essential.
Talent development to foster new-generation academic research leaders during the interpandemic period is eagerly anticipated.
Declaration of interest
T Ohashi is a full-time employee of Pfizer Japan Inc. M Nagashima and N Kawai are full-time employees of Pfizer R&D Japan. T Ohashi and M Nagashima hold stocks and stock options from Pfizer Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Medical writing and editorial assistance were provided by Mami Hirano, MS, of Cactus Life Sciences (part of Cactus Communications) and funded by Pfizer Japan Inc.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Touat M, Opatowski M, Brun-Buisson C, et al., A payer perspective of the hospital inpatient additional care costs of antimicrobial resistance in France: a matched case-control study. Appl Health Econ Health Policy. 2019;17(3):381–389.
- Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66.
- Murray CJ, Ikuta KS, Sharara F; Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655.
- Review on Antimicrobial Resistance [Internet]. Antimicrobial resistance: tackling a crisis for the health and wealth of nations; 2014 December [cited 2022 Aug 29]; [20 p.]. Available from: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
- World Health Organization. [Internet]. Antimicrobial resistance and the United Nations sustainable development cooperation framework: guidance for United Nations country teams. [cited 2022 Aug 29]; [ about 2 screens]. Available from: https://www.who.int/publications/i/item/9789240036024
- Kållberg C, Salvesen Blix H, Laxminarayan R. Challenges in antibiotic R&D calling for a global strategy considering both short- and long-term solutions. ACS Infect Dis. 2019;5(8):1265–1268.
- Jackson N, Czaplewski L, Piddock LJV. Discovery and development of new antibacterial drugs: learning from experience? J Antimicrob Chemother. 2018;73(6):1452–1459.
- Årdal C, Baraldi E, Theuretzbacher U, et al. Insights into early stage of antibiotic development in small- and medium-sized enterprises: a survey of targets, costs, and durations. J Pharm Policy Pract. 2018;11(1):8.
- Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892.
- Cama J, Leszczynski R, Tang PK, et al. To push or to pull? In a post-COVID world, supporting and incentivizing antimicrobial drug development must become a governmental priority. ACS Infect Dis. 2021;7(8):2029–2042.
- Duval RE, Grare M, Demoré B. Fight against antimicrobial resistance: we always need new antibacterials but for right bacteria. Molecules. 2019;24(17):3152.
- Outterson K, Orubu ESF, Rex J, et al., Patient access in fourteen high-income countries to new antibacterials approved by the US Food and Drug Administration, European Medicines Agency, Japanese Pharmaceuticals and Medical Devices Agency, or Health Canada, 2010-2020. Clin Infect Dis. 2022;74(7):1183–1190.
- Boyd NK, Teng C, Frei CR. Brief overview of approaches and challenges in new antibiotic development: a focus on drug repurposing. Front Cell Infect Microbiol. 2021;11:684515.
- Federal Ministry of Health. Breaking through the wall: a call for concerted action on antibiotics research and development; Berlin: Federal Ministry of Health; 2017. [cited 2022 Aug 29]; [84 p.]. Available from: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Gesundheit/Berichte/GUARD_Follow_Up_Report_Full_Report_final.pdf
- Littmann J, Viens AM. The ethical significance of antimicrobial resistance. Public Health Ethics. 2015;8(3):209–224.
- Payne DJ, Gwynn MN, Holmes DJ, et al. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6(1):29–40.
- Brown ED. Is the GAIN Act a turning point in new antibiotic discovery? Can J Microbiol. 2013;59(3):153–156.
- Yagisawa M. [Current status and prospective of antibacterial agents regarding countermeasure against antimicrobial resistance (AMR)]. Journal of Healthcare-associated Infection. 2019,12:55–71.Japanese.
- Kostyanev T, Bonten MJ, O’Brien S, et al. The Innovative Medicines Initiative’s New Drugs for Bad Bugs programme: European public-private partnerships for the development of new strategies to tackle antibiotic resistance. J Antimicrob Chemother. 2016;71(2):290–295.
- Balasegaram M, Piddock LJV. The Global Antibiotic Research and Development Partnership (GARDP) not-for-profit model of antibiotic development. ACS Infect Dis. 2020;6(6):1295–1298.
- US Food and Drug Administration [Internet]. Guidance document: antibacterial therapies for patients with an unmet medical need for the treatment of serious bacterial diseases; 2017 August [cited 2022 Aug 29]; [ about 2 screens]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/antibacterial-therapies-patients-unmet-medical-need-treatment-serious-bacterial-diseases
- European Medicines Agency [Internet]. European Medicines Agency. 1995-2022 Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections; 2013 October [cited 2022 Aug 29]; [20 p.]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/addendum-guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections_en.pdf
- Pharmaceuticals and Medical Devices Agency [Internet]. Guideline for clinical evaluation of antibacterial drugs (October 23, 2017, PSEHB/PED Notification No. 1023-3). Provisional Translation (as of 2020 March) [cited 2022 Aug 29]; [88 p.]. Available from: https://www.pmda.go.jp/files/000234350.pdf
- Pharmaceuticals and Medical Devices Agency [Internet]. [Reference information regarding guideline for clinical evaluation of antibacterial drugs (October 23, 2017, Administrative Notice)]. [cited 2022 Aug 29]; [5 p]. Japanese. Available from: https://www.pmda.go.jp/files/000221066.pdf
- Ministry of Health, Labour and Welfare of Japan. [Guidelines for PK/PD evaluation of antibacterial agents (December 25, 2015, ELD/PSEB Notification No. 1225-10)]. Jpn J Chemother. 2016;64(2):139–151. Japanese
- Pharmaceuticals and Medical Devices Agency [Internet]. Report on clinical evaluation of antimicrobial agents for AMR, October 4, 2019 (provisional translation). [cited 2022 Aug 29]; [23 p.]. Available from: https://www.pmda.go.jp/files/000233507.pdf
- Pharmaceuticals and Medical Devices Agency [Internet]. Tripartite meeting held between EMA, FDA and PMDA towards enhancing development for antibacterial agents. [cited 2022 Aug 29]; [ about 2 screens]. Available from: https://www.pmda.go.jp/english/int-activities/outline/0018.html
- Nambiar S, Cavaleri M, Sato J. Achieving regulatory alignment for anti-infective clinical trials. ACS Infect Dis. 2020;6(6):1308–1310.
- Pharmaceuticals and Medical Devices Agency [Internet]. [Results of re-evaluation of prescription drugs, fiscal year 2004, part 3 (September 30, 2004, PFSB Notification No. 0930002)]. [cited 2022 Aug 29]. Japanese. Available from: https://www.pmda.go.jp/files/000148071.pdf
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use [Internet]. Efficacy Guidelines. [cited 2022 Aug 29]; [ about 2 screens]. Available from: https://www.ich.org/page/efficacy-guidelines
- Pharmaceuticals and Medical Devices Agency [Internet]. Basic principles on global clinical trials (September 28, 2007, Notification No. 0928010) [cited 2022 Aug 29]; [11 p.]. Available from: https://www.pmda.go.jp/files/000157900.pdf
- Pharmaceuticals and Medical Devices Agency [Internet]. Basic principles on global clinical trials (reference cases. (Administrative Notice. 2012 Sept 5. cited 2022 Sept 5.12:]. (Administrative Notice. https://www.pmda.go.jp/files/000152969.pdf
- Pharmaceuticals and Medical Devices Agency [Internet]. Basic principles on global clinical trials (reference cases) (Administrative Notice, September 5, 2012). [cited 2022 Aug 29]; [12 p.]. Available at: https://www.pmda.go.jp/files/000152969.pdf
- Ushijima S, Matsumaru N, Tsukamoto K. Evaluation of drug lags in development initiation, new drug application and approval between Japan and the USA and the impact of local versus multi-regional clinical trials. Pharmaceut Med. 2021;35(4):253–260.
- Ohmagari N. National Action Plan on Antimicrobial Resistance (AMR) 2016-2020 and relevant activities in Japan. Glob Health Med. 2019;1(2):71–77.
- World Health Organization. [Internet]. Japan: national action plan on antimicrobial resistance (AMR) 2016-2020; 2016 April. World Health Organization; 2022. [cited 2022 Aug 29]; [ about 2 screens]. Available from: https://www.who.int/publications/m/item/japan-national-action-plan-on-antimicrobial-resistance-(amr)
- Gu Y, Fujitomo Y, Ohmagari N. Outcomes and future prospect of Japan’s National Action Plan on Antimicrobial Resistance (2016-2020). Antibiotics (Basel). 2021;10(11):1293.
- Japan Agency for Medical Research and Development [Internet]. About AMED. [cited 2022 Aug 29]; [about 2 screens]. Available from: https://www.amed.go.jp/en/aboutus/index.html
- Japan Agency for Medical Research and Development [Internet]. Japanese Initiative for Progress of Research on Infectious Disease for global Epidemic (J-PRIDE); 2019 Jul 12 [cited 2022 Aug 29]; [about 3 screens]. Available from: https://www.amed.go.jp/en/program/list/01/06/005.html
- Konno Y, Nagaoka S, Kimura I, et al. New world feline APOBEC3 potently controls inter-genus lentiviral transmission. Retrovirology. 2018;15(1):31.
- Mi-Ichi F, Yoshida H. Unique features of Entamoeba sulfur metabolism; compartmentalization, physiological roles of terminal products, evolution and pharmaceutical exploitation. Int J Mol Sci. 2019;20(19):4679.
- Watanabe S, Aiba Y, Tan XE, et al. Complete genome sequencing of three human clinical isolates of Staphylococcus caprae reveals virulence factors similar to those of S. epidermidis and S. capitis. BMC Genomics. 2018;19(1):810.
- Japan Agency for Medical Research and Development [Internet]. CiCLE: cyclic innovation for clinical empowerment; 2018. [cited 2022 Aug 29]; [ about 3 screens]. Available from: https://www.amed.go.jp/en/program/list/17/01/001.html
- Nagase H, Saitoh A. Research and development of κ opioid receptor agonists and δ opioid receptor agonists. Pharmacol Ther. 2020;205:107427.
- Shimoyama A, Di Lorenzo F, Yamaura H, et al. Lipopolysaccharide from gut-associated lymphoid-tissue-resident Alcaligenes faecalis: complete structure determination and chemical synthesis of its lipid A. Angew Chem Int Ed Engl. 2021;60(18):10023–10031.
- Yagishita S, Kato K, Takahashi M, et al. Characterization of the large-scale Japanese patient-derived xenograft (J-PDX) library. Cancer Sci. 2021;112(6):2454–2466.
- Japan Agency for Medical Research and Development [Internet]. Research Program on Emerging and Re-emerging Infectious Diseases; 2020 Oct 20 [cited 2022 Aug 29]; [ about 3 screens]. Available from: https://www.amed.go.jp/en/program/list/11/02/002.html
- Japan Agency for Medical Research and Development [Internet]. AMED support for R&D on the novel coronavirus disease (COVID-19) (summary); 2020 Nov 20 [cited 2022 Aug 29]; [ about 10 screens]. Available from: https://www.amed.go.jp/en/news/topics/covid-19.html
- Japan Agency for Medical Research and Development [Internet]. Joint Programming Initiative on Antimicrobial Resistance (JPIAMR); 2017 Apr 7 [cited 2022 Aug 29; [ about 3 screens]. Available from: https://www.amed.go.jp/en/aboutus/collaboration/jpiamr.html
- Tanaka M, Idei M, Sakaguchi H, et al. Achievements and challenges of the Sakigake designation system in Japan. Br J Clin Pharmacol. 2021;87(10):4027–4035.
- Matsushita S, Tachibana K, Kusakabe T, et al. Overview of the premarketing and postmarketing requirements for drugs granted Japanese conditional marketing approval. Clin Transl Sci. 2021;14(3):806–811.
- e-Gov Japanese law portal [Internet]. [Act on securing quality, efficacy and safety of products including pharmaceuticals and medical devices]. Act No. 145 of 1960, Amendment of Act No. 63 of 2019. [cited 2022 Aug 29]; [about 120 screens]. Japanese. Available from: https://elaws.e-gov.go.jp/document?lawid=335AC0000000145
- Maeda H. Japan’s special approval for emergency system during the COVID-19 pandemic. Clin Pharmacol Ther. 2022;111(3):551–558.
- Prime Minister’s Office of Japan [Internet]. Health/medical strategy promotion expert committee (30th). [cited 2022 Aug 29]; [ about 2 screens]. Japanese. Available from: https://www.kantei.go.jp/jp/singi/kenkouiryou/tyousakai/dai30/gijisidai.html
- Ministry of Health, Labour and Welfare of Japan [Internet]. Health science council (Pharmaceuticals and medical devices system subcommittee). [cited 2022 Aug 29]; [about 3 screens]. Japanese. Available from: https://www.mhlw.go.jp/stf/shingi/shingi-kousei_430263.html
- Ueda M, Tanimoto T, Murayama A, et al. Japan’s drug regulation during the COVID-19 pandemic: lessons from a case study of favipiravir. Clin Pharmacol Ther. 2022;111(3):545–547.
- Arima Y, Kanou K, Arashiro T, et al. Epidemiology of coronavirus disease 2019 in Japan: descriptive findings and lessons learned through surveillance during the first three waves. Jpn Med Assoc J. 2021;4(3):198–206.
- Uddin S, Imam T, Khushi M, et al. How did socio-demographic status and personal attributes influence compliance to COVID-19 preventive behaviours during the early outbreak in Japan? Lessons for pandemic management. Pers Individ Dif. 2021;175:110692.
- Shimizu K, Negita M. Lessons learned from Japan’s response to the first wave of COVID-19: a content analysis. Healthcare (Basel). 2020;8(4):426.
- Saito T, Muto K, Tanaka M, et al. Proactive engagement of the expert meeting in managing the early phase of the COVID-19 epidemic, Japan, February-June 2020. Emerg Infect Dis. 2021;27(10):1–9.
- Miethke M, Pieroni M, Weber T, et al. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem. 2021;5(10):726–749.
- Adaptive Platform Trials Coalition. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat Rev Drug Discov. 2019;18(10):797–807.
- US Food and Drug Administration [Internet]. Guidance document: adaptive designs for clinical trials of drugs and biologics guidance for industry. 2019 December [cited 2022 Aug 29]; [ about 2 screens]. about 2 screens: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/adaptive-design-clinical-trials-drugs-and-biologics-guidance-industry
- The RECOVERY Collaborative GroupHorby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
- WHO Solidarity Trial ConsortiumPan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19 - interim WHO Solidarity trial results. N Engl J Med. 2021;384(6):497–511.
- ClinicalTrials.gov [Internet]. Adaptive COVID-19 Treatment Trial (ACTT). [cited 2022 Aug 29]; [about 13 screens]. Available from: https://clinicaltrials.gov/ct2/show/NCT04280705
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — Final report. N Engl J Med. 2020;383(19):1813–1826.
- Collins FS, Stoffels P. Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): an unprecedented partnership for unprecedented times. JAMA. 2020;323(24):2455–2457.
- REMAP-CAP: a randomised, embedded, multi-factorial, adaptive platform trial for community-acquired pneumonia [Internet]. REMAP-CAP. [cited 2022 Aug 29]. Available from: https://www.remapcap.org/
- Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–1329.
- Kamata K, Jindai K, Ichihara N, et al. Why participation in an international clinical trial platform matters during a pandemic? Launching REMAP-CAP in Japan. J Intensive Care. 2021;9(1):34.
- Tsuruya N, Kawashima T, Shiozuka M, et al. Academia-industry cooperation in the medical field: matching opportunities in Japan. Clin Ther. 2018;40(11):1807–1812.
- Fukushima M, Austin C, Sato N, et al. The Global academic research organization network: data sharing to cure diseases and enable learning health systems. Learn Health Syst. 2018;3(1):e10073.
- AMR Action Fund [Internet]. AMR action fund. c2022. [cited 2022 Aug 29]. Available from 2022 Aug 29. https://www.amractionfund.com/
- Clancy CJ, Nguyen MH. Buying time: the AMR Action Fund and the state of antibiotic development in the United States 2020. Open Forum Infect Dis. 2020;7(11):ofaa464.
- Onyeaka H, Al-Sharify ZT, Ghadhban MY, et al. A review on the advancements in the development of vaccines to combat coronavirus disease 2019. Clin Exp Vaccine Res. 2021;10(1):6–12.
- Renwick MJ, Brogan DM, Mossialos E. A systematic review and critical assessment of incentive strategies for discovery and development of novel antibiotics. J Antibiot (Tokyo). 2016;69(2):73–88.
- Innovative Medicines Initiative [Internet]. ND4BB: new drugs for bad bugs. [cited 2022 Aug 29]; [about 7 screens]. Available from: https://www.imi.europa.eu/projects-results/project-factsheets/nd4bb
- CARB-X: combating antibiotic-resistant bacteria [Internet]. [cited 2022 Aug 29]. Available from: https://carb-x.org/
- US Department of Health and Human Services [Internet]. Biomedical advanced research and development authority. [cited 2022 Aug 29]; [ about 4 screens]. about 4 screens: https://www.phe.gov/about/barda/Pages/default.aspx
- Brogan DM, Mossialos E. Incentives for new antibiotics: the Options Market for Antibiotics (OMA) model. Global Health. 2013;9(1):58.
- Japan Pharmaceutical Manufacturers Association [Internet]; Yuasa A, Yoshida M, Tawaragi Y. Situation and issues of development of antimicrobial drugs in Japan and Europe and United States. [cited 2022 Aug 29]; [11 p.]. Available from: https://www.jpma.or.jp/english/globalhealth/infectious_diseases/amr/eki4g60000004bso-att/eplg5k000000067k.pdf
- GOV.UK [Internet]. G7 finance ministers’ statement on actions to support antibiotic development. [cited 2022 Aug 29]; [about 3 screens]. Available from: https://www.gov.uk/government/publications/g7-finance-ministers-statement-on-actions-to-support-antibiotic-development
- Japan Pharmaceutical Manufacturers Association [Internet]. Japan Pharmaceutical Manufacturers Association; c2006-2021. Recommendations on Antimicrobial Resistance (AMR) [cited 2022 Aug 29]; [about 2 screensAvailable from]: https://www.jpma.or.jp/english/globalhealth/infectious_diseases/amr/recommendations.html
- AMR Alliance Japan [Internet]. Our policy recommendations. [cited 2022 Aug 29]; [about 5 screens]. Available from: https://www.amralliancejapan.org/en/goals/
- Morikane K. Infection control in healthcare settings in Japan. J Epidemiol. 2012;22(2):86–90.
- Prime Minister of Japan and His Cabinet [Internet]. The prime minister in action: headquarters for healthcare policy. (June 1, 2021). [cited 2022 Aug 29]; [about 2 screens]. Available from: https://japan.kantei.go.jp/99_suga/actions/202106/_00001.html
- Ministry of Health, Labour and Welfare of Japan [Internet]. Study group on ensuring the availability of drugs for infectious disease crisis. [cited 2022 Aug 29]; [about 2 screens]. Japanese. Available from: https://www.mhlw.go.jp/stf/shingi/other-kenkou_kansennsyokikitaiou.html
- World Health Organization [Internet]. Preparing for pandemics [cited 2022 Aug 29]; [about 2 screens]. Available from: https://www.who.int/westernpacific/activities/preparing-for-pandemics
- Lu J, Sheldenkar A, Lwin MO. A decade of antimicrobial resistance research in social science fields: a scientometric review. Antimicrob Resist Infect Control. 2020;9(1):178.
- Itoh N, Akazawa N, Kanawaku E, et al. Effects of infectious disease consultation and antimicrobial stewardship program at a Japanese cancer center: an interrupted time-series analysis. PLoS One. 2022;17(1):e0263095.
- Maeda M, Miyake T, Inose R, et al. Bibliometric analysis of pharmacist’s research on antimicrobial stewardship in Japan: an interrupted time series analysis on the implementation of the certification system for infection control pharmacists. J Pharm Health Care Sci. 2021;7(1):38.
- Mukai S, Shigemura K, Yang YM, et al. Comparison between antimicrobial stewardship program and intervention by infection control team for managing antibiotic use in neurogenic bladder-related urinary tract infection patients: a retrospective chart audit. Am J Infect Control. 2022;50(6):668–672.
- Uda A, Shigemura K, Kitagawa K, et al. Effect of antimicrobial stewardship on oral quinolone use and resistance patterns over 8 years (2013-2020). Antibiotics (Basel). 2021;10(11):1426.
- Ntt D, Htl V, Nguyen CTK, et al. Community-based antibiotic access and use in six low-income and middle-income countries: a mixed-method approach. Lancet Glob Health. 2021;9(5):e610–e619.