ABSTRACT
Introduction
Invasive Candida Infections (ICIs) have undergone a series of significant epidemiological, pathophysiological, and clinical changes during the last decades, with a shift toward non-albicans species, an increase in the rate of exogenous infections and clinical manifestations ranging from candidemia to an array of highly invasive and life-threatening clinical syndromes. The long-acting echinocandin rezafungin exhibits potent in-vitro activity against most wild-type and azole-resistant Candida spp. including C.auris.
Areas covered
The following topics regarding candidemia only and ICIs were reviewed and addressed: i) pathogenesis; ii) epidemiology and temporal evolution of Candida species; iii) clinical approach; iv) potential role of the novel long-acting rezafungin in the treatment of ICIs.
Expert opinion
Authors’ expert opinion focused on considering the potential role of rezafungin in the evolving context of ICIs. Rezafungin, which combines a potent in-vitro activity against Candida species, including azole-resistant strains and C.auris, with a low likelihood of drug–drug interactions and a good safety profile, may revolutionize the treatment of candidemia/ICI. Indeed, it may shorten the length of hospital stays when clinical conditions allow and extend outpatient access to treatment of invasive candidiasis, especially when prolonged treatment duration is expected.
1. Introduction
Like many infectious diseases, Invasive Candida Infections (ICIs) have undergone a series of significant epidemiological and clinical changes during the last decades [Citation1]. Indeed, while ICIs were trivial mucocutaneous infections before the advent of cancer chemotherapy, nowadays ICIs range from candidemia to an array of highly invasive infections that cause a variety of life-threatening clinical syndromes [Citation2–4].
Moreover, in recent decades, ICIs have exhibited an increasing incidence rate (from approximately 2.18 cases per 100,000 inhabitants per year in the 1990s to 3.22 in the last decade for candidemia [Citation5]), primarily attributed to the expanding population of high-risk and fragile individuals. This population encompasses not only severely immunocompromised hosts such as hemato-oncological neutropenic patients and solid organ transplant recipients, but also critically ill patients and those with multiple comorbidities admitted to Internal Medicine (IM) wards [Citation6–11].
Although ICIs other than ‘candidemia only’ are mostly abdominal infections, all body sites can be affected by Candida and many other deep localizations can be observed [Citation2,Citation3]. Moreover, the increasing availability of drug-delivery or prosthetic devices (e.g. long-term central venous catheters) has resulted in this microorganism being involved in a variety of foreign body infections that significantly added to the complexity of ICI clinical manifestations [Citation12]. As a paradigmatic example, Candida spp represented up to 27% of all central-line – associated bloodstream infection (CLABSI) in US adult ICUs (from 0.216 to 0.281 density rates per 1,000 central-line days from 2011 to 2017) [Citation13].
In this scenario, Candida albicans remains the most frequent cause of ICI (ranging from approximately 40% to 60% [Citation1,Citation14,Citation15]), but the prevalence of other species, some of which exhibiting alarming features of antimicrobial resistance, has been increasing over time [Citation1,Citation5,Citation16].
Ultimately, ICIs, particularly when manifesting as candidemia, are still associated with unacceptably high crude mortality rates (approximately 40% overall) [Citation5] and significant increases in healthcare costs, underscoring the importance of early diagnosis and treatment [Citation17]. On the other hand, in recent years the antifungal drug arsenal has been enriched with new agents. Among these, rezafungin is a long-acting echinocandin that might have a favorable impact on the treatment of new emerging resistant Candida species as well as on healthcare costs [Citation18,Citation19].
Herein, we reviewed the recent changes in the epidemiology and clinical features of ICIs as well as the improvements brought by new tools for the diagnosis of these infections. Finally, we also provided some insights on the potential role of the long-acting echinocandin rezafungin in this complex scenario.
2. Background
This brief paragraph is focused on how Candida spp enter the bloodstream and/or cause infections (endogenous vs exogenous route), while a complete review of the pathogenesis of ICIs is out of the scope of this review.
ICIs commonly refer to bloodstream infections caused by Candida species. These infections typically occur when Candida organisms breach the damaged intestinal barrier, which can be caused by various factors such as cancer chemotherapy, surgery, bacterial toxins, and local vascular perfusion disorders (endogenous route) () [Citation4]. When these microorganisms enter the bloodstream, the resulting candidemia may be cleared by antifungal therapy and intravascular catheter removal or may persist long enough to cause endophthalmitis, pyelonephritis, peritonitis and intra-abdominal infections, meningitis, encephalitis, endocarditis, osteoarthritis, pneumonia, empyema, mediastinitis, and pericarditis [Citation2,Citation3]. The endogenous route mostly applies for C. albicans, Candida tropicalis, Candida glabrata (Nakaseomyces glabrata), Candida lusitaniae (Clavispora lusitaniae), Candida parapsilosis and, to a lesser extent, Candida krusei (Pichia kudriavzeveii) ().
Figure 1. Pathogenesis and hematogenous routes of Candida species bloodstream dissemination with possible secondary infections as endocarditis (A), retinitis (B), skin fungal vasculitis (C) with macular/papular lesion (D) and multifocal hepatitis (E). CVC: central venous catheter; PICC: peripherally inserted central catheter.
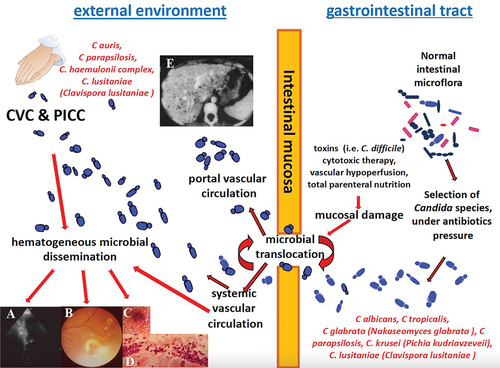
Table 1. Exogenous versus endogenous route of invasive Candida infection: differences in etiologies, epidemiology, and antifungal resistance.
New advancements in the pathophysiology of Candida have shown that this microorganism may also cause infections via an exogenous route, that is through healthcare workers’ hands or central line colonization and subsequent spread into the blood (). This is especially true for Candida auris, C. parapsilosis, Candida lusitaniae (C. lusitaniae) and Candida haemulonii, which often cause nosocomial outbreaks, frequently due to species resistant to antifungals and for which infection control measures are of paramount importance [Citation16,Citation20] ().
Considering C. parapsilosis as a possible cause of infection also through the endogenous route is justified by the presence of this species as a causative agent of hepatosplenic candidiasis [Citation21], the most common form of chronic disseminated candidiasis (CDC), which is often associated with negative blood cultures since hematogenous dissemination is limited to the portal vein system [Citation22].
For the purpose of this article, following recently adopted definitions [Citation23], we described the epidemiology, clinical aspects and therapy of candidemia without apparent secondary localizations (‘candidemia only’) separately from deep-seated ICIs, whether or not associated with Candida species isolated from the bloodstream.
3. Epidemiology of candidemia only
Candidemia is one of the most common healthcare-associated invasive fungal infections, with incidence varying according to geographical region, local epidemiology, and outbreak occurrence. A recent report indicates an overall pooled incidence rate of 3.88 cases per 100,000 inhabitants per year (ranging from 1.0 to 10.4). There is an observed increasing trend in incidence rates from the 1990s (median 2.18) compared to subsequent periods (median 4.67 in the year 2000–2010 and 3.22 in the last decade) [Citation5]. This increased incidence may be due to the increase in high-risk populations, such as critically ill and elderly patients and those with multiple comorbidities, along with nosocomial outbreaks.
Although the majority of the candidemia cases are hospital-acquired, with a reported incidence of 0.17–2.7 episodes per 1,000 discharges [Citation24], community-acquired candidemia is an emerging condition driven by the increasing use of long-term intravenous devices such as tunneled intravascular and peripherally-inserted central catheters [Citation3].
While C. albicans continues to be the most prevalent Candida species in both the adult and pediatric population, there has been a notable shift in species distribution over the past few decades. Specifically, there have been a decrease in the proportion of C. albicans and an increase in C. parapsilosis sensu lato and C. glabrata (N. glabrata). This change can be attributed to factors such as the use of antifungal medications, specific risk factors in patients such as immunosuppression, severity of the underlying conditions, chronic respiratory diseases, or nosocomial outbreaks [Citation1,Citation5]. This finding has important clinical implications, as C. parapsilosis and C. glabrata (N. glabrata) may show decreased susceptibility to echinocandins and azoles, respectively [Citation1].
Mareković and colleagues [Citation1] reported the distribution typical for Southern Europe, with C. parapsilosis ranking second and C. glabrata (N. glabrata) third in frequency. By contrast, in Northern Europe, United States, and Australia, C. albicans has been gradually replaced by C. glabrata (N. glabrata), ranking second in these geographical areas. Likewise, in the FUNGINOS survey conducted in Switzerland, Adam and colleagues observed a significant decrease from 60% to 53% in the proportion of C. albicans. At the same time, C. glabrata (N. glabrata) increased from 18% to 27%, particularly in the age group of 18–40 years and individuals above 65 years old. On the other hand, the frequency of other non-albicans Candida (NAC) species remained stable throughout the study period [Citation25].
A previous epidemiological study in high-risk patients with hematological malignancies by the Epidemiological Surveillance of Infections in Hemopathies (SEIFEM) group in Italy demonstrated that NACs accounted for 67% of the candidemia episodes (133 overall) and C. albicans for 33% only. The most frequent non-albicans species were C. parapsilosis, C. glabrata (N. glabrata), C. krusei (P. kudriavzeveii) (intrinsically resistant to fluconazole) and C. tropicalis. Strains resistant to at least one azole were 18%, mostly represented by NAC, with only one C. parapsilosis strain resistant to amphotericin B (AmB) and none to echinocandins [Citation11].
In IM wards, candidemia is a growing concern, as many more patients have risk factors for candidemia than in the past. In this setting, the most frequent pathogen was C. albicans (62%), followed by C. parapsilosis (17%), C. glabrata (N. glabrata) (13%), and C. tropicalis (5%), suggesting that the distribution in these patients is the same as in the past, at least in Italy [Citation14].
Furthermore, patients with early-onset candidemia may present with a new risk factor, represented by Chronic Obstructive Pulmonary Disease (COPD) [Citation26], and with significantly higher mortality due to treatment delays.
Clinical infections caused by NAC exceed those caused by C. albicans also in the Asia-Pacific region, with a high incidence of C. tropicalis [Citation27]. A nation-wide multicenter surveillance of NAC blood isolates was performed at seven university hospitals in Korea between 2010 and 2016: authors found that C. tropicalis was the most common NAC species (36.4%), followed by C. glabrata (N. glabrata) (28.5%), C. parapsilosis (24.7%), and C. krusei (P. kudriavzeveii) (2.6%). Compared the previous 6 years, the proportion of C. glabrata (N. glabrata) increased (from 21.3% to 28.5%) while that of C. parapsilosis decreased (from 36.5% to 24.7%). Fluconazole non-susceptible isolates accounted for 38.6% of isolates [Citation28]. Similar results have been reported in a multi-center prospective observational study carried out in 13 centers from Brunei, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam, where authors showed that non-albicans species were the most common isolates from bloodstream infections, with C. tropicalis as the predominant species. Overall, the most common species were C. albicans (35.9%), C. tropicalis (30.7%), C. parapsilosis (15.7%), and C. glabrata (N. glabrata) (13.6%) [Citation29]. Slightly differently, distribution of candidemia in a Malaysian tertiary care hospital revealed predominance of C. parapsilosis as the most common agent of candidemia (29.2%), followed by C. albicans (20.1%), C. tropicalis (18.7%), C. glabrata (N. glabrata) (6.0%) [Citation30].
Among agents of candidemia, diverse NAC species present some specific features which reflect different pathophysiology () and influence the observed differences in epidemiology.
Indeed, C. parapsilosis complex is a biofilm-producing and environmental contaminant species able to colonize central venous catheters (CVCs) and the hands of healthcare workers, causing systemic infections not only by the endogenous but also by the exogenous route (). These features may contribute to clonal outbreaks [Citation31] and underline the need of implementing measures to avoid the nosocomial spread of this species [Citation32].
Moreover, the ability of C. parapsilosis to produce biofilm seems to be related to an increase in virulence and to a worse outcome when these high-producing biofilm isolates are involved in candidemia [Citation33].
Antifungal susceptibility testing commonly shows borderline susceptibility to echinocandins; importantly, although fluconazole resistance was generally considered to be uncommon, up to one-third and one-quarter of the isolates recently showed resistance to fluconazole or voriconazole, respectively [Citation31]. A recent paper investigating candidemia in SARS-CoV-2 critically ill patients showed that the incidence of candidemia was significantly higher in critically ill COVID-19 patients on VV-ECMO than in critically ill COVID-19 patients who did not meet the criteria for VV-ECMO (16/45, 36% vs 13/93, 14%, respectively), and that C. parapsilosis accounted for the majority of the candidemia events [Citation34]. A 6-year retrospective study performed in China showed that C. parapsilosis was the predominant pathogen in patients with persistent candidemia [Citation35].
C. tropicalis is the species with the highest mortality rates in patients with candidemia, is increasingly reported in frequency and shows growing resistance to fluconazole [Citation36].
C. glabrata (N. glabrata) shows higher echinocandin resistance than other Candida species (1.7–3.5%), possibly due to the preferential use of echinocandins for the treatment of these infections, encouraged by increasing azole resistance in this species, ranging from 5.6% to 15.7% [Citation27,Citation37,Citation38]. Furthermore, C. glabrata (N. glabrata) showed a high propensity to readily mutate in vivo, especially in cases of intra-abdominal infections, where source control may be delayed or drug underexposure may be present [Citation37,Citation39].
Most commonly, C. tropicalis and C. glabrata (N. glabrata) cause candidemia after passing through damaged intestinal barrier ().
However, the most alarming agent for candidemia nowadays is C. auris, firstly reported in 2009 from the external ear canal of a patient in Japan and belonging to the critical priority group according to the recent WHO fungal priority pathogens list [Citation40,Citation41]. C. auris is characterized by several distinct features. Firstly, it has high transmissibility, having the ability to potentially colonize patients indefinitely and exhibiting resistance in the healthcare environment. This is due to prolonged survival on surfaces and its ability to resist common disinfectants [Citation42] (). Secondly, the identification of C. auris with conventional biochemical and microbiological techniques presents challenges [Citation43]. Thirdly, C. auris demonstrates a high rate of antifungal resistance, with almost universal resistance to fluconazole (90%), frequent resistance to AmB (30%), and the potential for resistance to echinocandins (5%), particularly in cases of pre-exposure. The emergence of pan-drug-resistance in C. auris is indeed alarming. Isolates resistant to three major classes of antifungal agents have recently been described in the US and other countries [Citation44–46]. A healthcare transmission of pan-resistant and echinocandin-resistant C. auris has been reported [Citation47], while a recent study analyzed the genome and drug-resistance profile of 19 isolates of C. auris collected over 72 days from a multi-visceral transplant recipient with refractory fungal peritonitis. Authors found that two C. auris isolates were resistant to four major classes of antifungal agents (azoles, echinocandins, polyene, and flucytosine) while several other strains exhibited resistance to three major drug classes [Citation48].
Lastly, C. auris has an unprecedented capacity to cause nosocomial outbreaks, which are difficult to control in many countries and can persist for extended periods, even years [Citation49,Citation50].
These outbreaks, frequently reported during the COVID-19 pandemic, occurred through environmental reservoirs (sometimes difficult to pinpoint, such as thermometers), extensive colonization in humans detected in axilla, groin, nares, rectum, and high rate of horizontal transmission [Citation51–53]. In Italy, the Liguria region reported a C. auris outbreak with at least 277 cases in eight healthcare facilities. The first of these cases was detected in one hospital in July 2019, and cases sporadically continued to occur in the same hospital [Citation54,Citation55]. In February 2020, C. auris was detected in an intensive care unit (ICU) dedicated to patients with severe COVID-19 in the same hospital, with a subsequent increase in cases throughout 2020 and 2021. Another 11 cases have occurred in facilities in the neighboring Emilia-Romagna region so far [Citation56]. C. auris infections have been described by a number of countries in Asia. Interestingly, the first C. auris candidemia was reported from a retrospective analysis of South Korean unidentified Candida isolates using rDNA sequencing. Later on, several reports and outbreaks of C. auris infections have been described in China, Hong Kong, and Taiwan [Citation57].
While a significantly higher risk for microbiologic recurrence within 60 days of completion of antifungal therapy was observed in C. auris patients, the outcome of patients with C. auris candidemia was similar to patients with candidemia due to other species [Citation58,Citation59].
Although still considered rare, the emergent C. haemulonii species complex (C. haemulonii sensu stricto, Candida duobushaemulonii, and Candida haemulonii var. vulnera) has been increasingly reported worldwide over the last 10 years, especially in patients with previous antimicrobial/antifungal therapy, those with malignant tumors, organ transplants, diabetes mellitus, and vascular diseases. Outbreaks in neonatal intensive care units have been reported in Korea and India, highlighting that this species may reach the blood by the exogenous route [Citation60]. Furthermore, the C. haemulonii species complex shows increased resistance to the available antifungal drugs such as fluconazole and AmB [Citation61].
Candida lusitaniae (C. lusitaniae), generally considered a low-frequency emerging pathogen, has been associated with peritonitis, meningitis, and urinary tract infections [Citation62] and can cause fatal infections in immunocompromised patients [Citation63]. In this setting, while representing approximately 1.3% of all candidemia episodes among cancer patients, C. lusitaniae caused up to 28% and 19% of fungemia and breakthrough fungemia caused by uncommon Candida species (19/68 and 7/37, respectively), with an overall mortality of 53% [Citation64]. Additionally, C. lusitaniae has been implicated in nosocomial acquisition [Citation20] and person-to-person transmission [Citation65]. Most importantly, C. lusitaniae is known for its ability to acquire in vivo drug resistance under antifungal treatment and multidrug resistance has also been reported [Citation66]. Very recently, the emergence of C. lusitaniae isolates with decreased micafungin susceptibility emerged during micafungin monotherapy was shown; furthermore, for the first time, cross-resistance to fluconazole despite no history of drug use was observed [Citation67].
Candida palmioleophila, often misidentified as Meyerozyma guilliermondii (Candida guilliermondii) or Candida famata, is an emerging pathogen in Denmark [Citation68]. Isolates of this species are still susceptible to echinocandins and resistant to azoles, especially fluconazole. Recently, two strains, misidentified as C. albicans, were described in the Campania region in Italy [Citation69].
4. Clinical approach to candidemia
The decision whether to initiate antifungal treatment for candidemia depends on several considerations, including epidemiology (community vs. hospital-acquired), setting (ICU, IM), risk factors (immunosuppression, severity of the underlying conditions, chronic respiratory diseases), severity of infection (septic shock), possibility of source control and treatment strategies (empiric, preemptive or targeted strategies, de-escalation) [Citation70–72].
However, one of the main drivers for better outcomes is represented by early antifungal treatment [Citation73,Citation74]. Indeed, a retrospective cohort of 230 patients with candidemia showed that mortality was lower if fluconazole was started on the same day of the index culture than if started later (15% vs 24% vs 37% vs 41% if started at day 1, day 2 or day 3 or later, respectively) [Citation75]. On the other hand, a > 12-hour delay in antifungal therapy initiation from the time of blood sample collection increased mortality (33.1% vs 11.1%) [Citation76], while receiving antifungal treatment within 72 h from index blood culture had a significant benefit on mortality (27% versus 40%) [Citation73].
Likewise, in patients with septic shock and candidemia, delayed antifungal treatment and failure to achieve source control were independently associated with hospital mortality [Citation74].
The decision to initiate early antifungal therapy should be guided by the use of biomarkers and/or risk factor-based prediction rules. This approach aims to identify patients at the highest risk of developing candidemia and, conversely, to avoid unnecessary antifungal treatment when results are negative. While blood cultures are considered the gold standard for diagnosing candidemia, their overall sensitivity is approximately 50% (ranging from 21% to 71%). Furthermore, blood cultures may require a significant amount of time to yield positive results, potentially leading to delays in initiating appropriate antifungal treatment [Citation77,Citation78].
As additional tools, non-culture-based assays may aid in guiding the diagnosis of candidemia. 1–3 β-D-glucan (BDG) has a pooled sensitivity and specificity of 80% and 82%, respectively, with the most accurate cutoff value >80 pg/mL [Citation79–81]. However, its main advantage is its high negative predictive value (98.7%), allowing early interruption of antifungal therapy if started empirically based on clinical suspicion and/or prediction scores [Citation82]. There are experiences and proposals for different cutoffs in IM Wards [Citation83].
T2Candida is a novel nanodiagnostic panel that uses T2 magnetic resonance (T2MR) and a dedicated instrument (T2Dx Instrument) to detect Candida directly in whole blood samples [Citation84,Citation85]. This instrument is able to identify the five most common Candida species [C. albicans, C. glabrata (N. glabrata), C. parapsilosis, C. tropicalis, C. krusei (P. kudriavzeveii)] [Citation86] with an estimated sensitivity and specificity of 91.1% and 99.4%, respectively [Citation87]. Interestingly, T2Candida can detect candidemia in 3–5 hrs., thus reducing the time to a negative result by some days. Along with its high negative predictive value (approximately 100%), T2 Candida negative results may suggest that antifungal therapy can be early withdrawn. In fact, stopping antifungal treatment when no longer necessary counteracts the detrimental effects of over-treatment in terms of selective pressure with a shift to resistant Candida species, emergence of resistance and increased costs [Citation88,Citation89].
Clinical prediction rules/scores have also been proposed for the identification of patients at high risk of candidemia necessitating early antifungal treatment and include, although not limited to, the Ostrosky-Zeichner or the Candida scores [Citation90–93]. Despite differences in sensitivity, they have high negative predictive value, ranging from 97% to 99.4% [Citation78]. Again, this feature allows not to start or early discontinue antifungal treatment when not necessary.
Several updated detailed guidelines for candidemia treatment can support healthcare professionals [Citation70,Citation72,Citation94]. Initial treatment with echinocandins is strongly recommended either in neutropenic or non-neutropenic candidemia patients, in more severe patients or where biofilm activity is needed [Citation10,Citation94,Citation95]. This recommendation is mainly based on trials comparing caspofungin or micafungin versus AmB (deoxycholate or liposomal, respectively), showing similar effectiveness but fewer adverse events [Citation96,Citation97], or a randomized controlled trial showing non-inferiority of anidulafungin versus fluconazole [Citation98]. A subsequent post-hoc analysis focused on C. albicans showed that global response was significantly better for anidulafungin than fluconazole and that anidulafungin was associated with significantly faster clearance of blood cultures [Citation99]. Furthermore, in approximately 2,000 patients from seven trials (three including patients with candidemia, four including patients with either candidemia or ICIs), echinocandins were associated with a significantly lower 30-day mortality rate than azoles or AmB [Citation100]. Notwithstanding the lower in-vitro activity vs. C. parapsilosis, echinocandins were associated with clinical effectiveness [Citation101,Citation102].
The echinocandin armamentarium currently available is being reinforced by the new long-acting, once weekly administered, rezafungin [Citation103], which demonstrated non-inferiority to caspofungin in the treatment of candidemia or ICIs regarding the primary endpoints of day-14 global cure (European Medicine Agency, EMA) and 30-day all-cause mortality (Food and Drugs Administration, FDA) [Citation23].
Compared to echinocandins, azoles may have advantages as a first-line therapy in stable patients if C. krusei (P. kudriavzeveii) or C. glabrata (N. glabrata) are not considered and in the absence of immunosuppression [Citation10,Citation94].
Interestingly, in patients admitted to IM wards and with septic shock due to candidemia, there are conflicting reports concerning echinocandin effectiveness over fluconazole, perhaps related to methodology, patient clinical complexity, frequencies of septic shock and treatment delays [Citation103–107]. Indeed, the initial echinocandin therapy does not seem to impact the outcome of candidemia patients with septic shock, while the benefit over fluconazole was mainly observed in non-septic patients [Citation108]. Similar results were obtained in a Spanish prospective study showing that both empirical and targeted treatment with fluconazole was not associated with increased 30-day mortality compared to echinocandins [Citation108]. On the other hand, echinocandins were associated with significantly higher survival as a definitive therapy in IM wards [Citation104].
Among azoles, isavuconazole should not be used in treating candidemia because of the excess of mortality observed in the trial vs. caspofungin [Citation106].
AmB deoxycholate or, preferably, lipid and especially liposomal formulations are fungicidal, active against Candida biofilm and able to penetrate the blood-brain barrier [Citation95,Citation109]. Despite these promising features, a recent meta-analysis revealed that the initial treatment of candidemia with echinocandins had a reduced mortality rate compared to polyenes [Citation95]. Furthermore, AmB use may be limited by the risk of nephrotoxicity and varying sensitivity of C. auris.
Echinocandins are the current preferred treatment options for C. auris infections [Citation16]. However, emergence of resistance and pan-fungal resistance have been reported [Citation16,Citation48,Citation55]. Amphotericin B may be an alternative to echinocandins since it has fungicidal activity and good biofilm penetration. Its main drawbacks include a relatively high rate of resistance in some C. auris clades, such as Clade I, potentially explaining the therapeutic failures observed in some clinical cases [Citation110]. Moreover, a case of in-vivo development of high-level AmB resistance during therapy was recently reported, and a novel mutation mechanism in the C. auris sterol-methyltransferase gene ERG6 was found [Citation111]. Delayed (or not executed) source control was another risk factor for the development of resistance to antifungals in C. auris [Citation49]. While some studies have reported susceptibility to azoles other than fluconazole, there are currently no established breakpoints for interpreting in vitro MIC values. In complex cases, especially when dealing with persistent infections or failure of echinocandins, alternative treatment options may be necessary. These may involve the use of combination therapies based on in-vitro and in vivo experiments such as colistin plus echinocandins [Citation112], isavuconazole plus echinocandins [Citation113,Citation114], posaconazole plus caspofungin [Citation115], isavuconazole plus colistin [Citation116], or novel agents, such as rezafungin (which has in vitro activity against strains resistant to other echinocandins), ibrexafungerp, or fosmanogepix may be considered [Citation5,Citation117–120].
The correct choice of the antifungal should be accompanied by the demonstration of negative blood cultures if previously positive, by at least 14 days of effective treatment and by appropriate de-escalation strategies, depending on clinical stability, isolates and guidelines, 3-to-7 days after the beginning of therapy, usually from echinocandins to fluconazole. As a matter of fact, it is safe to stepdown therapy to oral fluconazole or voriconazole as soon as patients are clinically stable and blood cultures are negative [Citation106,Citation121]. Nevertheless, early (day 5) de-escalation to fluconazole was only performed in a minority of cases despite no negative impact on patients’ outcome (in only 23% out of 235 eligible patients in a cohort from 3 RCTs including 1023 patients) [Citation122]. Compared to fluconazole, voriconazole is active against C. guilliermondii, C. glabrata (N. glabrata), and C. krusei (P. kudriavzeveii) with reduced susceptibility, or resistance, to fluconazole. The switch to oral azoles may undoubtedly present the advantage of allowing patient discharge, if feasible, while long-acting rezafungin can be a valid alternative in the case of azole resistance.
5. Epidemiology and clinical aspects of ICIs other than candidemia only
The epidemiology of ICIs (with or without concomitant candidemia) is still a difficult and unsolved enigma for the scientific community, due to variability in definitions, difficulties in differentiating colonization from infection and diagnostic issues [Citation123]. Indeed, only 17% of cases of deep-seated candidiasis were detected by blood cultures [Citation124], suggesting that many cases of ICIs could be under diagnosed [Citation125].
ICIs are most commonly observed in the ICU setting, where up to two-thirds of patients have concomitant candidemia, while 80% of patients without candidemia have intra-abdominal candidiasis (IAC) [Citation126,Citation127]. IAC accounts for most deep-seated cases, with approximately 30% occurring in critical care settings [Citation126,Citation127]. A multinational retrospective study conducted in several European ICUs showed a cumulative incidence of 7.07 episodes per 1,000 ICU admissions (5.52 and 1.84 episodes per 1,000 ICU admissions for candidemia and IAC, respectively). C. albicans was the most frequently isolated species (57%), followed by C. glabrata (N. glabrata) (21%) and C. parapsilosis (13%) [Citation15]. Of note, significant between-center variability and increased incidence over time were observed. Understanding ICI epidemiology in IM units is even more challenging, with a recent Italian study showing an overall incidence of 1.89 cases/1,000 hospital admissions [Citation14].
In general, ICIs other than ‘candidemia only’ include a variety of deep-seated infections that may be caused by fungal seeding in various organs as a consequence of hematogenous spread from a distant infectious focus [Citation21,Citation22,Citation128–133] or by direct contamination during surgery, trauma, or extension from a contiguous infected site [Citation123,Citation130,Citation134]. The majority of ICIs other than candidemia present the classical risk factors for invasive fungal diseases, such as previous or concomitant antibiotic therapy and immunosuppression often mediated by steroid therapy [Citation123,Citation130]. reports additional specific factors for each type of ICI [Citation21,Citation22,Citation128,Citation129,Citation132,Citation133,Citation138–141,Citation144–152,Citation154–158]. Along the same line, the spectrum of the Candida species involved varies according to the different type of infection. In general, C. albicans remains the most common pathogen, followed by C. glabrata (N. glabrata) and C. tropicalis in many deep-seated infections that usually represent a source of secondary candidemia, especially intra-abdominal and urinary tract infections [Citation135,Citation136,Citation138–140,Citation158]. On the other hand, C. parapsilosis is the second cause of all intravascular infections, shunt-related meningitis, and the leading cause of trauma or fracture-related Candida infections [Citation134,Citation141,Citation144,Citation145]. Of interest, C. dubliniensis may be considered an emerging pathogen since it has represented, in recent case series, not only an increasing cause of endophthalmitis and esophagitis, particularly in cases of infection related to intravenous drug addiction, but also a leading cause of Candida meningitis not related to intraventricular devices [Citation133,Citation147,Citation148].
Table 2. Underlying diseases and conditions, main Candida species involved, therapy options and management of different ICIs.
Some infections usually manifest with an acute onset, with septic shock being a possible ominous component of the initial clinical picture. It typically occurs in patients with ascending or emphysematous pyelonephritis [Citation140], but also in many intrabdominal infections [Citation135,Citation158] or serious intravascular infections [Citation130–132] such as peripheral vein septic thrombophlebitis [Citation145] or central vein septic thrombosis [Citation146]. In these instances, prompt adequate antifungal therapy, either directed against the offending pathogen or in terms of antimicrobial exposure in the infection sites, and surgical source control (when indicated, as outlined in ) are crucial for patient survival.
Other infections have a very insidious and misleading presentation. A typical example is that of Candida meningitis not related to intraventricular devices [Citation133,Citation147,Citation148]: this infection, nowadays more commonly sustained by C. dubliniensis, has an indolent course that mimics tubercular meningitis, and seems to be a late complication of a previous, apparently resolved, nosocomial candidemia or a side effect of intravenous drug addiction [Citation133,Citation147,Citation148]. Similarly, Candida prosthetic valve endocarditis is a typical biofilm-related infection that may be acquired during a candidemia episode in the early post cardio-surgery period [Citation132,Citation142]: despite apparent cure with standard antifungal therapy, Candida seeding on the prosthetic valve and surrounding endocardium persists and the infection manifests insidiously even after more than 1 year [Citation132,Citation143]. Under these circumstances, both the prompt recognition of the clinical syndrome and a correct approach to the microbiological diagnosis, along with the use of biomarkers, are of paramount importance for optimal management.
Despite being a very rare disease, hepatosplenic candidiasis is the most frequent form of chronic disseminated candidiasis (CDC) and typically occurs in patients with hematological malignancies and long-lasting neutropenia [Citation19,Citation20,Citation153]. CDC is now considered as a fungal immune reconstitution inflammatory syndrome (IRIS), because of the lack of microbiologically active lesions (often documented by negative cultures of liver biopsies and rare documentation of recent candidemia episodes), contrasting with a major inflammatory reaction and high levels of immune activation [Citation153]. Following the hypothesis that CDC may be driven by IRIS, the use of concomitant glucocorticosteroids may be considered for the management of this condition, along with long-term antifungals [Citation22].
In most ICIs other than candidemia, a proper therapeutic approach includes an initial treatment regimen, usually with intravenous fungicidal and effective anti-biofilm agents such as echinocandins and liposomal AmB [Citation94,Citation137]. Being hydrophilic agents, the former may have poor penetration across the blood-brain barrier, in the vitreous, and in the mesothelial cavities. Consequently, in the presence of deep-seated infections involving these body sites, fluconazole, voriconazole or liposomal AmB may be valuable alternatives or add-on therapies [Citation159]. In any case, as soon as clinical improvement, and clearance of a possible concomitant candidemia have been achieved with the initial treatment, intravenous or oral azoles, usually fluconazole or voriconazole, are the agents of choice for maintenance therapy [Citation160]. Various forms of source control are of paramount importance in many instances: surgical or percutaneous drainage or debridement, such as in secondary peritonitis or intrabdominal abscesses [Citation158,Citation161], and device removal with or without replacement, such as in prosthetic valve endocarditis or shunt-related meningitis [Citation94,Citation132,Citation150]. Peripheral vein thrombophlebitis, an infectious process that involves adventitia, intima, and adjacent thrombus, requires debridement or phlebotomy in most instances [Citation144]. On the other hand, in central vein septic thrombosis, which involves almost only the thrombus, anticoagulation, and antifungal therapy allow clinical cure in most cases [Citation145,Citation161].
In certain cases, maintenance antifungal therapy with oral azoles may be prolonged from 2 to 12 months, as in cases of hepatosplenic candidiasis [Citation141], osteomyelitis or disc space infection [Citation154–156]. Likewise, chronic suppressive therapy with oral azoles may be required in selected cases of intravascular device infections (prosthetic valve endocarditis, aortic graft infections, and cardiac implantable electronic device infections), where surgical device removal and replacement cannot be performed [Citation132]. In these cases, other therapeutic options should be considered when oral antifungal treatment is not suitable, as in the case of azole resistance. A reasonable approach to the above clinical settings may be the use of the novel long-acting echinocandin rezafungin [Citation162,Citation163].
6. Rezafungin
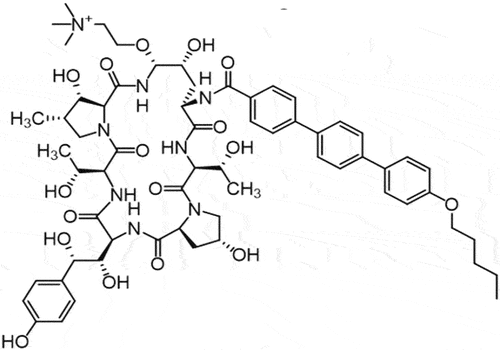
Rezafungin is a novel echinocandin derived from the precursor anidulafungin with an additional choline ether () [Citation164,Citation165]. Rezafungin shares the same mechanism of action as other echinocandins, which consists in inhibiting fungal cell wall synthesis by blocking the beta 1,3 D-glucan synthetase activity [Citation162].
6.1. In-vitro activity
Rezafungin activity against Candida spp. is similar to that of other members of the echinocandin class, exhibiting potent in-vitro activity against most wild-type and azole-resistant Candida spp [Citation166].
In a wide in-vitro study performed on several clinical Candida isolates in four different European Laboratories following the EUCAST method, the lowest rezafungin MICs (range, mg/L) were observed for C. albicans (0.002–0.125) and the highest for C. parapsilosis (0.063->4). Of note, unexplained laboratory variation was observed for C. albicans, including the quality control strains [Citation167].
Additionally, the in-vitro activity of rezafungin and comparators (caspofungin, anidulafungin, micafungin, and azoles) was analyzed on a worldwide collection performed on 2,205 invasive fungal isolates including 1,904 isolates from six Candida species and recovered from 2016 to 2018 by using Clinical and Laboratory Standards Institute (CLSI) broth microdilution methods. Authors found that rezafungin inhibited 99.8% of C. albicans isolates (MIC50/90, 0.03/0.06 μg/ml), 95.7% of C. glabrata (N. glabrata) isolates (MIC50/90, 0.06/0.12 μg/ml), 97.4% of C. tropicalis isolates (MIC50/90, 0.03/0.06 μg/ml), 100.0% of C. kruzei (P. kudriavzeveii) isolates (MIC50/90, 0.03/0.06 μg/ml), and 100.0% of C. dubliniensis isolates (MIC50/90, 0.06/0.12 μg/ml) at ≤0.12 μg/ml. All (329/329) C. parapsilosis isolates (MIC50/90, 1/2 μg/ml) were inhibited by rezafungin at ≤4 μg/ml [Citation168]. Indeed, C. parapsilosis was the least susceptible species in-vitro, with MICs up to 4 μg/mL [Citation166], while C. metapsilosis, C. orthopsilosis, and C. guilliermondii showed MICs between 0.5 and 1 μg/mL [Citation162]. Despite the lower in-vitro susceptibility for C. parapsilosis, treatment failures have not yet been described [Citation103].
Another study determined the in-vitro susceptibility of 689 clinical isolates of Candida species, including 19 rare Candida species, and Saccharomyces cerevisiae by using CLSI methods, showing excellent in-vitro activity against both wild-type and azole-resistant Candida species, as well as against S. cerevisiae [Citation169]. For C. lusitaniae (C. lusitaniae), MICs were 0.25 μg/mL [Citation162].
Overall, rezafungin had species-specific activity similar to other echinocandin, being equally or more active than fluconazole and amphotericin B against the most common Candida species, except C. parapsilosis [Citation166,Citation167]. Of note, rezafungin seems to be more potent in-vitro against C. auris than caspofungin and micafungin, with MIC 0.25 μg/mL [Citation16,Citation162,Citation170].
The selection of FKS mutants occurred at a similar low frequency for rezafungin as for anidulafungin and caspofungin. As expected, rezafungin shares with the other echinocandins the reduced activity against strains carrying mutations of the FKS1 and FKS2 genes [Citation16,Citation162]. Interestingly, fks1 mutations raised rezafungin MICs notably less than anidulafungin and micafungin MICs in C. auris [Citation166].
Rezafungin also exhibits high activity against Aspergillus spp., including azole-resistant strains, while it is not active against non-Aspergillus molds, Cryptococcus, Trichosporon, and Rhodotorula [Citation162,Citation170].
6.2. Pharmacokinetics
The major difference between rezafungin and the first-generation class of echinocandins is the much lower clearance rate, which prolongs the elimination half-life up to 6 to 10-fold compared to anidulafungin, caspofungin, or micafungin ().
Table 3. Pharmacokinetic parameters of rezafungin vs. older echinocandins.
This gives rezafungin a unique feature within the class of the echinocandins, namely the possibility of being administered once weekly instead of once daily, unlike all other available echinocandins. Rezafungin biotransformation is minimal in humans, and fecal excretion is the major route of elimination [Citation171]. It does not interact with the cytochrome P450 isoenzymes; therefore, it is not prone to drug–drug pharmacokinetic interactions, similarly to the other echinocandins [Citation172]. Specifically, it was recently shown that co-administration of rezafungin did not impact cyclosporine or mycophenolate mofetil pharmacokinetics [Citation173]. Rezafungin is minimally excreted by the renal route, and consequently, in an ex vivo bovine blood model, it was not shown to be cleared by continuous veno-venous hemofiltration [Citation174]. Interestingly, a population pharmacokinetic model based on data from a phase 1 study showed that none of several subject descriptors (including sex, infection status, serum albumin, and body surface area) affected the pharmacokinetic behavior of rezafungin in a clinically relevant manner [Citation175,Citation176].
6.3. Pharmacodynamics
Like first-generation echinocandins, the major pharmacodynamic determinant of rezafungin efficacy is the area under the concentration-time curve (AUC) to the MIC ratio [Citation177,Citation178].
In a neutropenic animal model of disseminated candidiasis caused by C. tropicalis and C. dubliniensis, free AUC/MIC ratio values ranging from 3 to 25 were associated with stasis, and values ranging from 4.3 to 62 were associated with 1-log kill [Citation179,Citation180]. It was estimated that a dosing regimen of 400 mg loading dose on week 1 followed by 200 mg once weekly may guarantee these AUC/MIC targets against the vast majority (>99%) of C. tropicalis and C. dubliniensis clinical isolates [Citation179,Citation180]. In a neutropenic mouse model of disseminated candidiasis caused by C. albicans, C. glabrata (N. glabrata) and C. parapsilosis, free AUC/MIC ratio values of 2.92, 0.07 and 2,61, respectively, were associated with stasis whereas values two- to four-fold higher than these were associated with 1-log kill [Citation179–181].
Studies on different dosing schedules for the same total weekly dose of rezafungin showed that once weekly dosing may guarantee more effective fungal killing than twice-weekly or once-daily regimens [Citation182]. Monte Carlo simulations conducted with three different intravenous rezafungin regimens (namely, single 400 mg dose; 400 mg week 1 followed by 200 mg weekly for 5 weeks; 400 mg weekly for 6 weeks) showed that the likelihood of attaining PK-PD targets against C. albicans and C. glabrata (N. glabrata) were always ≥90%. These analyses supported the use of single and once weekly rezafungin regimens for treating patients with candidemia and/or candidiasis due to C. albicans or C. glabrata (N. glabrata) [Citation183].
In a neutropenic mouse model of invasive candidiasis caused by C. auris, the free AUC/MIC ratio value for stasis was 1.88, whereas that for 1-log kill was 5.77 [Citation181]. Likewise, in a disseminated immunocompromised mouse model of candidiasis, rezafungin exhibited potent antifungal activity against C. auris, with significantly lower renal tissue fungal burden than AmB- and vehicle-treated mice [Citation184].
6.4. Registrative and real-life studies
The STRIVE trial was a phase II trial comparing adults with candidemia and/or ICI randomized to receive either rezafungin 400 mg once weekly or rezafungin 400 mg on week 1 followed by 200 mg once weekly or caspofungin 70 mg as a loading dose, followed by 50 mg daily for ≤4 weeks [Citation103]. The primary efficacy endpoint was overall cure at day 14 as demonstrated by mycological eradication and resolution of signs. The overall cure rate was highest for rezafungin 400/200 mg compared to rezafungin 400 mg or caspofungin (76.1% vs 60.5% vs 67.2%, respectively); likewise, the mortality rate was lowest for rezafungin 400/200 mg compared to rezafungin 400 mg or caspofungin (4.4% vs 15.8% vs 13.1%, respectively). Interestingly, candidemia was cleared earlier in patients on rezafungin than those receiving caspofungin. No significant safety issues were observed [Citation103]. The observed discrepancies between the two rezafungin dosing regimens were explained as a possible paradoxical effect at higher concentrations.
The subsequent ReSTORE trial was a multicenter, double-blind, double-dummy, randomized phase III trial including adults with candidaemia or ICI randomly assigned (1:1) to receive either intravenous rezafungin once weekly (400 mg in week 1, followed by 200 mg weekly, for a total of two to four doses) or intravenous caspofungin (70 mg loading dose on day 1, followed by 50 mg daily) for up to 4 weeks. The primary endpoints were global cure (consisting of clinical cure, radiological cure, and mycological eradication) at day 14 for the EMA and 30-day all-cause mortality for the FDA. The global cure rate at day 14 was 59% in the rezafungin group, compared to 61% in the caspofungin group, while 30-day mortality was 24% and 21% in the rezafungin and caspofungin groups, respectively, thus showing non-inferiority of rezafungin to caspofungin [Citation23].
A Phase III, multicenter, prospective, randomized, double-blind study is currently ongoing to evaluate the efficacy and safety of rezafungin (400 mg/200 mg once weekly) versus the standard antimicrobial regimen (daily azole prophylaxis with fluconazole or posaconazole and oral trimethoprim-sulfamethoxazole for anti-Pneumocystis jirovecii prophylaxis) for the prevention of invasive fungal diseases including Candida spp., Aspergillus spp., and P. jirovecii in subjects undergoing allogeneic blood and bone marrow transplantation. Fungal-free survival at day 90 is the primary outcome [Citation177].
A post-hoc analysis of the STRIVE and ReSTORE trials aimed to examine the outcomes of all-cause mortality in patients with candidemia/ICI and day-5 mycological eradication in patients with candidemia. The integrated analysis included 294 patients (139 in the rezafungin arm, 155 patients the in caspofungin arm) and showed that 30-day mortality was 18.7% and 19.4% for the rezafungin and caspofungin groups, respectively. Patients with candidemia exhibited higher mycological eradication rates at day 5 (80.0% for rezafungin vs 67.8% for caspofungin) [Citation23,Citation103,Citation185].
To date, there are only three reports of successful compassionate use of rezafungin in the treatment of patients with Candida spp infections: i) a multidrug-resistant C. glabrata (N. glabrata) mediastinal infection from vascular graft infection and retained foreign material, where rezafungin was administered for over 1 year, in the absence of adverse effects [Citation163]; ii) a refractory intra-abdominal candidiasis due to C. krusei (P. kudriavzeveii) in a liver transplant recipient, where rezafungin was administered for 12 weeks [Citation186] and iii) a chronic mucocutaneous candidiasis sustained by azole-resistant C. albicans in a 19-year-old man with STAT1-GOF immunodeficiency, where rezafungin was administered for 5 weeks [Citation187].
6.5. Safety
Rezafungin is characterized by higher chemical stability compared to anidulafungin, which may prevent the formation of reactive metabolites potentially causing toxicity [Citation164,Citation165,Citation172]. A preclinical 2-week repeat-dose comparison study in rats showed some degree of toxicity and hepatotoxicity with anidulafungin, but not with rezafungin [Citation172].
In a phase I, single-center, randomized, double-blind trial assessing the effects of intravenous rezafungin vs. intravenous placebo (with moxifloxacin as positive control) on the QT interval of the electrocardiogram, therapeutic (600 mg), and supratherapeutic (1,400 mg) rezafungin doses did not cause any clinically relevant QT interval prolongation [Citation178].
6.6. Economic impact
The health economic burden associated with candidemia/ICIs is largely due to prolonged hospital and ICU stays, contributing to more than half of the total costs [Citation17]. The once-weekly administration of rezafungin may possibly reduce hospitalization costs or length of stay. In this regard, the integrated analysis of the STRIVE and ReSTORE trials showed that, among patients hospitalized in the ICU, survivors receiving rezafungin had a reduction of 7.1 days in ICU length of stay (15.9 vs. 23 days, respectively). Although slightly reduced, this trend was confirmed after adjusting for mechanical ventilation (17.3 vs. 21.4 days in the rezafungin and caspofungin groups, respectively, with a difference of 4.1 days) [Citation185].
7. Expert opinion
Candidemia and ICI are major causes of in-hospital morbidity and mortality, accounting for prolonged ICU/hospital stays and leading to excessive healthcare economic burden [Citation188,Citation189]. The advances in healthcare as well as the evolution of patient risk factors and the ability of some Candida species to spread within the hospital environment and cause outbreaks have led to a change in the epidemiology of invasive fungal infections [Citation3–5,Citation38]. As a matter of fact, despite C. albicans being still the most common, a continuous increase in non-albicans species has been observed in recent years, along with the emergence of worrisome and drug resistant species such as C. auris [Citation16,Citation38,Citation127].
The shift toward non-albicans species may be influenced by the different and evolving pathophysiology behind the development of candidemia/ICIs. In fact, while the endogenous route (consisting of Candida species passing through an intestinal barrier damaged by a variety of insults) is mostly commonly observed for C. albicans, C. tropicalis, C. glabrata (N. glabrata), C parapsilosis, and C. krusei (P. kudriavzeveii), the exogenous route (consisting in healthcare workers’ hands, patients’ skin or central lines contamination and subsequent spread into the blood) applies, above all, for C. auris, C. parapsilosis, and C. haemulonii [Citation16,Citation22].
Among the increasing proportion of infections caused by non-albicans Candida species, those caused by the emerging C. auris are of particular concern, given its long-lasting ability to colonize patients and the environment and its high rate of resistance to antifungals. Indeed, up to 40% of C. auris isolates may be resistant to at least two classes of antifungals, and resistance to echinocandins, although to a lesser extent, may also occur [Citation16]. Prompt and adequate source control as well as the adoption of effective infection control measures are key strategies to manage infected patients and reduce cross-transmission and outbreaks.
C. parapsilosis and C. auris share the ability to colonize central lines and cause nosocomial outbreaks. One of the main concerns lies in the fact that up to one-third of the strains are resistant to fluconazole, thus limiting the available therapeutic options to AmB or, as an alternative, to echinocandins [Citation31,Citation190].
Furthermore, some ICIs, such as endovascular infections, in which source control is not feasible, require prolonged treatment, up to weeks or months.
In all the aforementioned conditions, the availability of long-acting rezafungin is of paramount importance [Citation162]. In general terms, the advent of long-acting antimicrobial agents (e.g. dalbavancin) has represented a real revolution in the field of infectious diseases, enabling outpatient treatment of complex infections and allowing therapeutic switch in patients suitable for hospital discharge [Citation189–192]. As for fungal infections, rezafungin combines the strengths of the echinocandin class with a prolonged elimination half-life, a low likelihood of drug–drug interactions and a robust safety profile, thus opening up several possibilities for its future use [Citation16,Citation162].
In particular, it may allow earlier hospital discharge when clinical conditions are favorable and extend outpatient access to treatment of invasive candidiasis, especially when prolonged treatment duration is expected. Rezafungin’s high activity against Candida spp., including species resistant to azoles or C. auris, makes it highly attractive for the treatment of candidemia/ICI. An additional advantage may be the potential effect on hospital stay duration and the costs associated with candidemia/ICI, such as those related to the need of daily infusions and the use of central lines. Unfortunately, there are still limited data available on these interesting aspects and more studies are needed to ascertain the cost-effectiveness of rezafungin over other antifungals. Its pharmacological characteristics make rezafungin attractive also for the prophylaxis of invasive fungal diseases in high-risk populations [Citation16,Citation177].
8. Conclusion
In the recent years, candidemia and ICIs have undergone an important change in their epidemiology, with a shift toward non-albicans species as causative agents. In the meantime, the emergence of C. auris as a worrisome agent of nosocomial outbreaks and the growing rate of antifungal resistance in some species highlight the dynamic aspects of this evolving condition, which may result from the different underlying pathophysiology (endogenous vs. exogenous route of infection). In this scenario, the novel long-acting echinocandin rezafungin, which combines a potent in-vitro activity against Candida species, including azole-resistant strains, with a low likelihood of drug–drug interactions and a good safety profile, may revolutionize the treatment of candidemia/ICI. Indeed, it may allow earlier hospital discharge and management of complex fungal infections in an outpatient setting, with a possible, although not yet quantifiable, reduction in associated costs and length of hospital stay.
Article highlights
The advances in healthcare, the evolution of patient risk factors and the ability of some Candida species to spread within the hospital environment have led to a change in the epidemiology of Invasive Candida Infections (ICIs) in the last decades.
The shift toward non-albicans species may be influenced by the different and evolving pathophysiology behind the development of candidemia/ICI.
Rezafungin is a novel long-acting echinocandin with a potent in-vitro activity against most Candida spp., including those azole-resistant and Candida auris.
Rezafungin combines the strengths of the echinocandin class with a prolonged elimination half-life, a low likelihood of drug–drug interactions and a robust safety profile.
Registrative studies showed non-inferiority of rezafungin to caspofungin, with earlier candidemia clearance in patients on rezafungin than those receiving caspofungin.
Compared to caspofungin, survivors hospitalized in the ICU receiving rezafungin showed a reduction in ICU length of stay.
Rezafungin may allow earlier hospital discharge and management of complex fungal infections in an outpatient setting, especially when prolonged treatment duration is expected.
A possible, although not yet quantifiable, reduction in the costs associated with ICIs and length of hospital stay may be obtained with rezafungin.
Declaration of interest
A Oliva participated to advisory boards or speaker’s bureau for MSD, Zambon and Angelini. F G De Rosa participated to advisory boards or speaker’s bureau for Gilead, MSD, Pfizer. M Mikulska participated to advisory boards or speaker’s bureau for Gilead, Janssen, Mundipharma, bioMerieux e Pfizer. F Pea participated to advisory boards or speaker’s bureau for Advanz Pharma, Angelini, BeiGene, Gilead, Menarini, MSD, Pfizer, Shionogi. M Sanguinetti participated to advisory boards or speaker’s bureau for Mundipharma, Pfizer, BioMerieux. C Tascini participated to advisory boards or speaker’s bureau for Gilead, MSD, Pfizer and Mundipharma. M Venditti participated to advisory boards or speaker’s bureau for Gilead, MSD and Mundipharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium for their review work. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors should have substantially contributed to the conception and design of the review article and interpreting the relevant literature and have been involved in writing the review article or revised it for intellectual content.
Additional information
Funding
References
- Mareković I, Pleško S, Rezo Vranješ V, et al. Epidemiology of candidemia: three-year results from a Croatian tertiary care hospital. Journal Of Fungi. 2021;7(4):267. doi: 10.3390/jof7040267
- McCarty T, White C, Pappas P. Candidemia and invasive candidiasis. Infect Dis Clin North Am. 2021;35:389–413. doi: 10.1016/j.idc.2021.03.007
- Pappas PG, Lionakis MS, Arendrup MC, et al. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. doi: 10.1038/nrdp.2018.26.
- Lamoth F, Lewis RE, Kontoyiannis DP. Investigational antifungal agents for invasive mycoses: a clinical perspective. Clin Infect Dis. 2022;75(3):534–544. Erratum in: Clin Infect Dis. n;76(4):779 doi: 10.1093/cid/ciab1070
- Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25(10):1200–1212. doi: 10.1016/j.cmi.2019.04.024
- Falcone M, Concia E, Iori I, et al. Identification and management of invasive mycoses in internal medicine: a road-map for physicians. Intern Emerg Med. 2014;9(5):501–511. doi: 10.1007/s11739-014-1077-4
- Luzzati R, Cavinato S, Deiana ML, et al. Epidemiology and outcome of nosocomial candidemia in elderly patients admitted prevalently in medical wards. Aging Clin Exp Res. 2015;27(2):131–137. doi: 10.1007/s40520-014-0251-x
- Briongos-Figuero LS, Hernanz-Román L, Pineda-Alonso M, et al. In-hospital mortality due to infectious disease in an internal medicine department. epidemiology and risk factors. Eur Rev Med Pharmacol Sci Feb. 2015;19(4):567–572.
- Puig-Asensio M, Ruiz-Camps I, Fernández-Ruiz M, et al. Epidemiology and outcome of candidaemia in patients with oncological and haematological malignancies: results from a population-based surveillance in Spain. Clin Microbiol Infect. 2015;21(5):.e491.1–.e491.10. doi: 10.1016/j.cmi.2014.12.027
- Scudeller L, Bassetti M, Concia E, et al. MEDICAL group; Società Italiana di Terapia Antinfettiva (SITA); Federazione delle Associazioni dei Dirigenti Ospedalieri Internisti (FADOI). MEDical wards Invasive Candidiasis ALgorithms (MEDICAL): Consensus proposal for management. Eur J Intern Med. 2016;34:45–53. doi: 10.1016/j.ejim.2016.07.007
- Posteraro B, De Carolis E, Criscuolo M, et al. SEIFEM group. Candidaemia in haematological malignancy patients from a SEIFEM study: Epidemiological patterns according to antifungal prophylaxis. Mycoses. 2020;63(9):900–910. doi: 10.1111/myc.13130
- Atamna A, Eliakim-Raz N, Mohana J, et al. Predicting candidemia in the internal medicine wards: a comparison with gram-negative bacteremia-a retrospectives study. Diagn Microbiol Infect Dis. 2019 Sep;95(1):80–83. Epub 2019 Apr 26. PMID: 31129007. doi: 10.1016/j.diagmicrobio.2019.04.007
- Novosad SA, Fike L, Dudeck MA, et al. Pathogens causing central-line-associated bloodstream infections in acute-care hospitals-United States, 2011-2017. Infect Control Hosp Epidemiol. 2020 Mar;41(3):313–319. Epub 2020 Jan 9. PMID: 31915083. doi: 10.1017/ice.2019.303
- Pieralli F, Dentali F, Giusti M, et al.; FADOI—IFI Registry Group. Clinical characteristics, management and outcome of patients with invasive candidiasis hospitalized in internal medicine units: findings from a registry by the Italian scientific society FADOI. Infection. 2021;49(2):277–285. doi: 10.1007/s15010-020-01535-z.
- Bassetti M, Giacobbe DR, Vena A, et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care. 2019;23(1):219. doi: 10.1186/s13054-019-2497-3
- Giacobbe DR, Magnasco L, Sepulcri C, et al. Recent advances and future perspectives in the pharmacological treatment of Candida auris infections. Expert Rev Clin Pharmacol. 2021;14(10):1205–1220. doi: 10.1080/17512433.2021.1949285
- Wan Ismail WNA, Jasmi N, Khan TM, et al. The economic burden of candidemia and invasive candidiasis: a systematic review. Value Health Reg Issues. 2020 May;21:53–58.
- Zhao Y, Perlin DS. Review of the novel echinocandin antifungal rezafungin: animal studies and clinical data. J Fungi (Basel). 2020;6(4):192. doi: 10.3390/jof6040192
- Lamoth F. Novel therapeutic approaches to invasive candidiasis: considerations for the clinician. Infect Drug Resist. 2023;16:1087–1097. doi: 10.2147/IDR.S375625
- Sanchez V, Vazquez JA, Barth-Jones D, et al. Epidemiology of nosocomial acquisition of Candida lusitaniae. J Clin Microbiol. 1992 Nov;30(11):3005–3008. PMID: 1360476; PMCID: PMC270571. doi: 10.1128/jcm.30.11.3005-3008.1992
- Pagano L, Mele L, Fianchi L, et al. Chronic disseminated candidiasis in patients with hematologic malignancies. Clinical features and outcome of 29 episodes. Haematologica. 2002;87(5):535–541.
- Cornely OA, Bangard C, Jaspers NI. Hepatosplenic candidiasis. Clin Liver Dis. 2015;6(2):47–50. doi: 10.1002/cld.491
- GR T 3rd, Soriano A, Cornely OA, et al. ReSTORE trial investigators. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet. 2023;401(10370):49–59. doi: 10.1016/S0140-6736(22)02324-8
- Prigitano A, Cavanna C, Passera M. Evolution of fungemia in an Italian region. J Mycol Med. 2020;30(1):100906. doi: 10.1016/j.mycmed.2019.1009063
- Adam KM, Osthoff M, Lamoth F, et al. Fungal infection network of Switzerland (FUNGINOS). Trends of the epidemiology of candidemia in Switzerland: A 15-Year FUNGINOS Survey. Open Forum Infect Dis. 2021;8(10):ofab471. doi: 10.1093/ofid/ofab471
- De Rosa FG, Trecarichi EM, Montrucchio C, et al. Mortality in patients with early- or late-onset candidaemia. J Antimicrob Chemother. 2013;68(4):927–935. doi: 10.1093/jac/dks480
- Pfaller MA, Diekema DJ, Turnidge JD, et al. Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997–2016. Open Forum Infect Dis. 2019;6(Suppl 1):S79–S94. doi: 10.1093/ofid/ofy358
- Ko JH, Jung DS, Lee JY, et al. Changing epidemiology of non-albicans candidemia in Korea. J Infect Chemother. 2019 May;25(5):388–391. doi: 10.1016/j.jiac.2018.09.016. Epub 2018 Oct 26. PMID: 30482698.
- Tan TY, Hsu LY, Alejandria MM, et al. Antifungal susceptibility of invasive Candida bloodstream isolates from the Asia-Pacific region. Med Mycol. 54(5): Epub 2016 Feb 11. PMID: 26868904:471–477. 2016 Jul 1. 10.1093/mmy/myv114
- Yamin D, Husin A, Harun A. Distribution of candidemia in Malaysian tertiary care hospital revealed predominance of Candida parapsilosis. Trop Biomed. 2020 Dec 1;37(4):903–910. PMID: 33612744. doi: 10.47665/tb.37.4.903
- Mesini A, Mikulska M, Giacobbe DR, et al. Changing epidemiology of candidaemia: Increase in fluconazole-resistant Candida parapsilosis. Mycoses. 2020;63(4):361–368. doi: 10.1111/myc.13050
- Castanheira M, Deshpande LM, Messer SA, et al. Analysis of global antifungal surveillance results reveals predominance of Erg11 Y132F alteration among azole-resistant Candida parapsilosis and Candida tropicalis and country-specific isolate dissemination. Int J Antimicrob Agents. 2020;55(1):105799. doi: 10.1016/j.ijantimicag.2019.09.003
- Soldini S, Posteraro B, Vella A, et al. Microbiologic and clinical characteristics of biofilm-forming Candida parapsilosis isolates associated with fungaemia and their impact on mortality. Clin Microbiol Infect. 2018;24(7):771–777. doi: 10.1016/j.cmi.2017.11.005
- Alessandri F, Ceccarelli G, Migliara G, et al. High incidence of candidemia in critically Ill COVID-19 patients supported by veno-venous extracorporeal membrane oxygenation: a retrospective study. J Fungi (Basel). 2023;9(1):119. doi: 10.3390/jof9010119
- Li Y, Gu C, Yang Y, et al. Epidemiology, antifungal susceptibility, risk factors, and mortality of persistent candidemia in adult patients in China: a 6-year multicenter retrospective study. BMC Infect Dis. 2023 Jun 1;23(1):369. PMID: 37264301; PMCID: PMC10233919. doi: 10.1186/s12879-023-08241-9
- Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019;25(7):792–798. doi: 10.1016/j.cmi.2019.03.028
- Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am j med. 2012;125(1 Suppl):S3–13. doi: 10.1016/j.amjmed.2011.11.001
- Tortorano AM, Prigitano A, Morroni G, et al. Candidemia: evolution of drug resistance and novel therapeutic approaches. Infect Drug Resist. 2021;14:5543–5553. doi: 10.2147/IDR.S274872
- Cleary JD, Garcia-Effron G, Chapman SW, et al. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob Agents Chemother. 2008;52(6):2263–2265. doi: 10.1128/AAC.01568-07
- Satoh K, Makimura K, Hasumi Y, et al. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53(1):41–44. Erratum in: Microbiol Immunol. 2018;62(3):205. doi: 10.1111/j.1348-0421.2008.00083.x
- WHO fungal priority pathogens list to guide research, development and public health action. https://www.who.int/publications/i/item/9789240060241. [cited 27 Jun 2023]
- Desoubeaux G, Coste AT, Imbert C, et al. Overview about Candida auris: What’s up 12 years after its first description? J Mycol Med. 2022;32(2):101248. doi: 10.1016/j.mycmed.2022.101248.
- Borman AM, Fraser M, Johnson EM. CHROMagarTM Candida Plus: A novel chromogenic agar that permits the rapid identification of Candida auris. Med Mycol. 2021;59(3):253–258. doi: 10.1093/mmy/myaa049
- Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691.
- O’Brien B, Liang J, Chaturvedi S, et al. Pan-resistant Candida auris: New York subcluster susceptible to antifungal combinations. Lancet Microbe. 2020;1(5):e193–e194. 10.1016/S2666-5247(20)30090–2. doi: 10.1016/S2666-5247(20)30090-2
- Ostrowsky B, Greenko J, Adams E, et al. Candida auris Isolates resistant to three classes of antifungal medications - New York, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:6–9. doi: 10.15585/mmwr.mm6901a2
- Lyman M, Forsberg K, Reuben J, et al. Notes from the field: transmission of pan-resistant and Echinocandin-resistant Candida auris in health care facilities—Texas and the District of Columbia, January–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1022–1023. doi: 10.15585/mmwr.mm7029a2
- Jacobs SE, Jacobs JL, Dennis EK, et al. Candida auris pan-drug-resistant to four classes of antifungal agents. Antimicrob Agents Chemother. 2022 Jul 19;66(7):e0005322. doi: 10.1128/aac.00053-22
- Mulet-Bayona JV, Salvador-García C, Tormo-Palop N, et al. Recurrent candidemia and isolation of echinocandin-resistant Candida auris in a patient with a long-term central catheter. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40(6):334–335. doi: 10.1016/j.eimc.2021.04.003
- Ben Abid F, Salah H, Sundararaju S, et al. Molecular characterization of candida auris outbreak isolates in Qatar from patients with COVID-19 reveals the emergence of isolates resistant to three classes of antifungal drugs. Clin Microbiol Infect. 2023:S1198-743X(23)00199–4. doi: 10.1016/j.cmi.2023.04.025
- Eyre DW, Sheppard AE, Madder H, et al. A candida auris outbreak and its control in an intensive care setting. N Engl J Med. 2018;379(14):1322–1331. doi: 10.1056/NEJMoa1714373
- Garcia-Bustos V, Cabanero-Navalon MD, Ruiz-Saurí A, et al. What do we know about candida auris? state of the art, knowledge gaps, and future directions. Microorganisms. 2021;9(10):2177. doi: 10.3390/microorganisms9102177
- Rossow J, Ostrowsky B, Adams E, et al. New york candida auris investigation workgroup. factors associated with candida auris colonization and transmission in skilled nursing facilities with ventilator Units, New York, 2016-2018. Clin Infect Dis. 2021;72(11):e753–e760. doi: 10.1093/cid/ciaa1462
- Di Pilato V, Codda G, Ball L, et al. Molecular epidemiological investigation of a nosocomial cluster of C. auris: Evidence of recent emergence in Italy and Ease of Transmission during the COVID-19 Pandemic. J Fungi (Basel). 2021;7(2):140. doi: 10.3390/jof7020140
- Codda G, Willison E, Magnasco L, et al. In vivo evolution to echinocandin resistance and increasing clonal heterogeneity in Candida auris during a difficult-to-control hospital outbreak, Italy, 2019 to 2022. Euro Surveill. 2023;28(14). doi: 10.2807/1560-7917.ES.2023.28.14.2300161
- ECDC. Rapid risk assessment. https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-candida-auris-outbreak-healthcare-facilities-northern-italy [cited 27 Jun 2023].
- Thatchanamoorthy N, Rukumani Devi V, Chandramathi S, et al. Candida auris: A mini review on epidemiology in healthcare facilities in Asia. J Fungi (Basel). 2022 Oct 26;8(11): 1126. PMID: 36354893; PMCID: PMC9696804. doi: 10.3390/jof8111126.
- Sayeed MA, Farooqi J, Jabeen K, et al. Comparison of risk factors and outcomes of Candida auris candidemia with non-Candida auris candidemia: A retrospective study from Pakistan. Med Mycol. 2020;58(6):721–729. doi: 10.1093/mmy/myz112
- Simon SP, Li R, Silver M, et al. Comparative outcomes of candida auris bloodstream infections: a multicenter retrospective case-control study. Clin Infect Dis. 2023;76(3):e1436–e1443. doi: 10.1093/cid/ciac735
- Colombo AL, Júnior JNA, Guinea J. Emerging multidrug-resistant Candida species. Curr Opin Infect Dis. 2017;30(6):528–538. doi: 10.1097/QCO.0000000000000411
- Ramos LS, Figueiredo-Carvalho MHG, Silva LN, et al. The threat called candida haemulonii species complex in rio de Janeiro State, Brazil: focus on antifungal resistance and virulence attributes. J Fungi (Basel). 2022;8(6):574. doi: 10.3390/jof8060574
- Mendoza-Reyes DF, Gómez-Gaviria M, Mora-Montes HM. Candida lusitaniae: biology, pathogenicity, virulence factors, diagnosis, and treatment. Infect Drug Resist. 2022 Aug 31;15:5121–5135. PMID: 36068831; PMCID: PMC9441179 10.2147/IDR.S383785
- Linkhorn RJ, Adelstein D, Spagnuolo PJ. Emergence of a new opportunistic pathogen, Candida lusitaniae. J Clin Microbiol. 1989;27(2):236–240. doi:10.1128/jcm.27.2.236-240.1989
- Jung DS, Farmakiotis D, Jiang Y, et al. Uncommon Candida species fungemia among cancer patients, Houston, Texas, USA. Emerg Infect Dis. 2015;21(11):1942–1950. doi: 10.3201/eid2111.150404
- Fowler SL, Rhoton B, Springer SC, et al. Evidence for person-to-person transmission of Candida lusitaniae in a neonatal intensive-care unit. Infect Control Hosp Epidemiol. 1998;19:343–345. doi: 10.2307/30141376
- Asner SA, Giulieri S, Diezi M, et al. Acquired multidrug antifungal resistance in candida lusitaniae during therapy. Antimicrob Agents Chemother. 2015;59(12):7715–7722. doi: 10.1128/AAC.02204-15
- Scott NE, Edwin Erayil S, Kline SE, et al.Rapid Evolution of Multidrug Resistance in a Candida lusitaniae Infection during Micafungin Monotherapy.Antimicrob Agents Chemother. 2023 Jul 10;e0054323. doi: 10.1128/aac.00543-23
- Arendrup MC, Bruun B, Christensen JJ, et al. National surveillance of fungemia in Denmark (2004 to 2009). J Clin Microbiol. 2011 Jan;49(1):325–334. doi: 10.1128/JCM.01811-10
- Casagrande Pierantoni D, Bernardo M, Mallardo E, et al. Candida palmioleophila isolation in Italy from two cases of systemic infection, after a CHROMagar and Vitek system misidentification as C. albicans. New Microbiol. 2020 Jan;43(1):47–50.
- Pappas PG, Kauffman CA, Andes D, et al. Infectious diseases society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the infectious diseases society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757
- Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102(3):433–444. doi: 10.3324/haematol.2016.152900
- Martin-Loeches I, Antonelli M, Cuenca-Estrella M, et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive care Med. 2019;45(6):789–805. doi: 10.1007/s00134-019-05599-w
- Grim SA, Berger K, Teng C, et al. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: correlation with outcomes. J Antimicrob Chemother. 2012;67(3):707–714. doi: 10.1093/jac/dkr511
- Kollef M, Micek S, Hampton N, et al. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54(12):1739–1746. doi: 10.1093/cid/cis305
- Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43(1):25–31. doi: 10.1086/504810
- Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49(9):3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005
- Clancy CJ, Nguyen MH, Kraft CS. Diagnosing Invasive Candidiasis. J Clin Microbiol. 2018;56(5):e01909–17. doi: 10.1128/JCM.01909-17
- Gonzalez-Lara MF, Ostrosky-Zeichner L. Invasive Candidiasis. Semin Respir Crit Care Med. 2020;41(1):3–12. doi: 10.1055/s-0040-1701215
- Karageorgopoulos DE, Vouloumanou EK, Ntziora F, et al. β-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52(6):750–770. doi: 10.1093/cid/ciq206
- Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-β-D-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50(1):7–15. doi: 10.1128/JCM.05267-11
- He S, Hang JP, Zhang L, et al. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-β-D-glucan for invasive fungal infection: Focus on cutoff levels. J Microbiol Immunol Infect. 2015;48(4):351–361. doi: 10.1016/j.jmii.2014.06.009
- Posteraro B, De Pascale G, Tumbarello M, et al. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1→3)-β-D-glucan assay, Candida score, and colonization index. Crit Care. 2011;15(5):R249. doi: 10.1186/cc10507
- Corcione S, Chasseur L, Lupia T, et al. The DIAGNOSTIC RELEVAnce of β-D-Glucan for candidemia within internal medicine wards. Diagnostics. 2022;12(9):2124. doi: 10.3390/diagnostics12092124
- Pfaller MA, Wolk DM, Lowery TJ. T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiol. 2016;11(1):103–117. doi: 10.2217/fmb.15.111
- Giannella M, Paolucci M, Roncarati G, et al. Potential role of T2Candida in the management of empirical antifungal treatment in patients at high risk of candidaemia: a pilot single-centre study. J Antimicrob Chemother. 2018;73(10):2856–2859. doi: 10.1093/jac/dky247
- Neely LA, Audeh M, Phung NA, et al. T2 magnetic resonance enables nanoparticle-mediated rapid detection of candidemia in whole blood. Sci Transl Med. 2013;5(182):182ra54. doi: 10.1126/scitranslmed.3005377
- Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis. 2015;60(6):892–899. doi: 10.1093/cid/ciu959
- Bizerra FC, Jimenez-Ortigosa C, Souza AC, et al. Breakthrough candidemia due to multidrug-resistant Candida glabrata during prophylaxis with a low dose of micafungin. Antimicrob Agents Chemother. 2014;58(4):2438–2440. doi: 10.1128/AAC.02189-13
- Cuervo G, Garcia-Vidal C, Nucci M, et al. Breakthrough candidaemia in the era of broad-spectrum antifungal therapies. Clin Microbiol Infect. 2016;22(2):181–188. doi: 10.1016/j.cmi.2015.09.029
- Paphitou NI, Ostrosky-Zeichner L, Rex JH. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med Mycol. 2005;43(3):235–243. doi: 10.1080/13693780410001731619
- León C, Ruiz-Santana S, Saavedra P, et al. Cava Study Group. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37(5):1624–1633. doi: 10.1097/CCM.0b013e31819daa14
- Hermsen ED, Zapapas MK, Maiefski M, et al. Validation and comparison of clinical prediction rules for invasive candidiasis in intensive care unit patients: a matched case-control study. Crit Care. 2011;15(4):R198. doi: 10.1186/cc10366
- Ostrosky-Zeichner L, Pappas PG, Shoham S, et al. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit. Mycoses. 2011;54(1):46–51. doi: 10.1111/j.1439-0507.2009.01756.x
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of america. Clin Infect Dis. 2016;62(4):e1–50. doi: 10.1093/cid/civ933
- Kato H, Hagihara M, Shibata Y, et al. Comparison of mortality between echinocandins and polyenes for an initial treatment of candidemia: A systematic review and meta-analysis. J Infect Chemother. 2021;27(11):1562–1570. doi: 10.1016/j.jiac.2021.06.017
- Mora-Duarte J, Betts R, Rotstein C, et al.; Caspofungin Invasive Candidiasis Study Group. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347(25):2020–2029. doi: 10.1056/NEJMoa021585
- Micafungin Invasive Candidiasis Working Group, Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369(9572):1519–1527.
- Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007 Jun 14;356(24):2472–2482. doi: 10.1056/NEJMoa066906
- Reboli AC, Shorr AF, Rotstein C, et al. Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis. 2011;11(1):261. doi: 10.1186/1471-2334-11-261
- Mycoses Study Group, Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54(8):1110–1122.
- Kale-Pradhan PB, Morgan G, Wilhelm SM, et al. Comparative efficacy of echinocandins and nonechinocandins for the treatment of Candida parapsilosis Infections: a meta-analysis. Pharmacotherapy. 2010;30(12):1207–1213. doi: 10.1592/phco.30.12.1207
- Fernández-Ruiz M, Aguado JM, Almirante B, et al. CANDIPOP Project; GEIH-GEMICOMED (SEIMC); REIPI. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clin Infect Dis. 2014;58(10):1413–1421. doi: 10.1093/cid/ciu158
- Thompson GR, Soriano A, Skoutelis A, et al. Rezafungin versus Caspofungin in a Phase 2, Randomized, Double-blind Study for the Treatment of Candidemia and Invasive Candidiasis: The STRIVE Trial. Clin Infect Dis. 2021;73(11):e3647–e3655. Erratum in: Clin Infect Dis. 2021;73(3):561-562. doi: 10.1093/cid/ciaa1380
- De Rosa FG, Corcione S, Filippini C, et al. The Effect on mortality of fluconazole or echinocandins treatment in candidemia in internal medicine wards [corrected]. Plos One. 2015;10(5):e0125149. Erratum in: PLoS One. 2015;10(6):e0131264. doi: 10.1371/journal.pone.0125149
- Cleveland AA, Harrison LH, Farley MM, et al. Declining incidence of candidemia and the shifting epidemiology of candida resistance in two US metropolitan areas, 2008–2013: results from population-based surveillance. Plos One. 2015;10(3):e0120452. doi: 10.1371/journal.pone.0120452
- Kullberg BJ, Viscoli C, Pappas PG, et al. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive candida infections: The ACTIVE Trial. Clin Infect Dis. 2019;68(12):1981–1989. doi: 10.1093/cid/ciy827
- Falcone M, Tiseo G, Gutiérrez-Gutiérrez B, et al. GISA (Italian Group for Antimicrobial Stewardship). Impact of initial antifungal therapy on the outcome of patients with candidemia and septic shock admitted to medical wards: a propensity score-adjusted analysis. Open Forum Infect Dis. 2019 Jul 16: 6(7):ofz251.
- López-Cortés LE, Almirante B, Cuenca-Estrella M, et al. Members of the CANDIPOP Project from GEIH-GEMICOMED (SEIMC) and REIPI. Empirical and targeted therapy of candidemia with fluconazole versus echinocandins: a propensity score-derived analysis of a population-based, multicentre prospective cohort. Clin Microbiol Infect. 2016;22(8):733.e1–8. doi: 10.1016/j.cmi.2016.05.008
- Rodrigues CF, Henriques M. Liposomal and deoxycholate amphotericin B formulations: effectiveness against biofilm infections of CANDIDa spp. Pathogens. 2017;6(4):62. doi: 10.3390/pathogens6040062
- Sharma D, Paul RA, Rudramurthy SM, et al. Impact of FKS1 genotype on echinocandin in vitro susceptibility in candida auris and in vivo response in a murine model of infection. Antimicrob Agents Chemother. 2022;66(1):e0165221. doi: 10.1128/AAC.01652-21
- Rybak JM, Barker KS, Muñoz JF, et al. In vivo emergence of high-level resistance during treatment reveals the first identified mechanism of amphotericin B resistance in Candida auris. Clin Microbiol Infect. 2022;28(6):838–843. doi: 10.1016/j.cmi.2021.11.024
- Bidaud AL, Djenontin E, Botterel F, et al. Colistin interacts synergistically with echinocandins against Candida auris. Int J Antimicrob Agents. 2020 Mar;55(3):105901. Epub 2020 Jan 17. PMID: 31954831. doi: 10.1016/j.ijantimicag.2020.105901
- Caballero U, Kim S, Eraso E, et al. In Vitro Synergistic Interactions of Isavuconazole and Echinocandins against Candida auris. Antibiotics (Basel). 2021 Mar 28;10(4):355. PMID: 33800601; PMCID: PMC8066733. doi: 10.3390/antibiotics10040355
- Nagy F, Tóth Z, Nyikos F, et al. In vitro and in vivo interaction of caspofungin with isavuconazole against Candida auris planktonic cells and biofilms. Med Mycol. 59(10): PMID: 34021571; PMCID: PMC8653632:1015–1023. 2021 Oct 4. doi: 10.1093/mmy/myab032
- Balla N, Kovács F, Balázs B, et al. SynergistiC interaction of caspofungin combined with posaconazole against fks wild-type and mutant candida auris planktonic cells and biofilms. Antibiotics (Basel). 11(11): PMID: 36421245; PMCID: PMC9686983:1601. 2022 Nov 11. doi: 10.3390/antibiotics11111601
- Schwarz P, Bidaud AL, Dannaoui E. In vitro synergy of isavuconazole in combination with colistin against Candida auris. Sci Rep. 2020 Dec 8;10(1):21448. PMID: 33293607; PMCID: PMC7722718. doi: 10.1038/s41598-020-78588-5
- Treviño-Rangel RJ, González GM, Montoya AM, et al. Recent Antifungal Pipeline Developments against Candida auris: A Systematic Review. J Fungi (Basel). 2022;8(11):1144. doi: 10.3390/jof8111144
- Wiederhold NP, Najvar LK, Olivo M, et al. Ibrexafungerp Demonstrates in vitro Activity against Fluconazole-Resistant Candida auris and in vivo Efficacy with Delayed Initiation of Therapy in an Experimental Model of Invasive Candidiasis. Antimicrob Agents Chemother. 2021;65(6):e02694–20. doi: 10.1128/AAC.02694-20
- Wiederhold NP, Najvar LK, Shaw KJ, et al. Efficacy of Delayed Therapy with Fosmanogepix (APX001) in a Murine Model of Candida auris Invasive Candidiasis. Antimicrob Agents Chemother. 2019;63(11):e01120–19. doi: 10.1128/AAC.01120-19
- Pfaller MA, Huband MD, Flamm RK, et al. Antimicrobial activity of manogepix, a first-in-class antifungal, and comparator agents tested against contemporary invasive fungal isolates from an international surveillance programme (2018-2019). J Glob Antimicrob Resist. 2021;26:117–127. doi: 10.1016/j.jgar.2021.04.012
- Vazquez J, Reboli AC, Pappas PG, et al. Evaluation of an early step-down strategy from intravenous anidulafungin to oral azole therapy for the treatment of candidemia and other forms of invasive candidiasis: results from an open-label trial. BMC Infect Dis. 2014;14(1):97. doi: 10.1186/1471-2334-14-97
- Moreno-García E, Puerta-Alcalde P, Gariup G, et al. Early Stepdown from Echinocandin to Fluconazole Treatment in Candidemia: A Post Hoc Analysis of Three Cohort Studies. Open Forum Infect Dis. 2021;8(6):ofab250. Erratum in: Open Forum Infect Dis. 2022;9(6):ofac180. doi: 10.1093/ofid/ofab250
- Soulountsi V, Schizodimos T, Kotoulas SC. Deciphering the epidemiology of invasive candidiasis in the intensive care unit: is it possible? Infection. 2021;49(6):1107–1131. doi: 10.1007/s15010-021-01640-7
- Nguyen MH, Wissel MC, Shields RK, et al. Performance of Candida real-time polymerase chain reaction, β-D-glucan assay, and blood cultures in the diagnosis of invasive candidiasis. Clin Infect Dis. 2012;54(9):1240–1248. doi: 10.1093/cid/cis200
- Fortún J, Meije Y, Buitrago MJ, et al. Clinical validation of a multiplex real-time PCR assay for detection of invasive candidiasis in intensive care unit patients. J Antimicrob Chemother. 2014;69(11):3134–3141. doi: 10.1093/jac/dku225
- Bassetti M, Righi E, Ansaldi F, et al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive care Med. 2015;41(9):1601–1610. doi: 10.1007/s00134-015-3866-2
- Logan C, Martin-Loeches I, Bicanic T. Invasive candidiasis in critical care: challenges and future directions. Intensive care Med. 2020;46(11):2001–2014. doi: 10.1007/s00134-020-06240-x
- Tirpack AR, Duker JS, Baumal CR. An Outbreak of Endogenous Fungal Endophthalmitis Among Intravenous Drug Abusers in New England. JAMA Ophthalmol. 2017;135(6):534–540. doi: 10.1001/jamaophthalmol.2017.0650
- Haseeb AA, Elhusseiny AM, Siddiqui MZ, et al. Fungal Endophthalmitis: A Comprehensive Review. J Fungi (Basel). 2021;7(11):996. doi: 10.3390/jof7110996
- Venditti M. Clinical aspects of invasive candidiasis: endocarditis and other localized infections. Drugs. 2009;69(1):39–43. doi: 10.2165/11315610-000000000-00000
- Venditti M, De Bernardis F, Micozzi A, et al. Fluconazole treatment of catheter-related right-sided endocarditis caused by Candida albicans and associated with endophthalmitis and folliculitis. Clin Infect Dis. 1992;14(2):422–426. doi: 10.1093/clinids/14.2.422
- Giuliano S, Guastalegname M, Russo A, et al. Candida endocarditis: systematic literature review from 1997 to 2014 and analysis of 29 cases from the Italian Study of Endocarditis. Expert Rev Anti Infect Ther. 2017;15(9):807–818. doi: 10.1080/14787210.2017.1372749
- van Hal SJ, Stark D, Harkness J, et al. Candida dubliniensis meningitis as delayed sequela of treated C. dubliniensis fungemia. Emerg Infect Dis. 2008;14(2):327–329. doi: 10.3201/eid1402.070985
- De Meo D, Cera G, Ceccarelli G, et al. Candida fracture-related infection: a systematic review. J Bone Jt Infect. 2021;6(7):321–328. doi: 10.5194/jbji-6-321-2021
- Majeed A, Ullah W, Zahid U, et al. Persistent spontaneous fungal peritonitis secondary to Candida albicans in a patient with alcoholic cirrhosis and review of the literature. BMJ Case Rep. 2016;2016:bcr2016216979. doi: 10.1136/bcr-2016-216979
- Hwang SY, Yu SJ, Lee JH, et al. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis. 2014;33(2):259–264. doi: 10.1007/s10096-013-1953-2
- Bassetti M, Marchetti M, Chakrabarti A, et al. A research agenda on the management of intra-abdominal candidiasis: results from a consensus of multinational experts. Intensive care Med. 2013;39(12):2092–2106. doi: 10.1007/s00134-013-3109-3
- Kauffman CA. Diagnosis and management of fungal urinary tract infection. Infect Dis Clin North Am. 2014;28(1):61–74. doi: 10.1016/j.idc.2013.09.004
- Tan WP, Turba UC, Deane LA. Renal fungus ball: a challenging clinical problem. Urologia. 2017;84(2):113–115. doi: 10.5301/uro.5000201
- Ideguchi S, Yamamoto K, Ikeda A, et al. A case of bilateral emphysematous pyelonephritis caused by Candida albicans. J Infect Chemother. 2019;25(4):302–306. doi: 10.1016/j.jiac.2018.10.011
- Rivoisy C, Vena A, et al; French Mycoses Study Group and Grupo de Apoyo al Manejo de las Endocarditis en España (GAMES). Prosthetic Valve Candida spp. Endocarditis: New Insights into Long-term Prognosis-The ESCAPE Study. Clin Infect Dis. 2018;66(6):825–832. doi:10.1093/cid/cix913
- Falcone M, Barzaghi N, Carosi G, et al. Candida infective endocarditis: report of 15 cases from a prospective multicenter study. Medicine (Baltimore). 2009;88(3):160–168. Italian Study on Endocarditis (SEI). doi: 10.1097/MD.0b013e3181a693f8
- Russo A, Falcone M, Picciarella A, et al. Candidaemia after heart valve replacement surgery: recurrence as prosthetic valve endocarditis is an expected over one-year complication. Clin Microbiol Infect. 2016;22(5):466–467. doi: 10.1016/j.cmi.2016.01.019
- Torres-Rojas JR, Stratton CW, Sanders CV, et al. Candidal suppurative peripheral thrombophlebitis. Ann Intern Med. 1982;96(4):431–435. doi: 10.7326/0003-4819-96-4-431
- Caccese R, Carfagna P, Pistilli N, et al. Candidal thrombophlebitis of central veins: case report and review. Med Mycol. 2012;50(3):299–304. doi: 10.3109/13693786.2011.604046
- Strinden WD, Helgerson RB, Maki DG. Candida septic thrombosis of the great central veins associated with central catheters Clinical Features And Management. Ann Surg. 1985;202(5):653–658. doi: 10.1097/00000658-198511000-00019
- Tahir M, Peseski AM, SJ J. Case Report: Candida dubliniensis as a Cause of Chronic Meningitis. Front Neurol. 2020;11:601242. doi: 10.3389/fneur.2020.601242
- Yamahiro A, Lau KH, Peaper DR, et al. Meningitis Caused by Candida Dubliniensis in a Patient with Cirrhosis: A Case Report and Review of the Literature. Mycopathologia. 2016;181(7–8):589–593. doi: 10.1007/s11046-016-0006-7
- Fennelly AM, Slenker AK, Murphy LC, et al. Candida cerebral abscesses: a case report and review of the literature. Med Mycol. 2013;51(7):779–784. doi: 10.3109/13693786.2013.789566
- Kelly L, Walsh J, Skally M, et al. Candida meningitis/ventriculitis over a decade. Increased morbidity and length of stay a concern. Br J Neurosurg. 2023;37(2):227–230. doi: 10.1080/02688697.2022.2054947
- López-Dupla M, Mora Sanz P, Pintado García V, et al. Clinical, endoscopic, immunologic, and therapeutic aspects of oropharyngeal and esophageal candidiasis in HIV-infected patients: a survey of 114 cases. Am J Gastroenterol. 1992;87(12):1771–1776.
- Chen YH, Jao TM, Shiue YL, et al. Prevalence and risk factors for Candida esophagitis among human immunodeficiency virus-negative individuals. World J Clin Cases. 2022;10(30):10896–10905. doi: 10.12998/wjcc.v10.i30.10896
- Candon S, Rammaert B, Foray AP, et al. Chronic Disseminated Candidiasis During Hematological Malignancies: An Immune Reconstitution Inflammatory Syndrome with Expansion of Pathogen-Specific T Helper Type 1 Cells. J Infect Dis. 2020;221(11):1907–1916. doi: 10.1093/infdis/jiz688
- McLeod N, Fisher M, Lasala PR. Vertebral osteomyelitis due to Candida species. Infection. 2019;47(3):475–478. doi: 10.1007/s15010-019-01294-6
- Slenker AK, Keith SW, Horn DL. Two hundred and eleven cases of Candida osteomyelitis: 17 case reports and a review of the literature. Diagn Microbiol Infect Dis. 2012;73(1):89–93. doi: 10.1016/j.diagmicrobio.2012.02.004
- Gamaletsou MN, Rammaert B, Brause B, et al. International Consortium for Osteoarticular Mycoses Osteoarticular Mycoses. Clin Microbiol Rev. 2022 Dec 21;35(4):e0008619. doi: 10.1128/cmr.00086-19
- Koutserimpas C, Zervakis SG, Maraki S, et al. Non-albicans Candida prosthetic joint infections: A systematic review of treatment. World J Clin Cases. 2019;7(12):1430–1443. doi: 10.12998/wjcc.v7.i12.1430
- Vergidis P, Clancy CJ, Shields RK, et al. Intra-Abdominal Candidiasis: The Importance of Early Source Control and Antifungal Treatment. Plos One. 2016;11(4):e0153247. doi: 10.1371/journal.pone.0153247
- Pea F. Current pharmacological concepts for wise use of echinocandins in the treatment of Candida infections in septic critically ill patients. Expert Rev Anti Infect Ther. 2013;11(10):989–997. doi: 10.1586/14787210.2013.836058
- Pappas PG. Lessons learned in the multistate fungal infection outbreak in the United States. Curr Opin Infect Dis. 2013;26(6):545–550. doi: 10.1097/QCO.0000000000000013
- Wilson Dib R, Chaftari AM, Hachem RY, et al. Catheter-Related Staphylococcus aureus Bacteremia and Septic Thrombosis: The Role of Anticoagulation Therapy and Duration of Intravenous Antibiotic Therapy. Open Forum Infect Dis. 2018;5(10):ofy249. doi: 10.1093/ofid/ofy249
- Garcia-Effron G. Rezafungin-Mechanisms of Action, Susceptibility and Resistance: Similarities and Differences with the Other Echinocandins. J Fungi (Basel). 2020;6(4):262. doi: 10.3390/jof6040262
- Adeel A, Qu MD, Siddiqui E, et al. Expanded Access Use of Rezafungin for Salvage Therapy of Invasive Candida glabrata Infection: A Case Report. Open Forum Infect Dis. 2021;8(11):ofab431. doi: 10.1093/ofid/ofab431
- Krishnan BR, James KD, Polowy K, et al. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J Antibiot (Tokyo). 2017;70(2):130–135. doi: 10.1038/ja.2016.89
- Szymański M, Chmielewska S, Czyżewska U, et al. Echinocandins - structure, mechanism of action and use in antifungal therapy. J Enzyme Inhib Med Chem. 2022;37(1):876–894. doi: 10.1080/14756366.2022.2050224
- Helleberg M, Jørgensen KM, Hare RK, et al. Rezafungin in vitro Activity against Contemporary Nordic Clinical Candida Isolates and Candida auris Determined by the EUCAST Reference Method. Antimicrob Agents Chemother. 2020 Mar 24;64(4):e02438–19. doi: 10.1128/AAC.02438-19
- Arendrup MC, Meletiadis J, Zaragoza O, et al. Multicentre determination of rezafungin (CD101) susceptibility of Candida species by the EUCAST method. Clin Microbiol Infect. 2018 Nov;24(11):1200–1204. doi: 10.1016/j.cmi.2018.02.021
- Pfaller MA, Carvalhaes C, Messer SA, et al. Activity of a Long-Acting Echinocandin, Rezafungin, and Comparator Antifungal Agents Tested against Contemporary Invasive Fungal Isolates (SENTRY Program, 2016 to 2018). Antimicrob Agents Chemother. 2020 Mar 24;64(4):e00099–20. doi:10.1128/AAC.00099-20
- Tóth Z, Forgács L, Locke JB, et al. In vitro activity of rezafungin against common and rare Candida species and Saccharomyces cerevisiae. J Antimicrob Chemother. 2019 Dec 1;74(12):3505–3510. doi: 10.1093/jac/dkz390
- Hoenigl M, Sprute R, Egger M, et al. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs. 2021;81(15):1703–1729. doi: 10.1007/s40265-021-01611-0
- Ong V, Wills S, Watson D, et al. Metabolism, Excretion, and Mass Balance of [14C]-Rezafungin in Animals and Humans. Antimicrob Agents Chemother. 2022;66(1):e0139021. doi: 10.1128/AAC.01390-21
- Ong V, Hough G, Schlosser M, et al. Preclinical Evaluation of the Stability, Safety, and Efficacy of CD101, a Novel Echinocandin. Antimicrob Agents Chemother. 2016;60(11):6872–6879. doi: 10.1128/AAC.00701-16
- Flanagan S, Walker HE, Ong V. Coadministration of Rezafungin Does Not Impact the Pharmacokinetics of Cyclosporine, Ibrutinib, Mycophenolate Mofetil, or Venetoclax. Open Forum Infect Dis. 2022 Dec;9(Supplement_2):ofac492. doi: 10.1093/ofid/ofac492.1324
- Flanagan S, Jandourek A, Ong V, et al. Coadministration of rezafungin does not impact cyclosporine or mycophenolate mofetil pharmacokinetics. Bone Marrow Transplant. 2022;57:455–456.
- Jang SM, Hough G, Mueller BA. Ex vivo Rezafungin Adsorption and Clearance During Continuous Renal Replacement Therapy. Blood Purif. 2018;46(3):214–219. doi: 10.1159/000489212
- Flanagan S, Goodman DB, Jandourek A, et al. Lack of Effect of Rezafungin on QT/QTc Interval in Healthy Subjects. Clin Pharmacol Drug Dev. 2020 May;9(4):456–465.
- Ham YY, Lewis JS, Thompson GR. Thompson GR 3rd. Rezafungin: a novel antifungal for the treatment of invasive candidiasis. Future Microbiol. 2021 2nd;16(1):27–36. doi: 10.2217/fmb-2020-0217
- Lakota EA, Ong V, Flanagan S, et al. Population Pharmacokinetic Analyses for Rezafungin (CD101) Efficacy Using Phase 1 Data. Antimicrob Agents Chemother. 2018;62(6):e02603–17. Erratum in: Antimicrob Agents Chemother. 2019;63(4). doi: 10.1128/AAC.02603-17
- Lepak AJ, Zhao M, Andes DR. Determination of Pharmacodynamic Target Exposures for Rezafungin against Candida tropicalis and Candida dubliniensis in the Neutropenic Mouse Disseminated Candidiasis Model. Antimicrob Agents Chemother. 2019;63(11):e01556–19. doi: 10.1128/AAC.01556-19
- Lepak AJ, Zhao M, VanScoy B, et al. Pharmacodynamics of a Long-Acting Echinocandin, CD101, in a Neutropenic Invasive-Candidiasis Murine Model Using an Extended-Interval Dosing Design. Antimicrob Agents Chemother. 2018;62(2):e02154–17. doi: 10.1128/AAC.02154-17
- Lepak AJ, Zhao M, Andes DR. Pharmacodynamic Evaluation of Rezafungin (CD101) against Candida auris in the Neutropenic Mouse Invasive Candidiasis Model. Antimicrob Agents Chemother. 2018;62(11):e01572–18. doi: 10.1128/AAC.01572-18
- Lakota EA, Bader JC, Ong V, et al. Pharmacological Basis of CD101 Efficacy: Exposure Shape Matters. Antimicrob Agents Chemother. 2017;61(11):e00758–17. doi: 10.1128/AAC.00758-17
- Bader JC, Lakota EA, Flanagan S, et al. Overcoming the Resistance Hurdle: Pharmacokinetic-Pharmacodynamic Target Attainment Analyses for Rezafungin (CD101) against Candida albicans and Candida glabrata. Antimicrob Agents Chemother. 2018;62(6):e02614–17. doi: 10.1128/AAC.02614-17
- Hager CL, Larkin EL, Long LA, et al. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother. 2018;73(8):2085–2088. doi: 10.1093/jac/dky153
- Bielicka I, Dickerson S, Manamley N, et al. CO89 Integrated Analysis of Efficacy and ICU Length of Stay Data from Phase 2 and Phase 3 Trials of Rezafungin for the Treatment of Invasive Candidiasis and/or Candidemia. Value Health. 2002;25(12):S35. doi: 10.1016/j.jval.2022.09.168
- Pechacek J, Yakubu I, Vissichelli NC, et al. Successful expanded access use of rezafungin, a novel echinocandin, to eradicate refractory invasive candidiasis in a liver transplant recipient. J Antimicrob Chemother. 2022 Aug 25;77(9):2571–2573. doi: 10.1093/jac/dkac206
- Melenotte C, Ratiney R, Hermine O, et al. Successful Rezafungin Treatment of an Azole-Resistant Chronic Mucocutaneous Candidiasis in a STAT-1 Gain-of-Function Patient. J Clin Immunol. 2023;43(6):1182–1184. doi: 10.1007/s10875-023-01519-2
- Martini C, Torelli R, de Groot T, et al. Prevalence and Clonal Distribution of Azole-Resistant Candida parapsilosis Isolates Causing Bloodstream Infections in a Large Italian Hospital. Front Cell Infect Microbiol. 2020;10:232. doi: 10.3389/fcimb.2020.00232
- Volpicelli L, Venditti M, Oliva A. Acute bacterial skin and skin structure infections in pediatric patients: potential role of dalbavancin. Expert Rev Anti Infect Ther. 2023;21(4):329–341. doi: 10.1080/14787210.2023.2182769
- Andreoni M, Bassetti M, Corrao S, et al. The role of dalbavancin for Gram positive infections in the COVID-19 era: state of the art and future perspectives. Expert Rev Anti Infect Ther. 2021;19(9):1125–1134. doi: 10.1080/14787210.2021.1894130
- Oliva A, Stefani S, Venditti M, et al. Biofilm-Related Infections in Gram-Positive Bacteria and the Potential Role of the Long-Acting Agent Dalbavancin. Front Microbiol. 2021;12:749685. doi: 10.3389/fmicb.2021.749685
- Oliva A, Carbonara S, Cianci V, et al. Direct or early Discharge of Acute Bacterial Skin and Skin Structure Infection patients from the Emergency Department/Unit: place in therapy of dalbavancin. Expert Rev Anti Infect Ther. 2023;31:1–19. doi: 10.1080/14787210.2023.2214727