ABSTRACT
Introduction: Calmodulin (CaM) is a highly conserved Ca2+-binding protein that is exceptionally abundant in the brain. In the presynaptic compartment of neurons, CaM transduces changes in Ca2+ concentration into the regulation of synaptic transmission dynamics.
Areas covered: We review selected literature including published CaM interactor screens and outline established and candidate presynaptic CaM targets. We present a workflow of biochemical and structural proteomic methods that were used to identify and characterize the interactions between CaM and Munc13 proteins. Finally, we outline the potential of ion mobility-mass spectrometry (IM-MS) for conformational screening and of protein-protein cross-linking for the structural characterization of CaM complexes.
Expert commentary: Cross-linking/MS and native MS can be applied with considerable throughput to protein mixtures under near-physiological conditions, and thus effectively complement high-resolution structural biology techniques. Experimental distance constraints are applicable best when obtained by combining different cross-linking strategies, i.e. by using cross-linkers with different spacer length and reactivity, and by using the incorporation of unnatural photo-reactive amino acids. Insights from structural proteomics can be used to generate CaM-insensitive mutants of CaM targets for functional studies in vitro or ideally in vivo.
1. Introduction
Calcium ions (Ca2+) are central cellular messengers in an extraordinarily high number of cellular processes, including gene transcription, cell proliferation, apoptosis, muscle contraction, and mitochondrial function. How can Ca2+ exert an effect on so many processes? Multiple proteins can directly bind Ca2+ via Ca2+-binding domains in their primary sequence (e.g. EF hands, C2 domains). Alternatively, an array of proteins have evolved that bind Ca2+ across a broad concentration range and additionally multiple target proteins, thereby regulating their activity in a Ca2+-dependent manner. A large group of such proteins, typically grouped as the EF hand superfamily, is expressed in neurons [Citation1–Citation3]. The prototype of this group is calmodulin (CaM), a small acidic protein (148 amino acids, ~17 kDa) with an exceptionally high sequence conservation among eukaryotes [Citation4], and sequence identity across vertebrates. CaM is expressed ubiquitously in all eukaryotic cell types. The high concentrations of CaM in the brain (up to 100 μM) [Citation5–Citation7] may reflect its importance for nervous system function. Among the myriad of cellular functions regulated by CaM [Citation8], we focus here on synaptic transmission.
CaM consists of an N- and a C-terminal globular domain, each containing two Ca2+-binding helix-loop-helix motifs of the EF-hand type. These domains (here referred to as N- and C-lobes) have a considerable backbone flexibility that is key to their ability to bind a wide range of targets [Citation9] and are connected by a flexible linker. Ca2+ binds to CaM in a cooperative manner (Kd = 5 · 10–7 M to 5 · 10–6 M), with the C-lobe EF hands having a three- to fivefold higher affinity for Ca2+ than the N-lobe EF hands [Citation10]. CaM undergoes dramatic structural rearrangements upon Ca2+ binding, including conformational changes within the EF hands and the repositioning of the N- and C-lobes [Citation11]. These conformational changes expose hydrophobic surfaces where protein–protein interaction can occur. It is therefore postulated that the various Ca2+-bound conformational states of CaM allow for unique interactions with target proteins [Citation11–Citation13].
A recent analysis of the >80 unique CaM complex structures deposited in the Protein Data Bank (PDB) as of 2013 [Citation11] impressively highlights, that a defined consensus sequence for CaM binding does not exist, and that target sequences often show very low homology. Nonetheless, CaM-binding sites do share several characteristic features, including a high helical propensity, a net positive charge, and the presence of hydrophobic anchor residues. The spacing of the hydrophobic anchor residues historically serves as criterion to the classification of CaM-binding motifs, such as in the prototypic 1–14 and 1–5–10 motifs of myosin light chain kinase (MLCK) and CaM-dependent protein kinase II (CaMKII), respectively. The recognition of such features has been implemented into bioinformatic tools for the prediction of CaM-binding sites [Citation14,Citation15], and novel concepts in computational biology (e.g. conversion of biological sequences into feature vectors to be combined with machine-learning algorithms [Citation16,Citation17]) may lead to refined, ideally web server-based prediction tools.
CaM can bind its target proteins either in a Ca2+-free form (apo-CaM) or in one of its Ca2+-bound forms. The interactions are tightly dependent on the surrounding Ca2+-concentration and can range from transient to irreversible. Thus, a variety of regulation modes by CaM have been described [Citation10]. Prominent examples include the activation of enzymes like CaMKII and calcineurin, where the binding of Ca2+/CaM interferes with an interaction between an autoinhibitory domain and the catalytic domain [Citation18,Citation19], and inositol-1,4,5-trisphosphate receptors (IP3 R), which are inhibited by the binding of Ca2+/CaM [Citation20]. Further structural rearrangements in CaM occur upon binding to the target protein. For example, in the well-studied cases of CaM-dependent kinases like MLCK (1–14 motif) and CaMKII (1–5–10 motif), binding leads to a rather compact CaM structure in the complexes [Citation21–Citation23]. In contrast, when the flexible linker region between the lobes is fully extended, CaM can adopt an open conformation, and the anchoring residues in the target protein can theoretically be as far as 70 Å apart [Citation11].
CaM has been functionally optimized through evolution to bind to a large number of target proteins with high specificity [Citation24]. Accordingly, a wide variety of methods have been applied for the experimental discovery of CaM binding partners, including probing of expression libraries [Citation25], mRNA display techniques [Citation26], affinity chromatography followed by mass spectrometric protein identification [Citation27], and protein arrays [Citation28], and hundreds of proteins are now suggested to bind to CaM. However, a tremendous amount of research is necessary for the experimental verification of such interactions and, more importantly, for the investigation of their biological significance. Consequently, only a fraction of these putative interactions has been experimentally verified.
In the present review, we focus on the interactions of CaM in the presynaptic compartment of neurons where CaM serves as a regulator of multiple processes in the synaptic vesicle (SV) cycle. Understanding the network of interactions between Ca2+, CaM, and the presynaptic target proteins is essential for understanding the function of these proteins and the dynamics of synaptic transmission. We summarize a list of established presynaptic CaM targets, and of candidate presynaptic CaM targets that we extracted from published CaM interactor screens (). We then present a workflow of biochemical and structural proteomic methods that we have used to identify and characterize the interaction between the presynaptic Munc13 proteins and CaM, and to gain insight into the structure of their complexes. Finally, we provide an outlook on the potential of ion mobility-mass spectrometry (IM-MS) as a tool for conformational screening and discuss peptide–protein vs. protein–protein cross-linking techniques for the structural characterization of CaM complexes.
Table 1. Potential and established presynaptic CaM targets. The table contains a list of presynaptic CaM-binding proteins from the Calmodulin Target Database (Ikura group, I [Citation15]) and from three recent CaM interactor screens (James group, J [Citation27]; Liu group, L [Citation26]; Cahill group, C [Citation28]. Proteins were selected as presynaptic based on available information in the UniProtKB database, prior knowledge, and comprehensive literature search. For each entry, we list the protein name, UniProtKB accession number, gene name, source organism (SO; Mus musculus, M; Rattus norvegicus, R; Homo sapiens, H), molecular weight (MW), and refer to the database/screen (DB/S) where the CaM interaction was described. For proteins that are directly associated with synaptic vesicles (SVs) or with the SV cycle, we also list the step in which they are presumed to exert their effect. In cases where the interaction with CaM was biochemically confirmed or where the CaM-binding site is identified, the PubMed ID (PMID) is given to indicate key papers where these findings were made.
2. Calmodulin at the presynapse
2.1. Calmodulin targets within the synaptic vesicle cycle
Neurons of the mammalian brain are connected to form large networks, in which the computation of neuronal activity occurs. Neurons are connected via specialized structures called synapses, where the information from a transmitting, presynaptic neuron is conveyed to a receiving, postsynaptic neuron. At the basis of this process lies the presynaptic SV cycle [Citation29] that occurs repeatedly in billions of synapses of the mammalian brain and is orchestrated by hundreds of proteins, many of which are regulated by Ca2+. In chemical synapses, the information arrives at the synapse in the form of a transient membrane depolarization. This electric signal evokes the opening of voltage-gated Ca2+ channels, giving rise to a dramatic increase in the presynaptic Ca2+ concentration, from basal presynaptic Ca2+ levels as low as ~25 nM [Citation30] to a level of >1 μM estimated during high-frequency trains [Citation31]. The influx of Ca2+ from the extracellular space drives the fusion of neurotransmitter-filled SVs with the presynaptic plasma membrane. Released neurotransmitter binds to receptors on the postsynaptic neuron and this can generate the electrical signal in the postsynaptic neuron. After fusion, the SV membrane and its proteins, which are now imbedded in the presynaptic membrane, are endocytosed and recycled for reuse. SVs are refilled with neurotransmitter, translocated to the plasma membrane and tethered to it. A subset of these SVs is then prepared for the next round of fusion in a step called priming [Citation32]. Primed SVs form the pool of readily releasable SVs (RRP) and are capable of fusing with the plasma membrane at the next depolarization-induced Ca2+ influx.
The influx of Ca2+ into the presynaptic compartment creates a steep and local Ca2+ gradient that decays with the distance from the Ca2+ channels and within milliseconds after the channel opening. Periods of repetitive activity, however, result in accumulation of residual Ca2+ that is slowly cleared during periods of rest [Citation31]. This broad-range Ca2+ signal drives and coordinates the various steps of the SV cycle by regulating the activation and inactivation of multiple proteins, depending on their Ca2+ sensitivity and distance from the Ca2+ channels. CaM serves as a key player in this regulation, and two major functions have been proposed for CaM at the presynapse: (i) as a sensor protein that distributes the Ca2+ signal in its variable amplitudes amongst downstream targets, transforming it into specific changes in function, and (ii) as a fast Ca2+ buffer [Citation33,Citation34], binding Ca2+ with high affinity. Together, these roles converge to affect multiple parameters of synaptic transmission. In we summarize a list of presynaptic proteins to which CaM binding has been experimentally demonstrated, or where a putative interaction was identified in an array of screens [Citation26–Citation28] and still needs to be experimentally confirmed. Despite the key regulatory role of CaM in synaptic transmission, there are no estimates on its concentration in the presynapse to the best of our knowledge. The estimates prevailing in the literature [Citation5,Citation33] refer to the total CaM concentration, which is derived from the analysis of whole brain lysates, including neuronal and glial cells.
The expression of CaM is mediated by three genes encoding identical proteins and is essential for cell survival, making it impossible to study knock-out models. Two recent papers describe the effects of reduced CaM expression on synaptic transmission using a knock-down (KD) strategy in cortical neurons [Citation35,Citation36]. A reduction of 70% in CaM expression levels led to a specific and exclusive effect on presynaptic function in the form of a decreased synaptic strength (defined as the relative amplitude of the postsynaptic response that is generated by the activity of the presynaptic neuron). This result supports the notion of a role of high CaM concentration in enhancing synaptic transmission, but is somewhat contradictory to the proposed role of high CaM concentration in Ca2+ buffering. The authors suggest that the reduced synaptic strength results from an effect of CaM on the SV release machinery at the level of the Ca2+-dependent triggering of release, and show that the expression of a constitutively active CaMKII(alpha) rescued the deficiency. This suggests that a downstream phosphorylation of CaM-bound CaMKII(alpha) target(s), rather than a direct interaction of CaM with a target protein, is essential for the effect of CaM on synaptic strength. CaMKII has been shown to predominantly act as a postsynaptic second messenger in longer lasting forms of synaptic plasticity, and its multiple downstream targets have been reviewed extensively [Citation37]. Synatotagmin 1, the Ca2+ sensors for fast, synchronous SV fusion has a phosphorylation site that can be phosphorylated by protein kinase C (PKC) and CaMKII [Citation38,Citation39], but a deletion of this site did not lead to a reduction in synaptic strength in neurons expressing the mutated protein [Citation40]. Moreover, the possibility of a direct interaction of synaptotagmin 1 and 2 with CaM was recently excluded [Citation41]. Two prominent presynaptic CaMKII targets are synapsin-1a and rabphilin 3A [Citation37], which are phosphorylated in an activity-dependent manner, but involved in SV mobilization upstream of fusion. Active CaMKII has also been suggested to bind and regulate syntaxin 1 [Citation42], a SNARE (soluble NSF attachment protein receptor [Citation43]) protein. Syntaxin 1 is essential for SV docking and fusion because it interacts with synaptobrevin1/2 and SNAP 25 to form trans-SNARE complexes, creating a bridge between the SV membrane and the plasma membrane. Reduced synaptic strength can also result from reduced Ca2+ influx via the presynaptic P/Q voltage-gated Ca2+ channels, which bind both apo-CaM and Ca2+-loaded CaM. The C-lobe of CaM interacts with the IQ domain of the channel with high affinity, and upon Ca2+ entry this interaction leads to a Ca2+-dependent facilitation of the current. The N-lobe of CaM interacts with a different CaM-binding domain in the channel with lower affinity, leading to a Ca2+-dependent inactivation of the current [Citation44]. However, it is unlikely that CaM KD affects these interactions, which are reported to occur with very high affinity. Of note, CaM KD also leads to a change in the transcription of some 250 genes, including a fivefold increase in the transcription of synaptotagmin 2 [Citation36], making the effects of the CaM KD complicated to dissect.
While a CaM KD strategy affects every compartment of the neuron, selective interference with presynaptic CaM is possible in a few specialized synapses, where the presynaptic compartment is large enough for direct infusion of CaM inhibitors via a micropipette (i.e. the recording unit of the patch-clamp set-up). One such giant synapse is the calyx of Held, a glutamatergic brainstem synapse of the auditory pathway. In the calyx of Held, CaM inhibitors slow down the rate of endocytosis, but it is under debate whether CaM is mandatory for all forms of endocytosis [Citation45,Citation46]. This effect is thought to be mediated by the dephosphorylation of endocytic proteins by calcineurin, a Ca2+/CaM-activated phosphatase [Citation45], and MLCK was also suggested to be involved [Citation47]. Interestingly, introduction of CaM inhibitors into the calyx of Held leads to the decoupling of the endocytosis of the presynaptic membrane from that of synaptotagmin 2, indicating that CaM can selectively differentiate between the recycling mechanisms for membranes and vesicle proteins [Citation48]. By using CaM inhibitors in the calyx of Held, a crucial role for CaM was also demonstrated in the recovery of the RRP, the pool of primed SVs that are ready to undergo fusion. In particular, the recovery rate of the rapidly releasing, slowly recovering SV pool was hindered in the presence of CaM inhibitors [Citation49].
To further understand such effects at the molecular level, key residue(s) involved in CaM binding can be mutated in candidate proteins, making them CaM-insensitive. Such a strategy, which is highly specific, but requires prior knowledge of the CaM-binding site in a putative candidate, was used to abolish the interaction of the SV priming proteins of the Munc13 family with CaM. Munc13s are essential for the generation of the RRP. As described in more detail in the next section, Munc13s all bind to CaM in a Ca2+-dependent manner [Citation50,Citation51]. Abolishing CaM binding by mutating residues in the CaM-binding site of all Munc13 isoforms lead to a slower refilling rate of the RRP, resulting in a reduced synaptic strength during and after trains of synaptic activity in cultured hippocampal neurons expressing the respective mutant isoform [Citation50,Citation51]. To demonstrate the importance of this regulation in intact neuronal networks, a knock-in mouse line was generated where the interaction between Munc13-1 and CaM was abolished by a single point mutation of the anchoring residue at motif position 1 of the Munc13-1 CaM-binding site. Calyx of Held synapses were studied, and reduced replenishment rate of the RRP, together with reduced synaptic strength during and after high-frequency activity were observed [Citation52].
Other prominent presynaptic CaM targets include the Ca2+-dependent K+ channels of the SK family, where CaM functions as a permanently bound, regulatory unit of the channel and mediates Ca2+-dependent channel activation. SK channels are important for the termination of the action potential, which consequently limits the influx of Ca2+ into the presynapse and ends SV fusion [Citation53]. Myosin 5a is an unconventional myosin motor protein involved in transport of organelles along actin filaments. It consists of a pair of heavy chains, each containing six IQ motifs to which CaM is bound. At submicromolar Ca2+ concentrations, myosin 5a is active, but at higher concentrations CaM dissociates from the heavy chains, leading to inhibition of motility, at least in vitro [Citation54]. Myosin 5a is implicated in regulating SV exocytosis and synaptic plasticity via multiple pre- and postsynaptic roles including SV mobilization [Citation54] and a putative interaction with syntaxin [Citation55]. Members of the Rab3 protein family are GTP-binding proteins associated with SVs that exert a complex effect on SV release probability and recovery from SV depletion in hippocampal neurons [Citation56]. An interaction of Rab3s with CaM was demonstrated biochemically [Citation57] but the physiological role of this interaction remains unclear and controversial [Citation58,Citation59]. Synaptobrevin 2 is an essential SV-protein for the fusion of SVs, as it constitutes a part of the SNARE complex. Synaptobrevin 2 was shown to bind CaM in a Ca2+-dependent manner [Citation60,Citation61] and microinjection of the CaM-binding peptide of synaptobrevin 2 lowered the exocytotic frequency. However, in vitro assays measuring SNARE complex formation in the presence of Ca2+/CaM [Citation62,Citation63] gave rise to conflicting results, and it remains unclear whether SNARE-mediated SV fusion is modulated by CaM. Another candidate for Ca2+/CaM-dependent regulation of SNARE complex assembly is the neuron-specific vesicular ATPase V0 subunit a1 (V100), which is part of the multisubunit proton pump that acidifies vesicles. While Ca2+/CaM regulation of the V100 isoform in the fruit fly Drosophila melanogaster is not required for vesicle acidification, it was suggested to control SNARE complex assembly for a subset of SVs that are then spontaneously released [Citation64,Citation65]. Mover, a regulator of presynaptic release probability, was recently shown to bind CaM [Citation66], but the implications of this interaction remain to be studied. An interesting recent finding is the interaction of CaM with synaptotagmin 7, a protein implicated in synchronous and asynchronous SV release [Citation67], Ca2+-dependent recovery of SVs [Citation41], and in the facilitation of synaptic transmission [Citation68]. This interaction was biochemically demonstrated, but its physiological implications remain to be studied.
Taken together, CaM is a key protein that interacts with multiple presynaptic targets to produce a complex effect on synaptic transmission. The identity of these targets and the physiological effect of their interaction with CaM have been hard to resolve. In the next section, we review in detail the biochemical and structural characterization of the Ca2+/CaM-dependent regulation of the SV priming process by Munc13s, which is one of the well-understood examples, not only from a physiological, but also from a structural point of view.
2.2. The Munc13 case
The Munc13 protein family comprises of four neuronal isoforms (Munc13-1, ubMunc13-2, bMunc13-2 and Munc13-3 [Citation69,Citation70]), which display a neuronal subtype-specific expression pattern and a presynaptic localization. Munc13-1 is the major isoform and is expressed in essentially all neurons (exceptions are described in [Citation71,Citation72]). The presence of Munc13s is essential for the completion of the SV cycle: in synapses lacking Munc13s SVs fail to attach to the plasma membrane due to a block of trans-SNARE complex assembly [Citation73–Citation76]. The functional consequence of this failure is a complete block of spontaneous and evoked synaptic transmission, resulting in the immediate death of Munc13-1/2 double-knock-out mice after birth [Citation77]. Munc13s are regulated in an activity-dependent manner via a diacylglycerol- and phorbol ester-binding C1 domain [Citation78,Citation79], a Ca2+/CaM-binding domain [Citation50], and, as discovered more recently, a C2 domain (C2B) that binds to Ca2+ and phospholipids [Citation80]. Together, these regulatory domains modulate Munc13 function to affect multiple parameters of synaptic transmission, including the size of the RRP, the rate of RRP recovery from depletion, synaptic strength, and the SV release probability. Alterations in synaptic strength during synaptic activity are collectively named short-term synaptic plasticity (STP) and, at the molecular level, can be interpreted as transient effects of the residual Ca2+ on the pre- and postsynaptic molecular machinery [Citation81,Citation82]. STP is of fundamental importance for many brain functions, including neuronal network oscillations, the computation of motor, somatosensory, and auditory inputs, or working memory [Citation83]. Indeed, Munc13 activity is essential for various parameters of STP in neurons in culture and in slice preparation [Citation50–Citation52,Citation79,Citation80,Citation84]. The central and essential role of Munc13 in synaptic transmission emerged recently also in form of its involvement in human neurological and neuropsychiatric disease. A gain of function Munc13-1 mutation was recently identified in a patient with a dyskinetic movement disorder, delayed cognitive development, and autistic features [Citation85]. Another patient was identified with a complete loss of Munc13-1 due to a homozygous nonsense mutation, suffering from a fatal syndrome with microcephaly, cortical hyperexcitability, and myasthenia [Citation86]. In view of the above, studying the regulation of Munc13s is essential for the understanding of physiology and pathophysiology of synaptic transmission.
The interaction between the Munc13-1 homolog Unc13A and CaM, along with the CaM-binding sequence involved, was initially identified by probing a retinal expression library from Drosophila melanogaster [Citation25]. Subsequently, the interaction was identified for the mouse/rat Munc13-1 in a yeast two hybrid screen [Citation50]. Based on the homology with the CaM-binding site of Unc13A, the putative CaM-binding sites in Munc13-1 and in the highly homologous ubMunc13-2 were identified, and corresponding photo-reactive peptides were synthesized by incorporation of a benzophenone moiety [Citation87,Citation88]. These peptides were used for photoaffinity labeling (PAL) of CaM to demonstrate stoichiometric CaM binding to this minimal CaM-binding site. This interaction was then confirmed for the full-length protein by cosedimentation assays, demonstrating the usefulness of short peptides as protein mimetics for initial biochemical validation, at least in this particular case. A more challenging task was to identify and characterize CaM binding in the related isoforms bMunc13-2 and Munc13-3 as their N-terminal parts lack sequence homology with Munc13-1 and ubMunc13-2. A computational prediction of CaM-binding site(s) based on the primary protein sequence [Citation15] identified two potential sites in each isoform [Citation89]. PAL of CaM with these four potential CaM-binding peptides showed that at the peptide level, all four sites were able to bind CaM. However, in cosedimentation experiments only one site in each isoform was functional [Citation51]. Therefore, results from bioinformatic prediction of CaM-binding sites and from cross-linking with short peptides have to be interpreted with caution and always backed up by biochemical confirmation at the protein level.
During our initial PAL studies, we found that the affinity to CaM is not compromised when the first hydrophobic anchor residue of the CaM-binding peptides (Trp in Munc13-1 and ubMunc13-2; Phe in bMunc13-2 and Munc13-3) is replaced by the photo-reactive amino acid para-benzoyl-phenylalanine (Bpa), most likely because the hydrophobic, bulky, and aromatic features of the anchor position remained largely unchanged ((a)). This is in contrast to other peptide-protein interaction systems, where peptidic ligands can often only be modified at their termini without loss of biological activity (e.g. peptide hormones [Citation88]). We designed the photoreactive Munc13 peptides in a way that the photophore is incorporated into a position known to be relevant for binding, thereby increasing the likelihood that the interface between the CaM-binding site and CaM is targeted and that the distance constraints derived from MS analysis of the photoadducts indeed represent direct contact sites between Munc13 and CaM. To gain first insights into the structure of CaM/Munc13 complexes, we combined PAL with chemical cross-linking (XL) using different types of bifunctional reagents. In contrast to PAL, where the reactive group is directly incorporated into the peptide sequence as a modified amino acid, XL reagents introduce a covalent bond between the functional groups of two amino acids that are in spatial proximity within the CaM/peptide complex (see below for details). Common to both approaches is that by MS analysis of the covalent complexes, the distance between the covalently connected amino acids is derived, providing experimental constraints for molecular modeling approaches. PAL-MS reveals constraints that are typically low in number, but tight in distance, whereas the information obtained by XL-MS is often complementary, i.e. giving rise to several constraints with rather loose distances ((a)). In our view, it is the combination of these complementary cross-linking strategies that leads to the most meaningful input for the distance constraint-directed generation of structural models.
Figure 1. Characterization of the CaM-Munc13 interaction.
(a.) Photoaffinity labeling (PAL) and chemical cross-linking (XL) complement each other in the generation of structural models. As exemplified here on the basis of the molecular modeling of the CaM/Munc13 peptide complexes, the use of photoreactive peptides containing Bpa instead of the hydrophobic anchor residue Trp or Phe (see structures for similarity of the amino acid side chains) at position 1 of the CaM-binding motif, typically reveals a low number of tight constraints, resulting in the docking solutions shown in grey (left). The contact site of the anchor residue can be positioned with high precision, whereas the peptide C-terminus can adopt many orientations due to the lack of constraints for this region. In contrast, the structural model derived from XL alone (right) has less degrees of freedom with regard to ligand orientation and overall structure, indicating that the increase in constraint number balances the increase in constraint length. Nevertheless, the absence of tight constraints from PAL hampered the exact localization of the peptide N-terminus as indicated by a relatively high deviation from the best-matching structure in this region (CaM, green; nNOS peptide, red; PDB 2O60). High-quality structural models were finally obtained when the two complementary sets of constraints from PAL and XL were combined and led to the conclusion that all Munc13 isoforms employ a common mode of binding, despite a lack of sequence homology between their CaM-binding sites. In the sequence alignment, bold font represents the peptides used in the initial cross-linking experiments. Residues homologous to Munc13-1 are underlined. Position 1 of the CaM-binding motifs is marked by an asterisk. Structural models were modified from [Citation51,Citation90].(b.) Sequence alignment of C-terminally extended CaM-binding motifs in unc-13 proteins from different species. Hydrophobic anchor residues in motif position 1, 5, 8 (red) and 26 (green) are marked if present. Residues homologous to Munc13-1 from Homo sapiens are underlined. The following protein sequences were used (NCBI accession in parentheses): Homo sapiens, protein unc-13 homolog A (NP_001073890); Rattus norvegicus, protein unc-13 homolog A (NP_074052); Mus musculus, protein unc-13 homolog A (NP_001025044); Danio rerio, protein unc-13 homolog A (NP_001038630); Caenorhabditis elegans, UNC-13 (AAA93094); Drosophila melanogaster, unc-13 isoform A (NP_651949).
![Figure 1. Characterization of the CaM-Munc13 interaction.(a.) Photoaffinity labeling (PAL) and chemical cross-linking (XL) complement each other in the generation of structural models. As exemplified here on the basis of the molecular modeling of the CaM/Munc13 peptide complexes, the use of photoreactive peptides containing Bpa instead of the hydrophobic anchor residue Trp or Phe (see structures for similarity of the amino acid side chains) at position 1 of the CaM-binding motif, typically reveals a low number of tight constraints, resulting in the docking solutions shown in grey (left). The contact site of the anchor residue can be positioned with high precision, whereas the peptide C-terminus can adopt many orientations due to the lack of constraints for this region. In contrast, the structural model derived from XL alone (right) has less degrees of freedom with regard to ligand orientation and overall structure, indicating that the increase in constraint number balances the increase in constraint length. Nevertheless, the absence of tight constraints from PAL hampered the exact localization of the peptide N-terminus as indicated by a relatively high deviation from the best-matching structure in this region (CaM, green; nNOS peptide, red; PDB 2O60). High-quality structural models were finally obtained when the two complementary sets of constraints from PAL and XL were combined and led to the conclusion that all Munc13 isoforms employ a common mode of binding, despite a lack of sequence homology between their CaM-binding sites. In the sequence alignment, bold font represents the peptides used in the initial cross-linking experiments. Residues homologous to Munc13-1 are underlined. Position 1 of the CaM-binding motifs is marked by an asterisk. Structural models were modified from [Citation51,Citation90].(b.) Sequence alignment of C-terminally extended CaM-binding motifs in unc-13 proteins from different species. Hydrophobic anchor residues in motif position 1, 5, 8 (red) and 26 (green) are marked if present. Residues homologous to Munc13-1 from Homo sapiens are underlined. The following protein sequences were used (NCBI accession in parentheses): Homo sapiens, protein unc-13 homolog A (NP_001073890); Rattus norvegicus, protein unc-13 homolog A (NP_074052); Mus musculus, protein unc-13 homolog A (NP_001025044); Danio rerio, protein unc-13 homolog A (NP_001038630); Caenorhabditis elegans, UNC-13 (AAA93094); Drosophila melanogaster, unc-13 isoform A (NP_651949).](/cms/asset/0ef9ffe7-f70d-447a-b789-f801d7c02433/ieru_a_1275966_f0001_oc.jpg)
Conceptual and methodical aspects of cross-linking/MS approaches such as cross-link generation and enrichment, mass spectrometric identification of cross-linked peptides, data analysis algorithms, and molecular modeling have been reviewed extensively [Citation91–Citation98] and will be covered only briefly in the last section of this article. Here, we would like to point out two specific workflow refinements that facilitated the characterization of CaM/peptide complexes in particular and may be applicable to other systems in general. First, identification of cross-linked peptides by MS is considerably facilitated by the use of stable isotope labeling, which can be introduced via deuterated amine-reactive reagents [Citation99], 13C-labeled Bpa [Citation100] or the inclusion of 15N-CaM as reference protein [Citation90]. Second, pinpointing the cross-linked sites down to the level of individual amino acid residues is greatly facilitated by the use of complementary mass spectrometric strategies including different ionization and fragmentation techniques. As branched cross-linked peptides often require multiple charging for a reasonable fragmentation yield, on-line electrospray (ES)-MS is most commonly applied in cross-linking workflows and the complementarity of different fragmentation modes including collision-induced, higher-energy collisional, and electron transfer dissociation (CID, HCD, ETD), as well as hybrid versions thereof are beginning to be explored [Citation100–Citation103]. Matrix-assisted laser desorption/ionization (MALDI)-MS as an off-line method, although generating mainly singly charged ions, is a powerful alternative enabling the sequence analysis of cross-linked peptides without temporal constraints or even on-target chemical treatments, e.g. with CNBr for cleavage at Met residues or confirmation of Bpa-Met-photoadducts [Citation90,Citation104]. This particular linkage is frequently observed in PAL experiments with CaM for several reasons: (i) CaM is a Met-rich protein (6% in contrast to the ~1% average), (ii) Met residues constitute virtually half of its hydrophobic binding interfaces that are exposed to the solvent upon Ca2+ binding and thus play a key role in target recognition [Citation24,Citation105], and (iii) the methyl group of the Met side chain is one of the major targets of the benzophenone radical in line with its preference to react with C-H bonds adjacent to heteroatoms [Citation87].
PAL-MS and XL-MS of CaM with ~20 amino acid peptides representing the minimal CaM-binding sites of all four Munc13 isoforms, followed by molecular modeling, revealed a common binding mode for all Munc13 proteins ((a)), indicating that the Ca2+/CaM-dependent regulation of priming activity is structurally conserved throughout the entire Munc13 protein family [Citation51,Citation90]. The common model structure is of overall compact nature and is reminiscent of the CaM/neuronal NO synthase (nNOS) peptide complex (PDB 2O60), in which CaM wraps around the target peptide by binding through an 1–5–8 motif (or a derivative thereof) in an antiparallel orientation (i.e. the C-terminal part of the target is engaged with the N-lobe of CaM, while the N-terminal part of the target is engaged with the C-lobe of CaM). The functional role of CaM binding is also conserved in all Munc13 isoforms: during periods of high frequency activity, steady-state synaptic strength is increased by Ca2+/CaM binding, which results in a unique pattern of STP induced by the expression of each isoform [Citation50–Citation52]. Taken together, this led us to postulate that the composition of Munc13 isoforms in a neuron contributes to its STP characteristics. Evolution of four Munc13 isoforms enables the mammalian brain to differentially shape STP outputs in distinct synapses and thereby fine-tune neuronal plasticity. Although certainly challenging because of the promiscuity of CaM, a long-term aim of structural studies of CaM/Munc13 complexes is the design of small molecule inhibitors to specifically interfere with this interaction in vivo. Such inhibitors can be useful for mechanistic studies on Munc13 function and may also have therapeutic implications where negative pharmacological interference with SV fusogenicity is desired to dampen strong synaptic activity.
2.3. A novel calmodulin-binding motif in Munc13s
While binding of the N-terminal part of the Munc13 peptides to the C-lobe of CaM was clearly evident in the structural models and supported by numerous experimental constraints, we never reached the same level of confidence for the contacts between the C-terminus of the peptides and the N-lobe of CaM. In contrast to the 1–5–8–14 motif of nNOS, the hydrophobic anchor residue in motif position 14 is missing in Munc13-1 and ubMunc13-2, resulting in more degrees of freedom when positioning the C-terminal part of the Munc13 peptides. We therefore C-terminally elongated the CaM-binding peptides derived from Munc13-1 and ubMunc13-2 to include an additional conserved hydrophobic cluster downstream of the 1–5–8 motif. The resulting structural models from PAL and NMR spectroscopy confirmed our initial model with regard to the C-lobe, but also revealed an additional conserved hydrophobic contact between a Trp residue in position 31 of the peptide (corresponding to position 26 of the motif) and the N-lobe, thereby defining a novel 1–5–8–26 CaM recognition motif in the 34-amino acid Munc13 peptides [Citation106]. With a spacing of 41 Å between the hydrophobic anchor residues, the 1–5–8–26 motif represents the longest linear motif described among peptides so far. While this motif is highly conserved in mammalian and zebrafish Munc13-1 sequences, homology drops considerably in lower species, such as fruit fly and nematode where CaM-Munc13 binding was established experimentally [Citation25,Citation107]. However, the spacing of the anchoring residues is at least partly conserved, and in the nematode even the characteristic C-terminal hydrophobic cluster is apparent ((b)).
A hallmark of the complex between CaM and the C-terminally elongated Munc13-1 peptide (PDB 2KDU) is its modular architecture [Citation106]. The interacting modules form separate structural elements that are connected via flexible linkers in both, CaM and Munc13 peptide, thus allowing them to adopt a characteristic extended conformation. Corroborative PAL-MS experiments readily confirmed this open structure of the complex at near-physiological protein concentration and buffer conditions for C-terminally elongated peptides derived from both, Munc13-1 and ubMunc13-2, highlighting the advantages and the complementary nature of proteomic methods even when high-resolution structures are available.
NMR titration experiments indicated that the modular architecture and the extended conformation may underlie a sequential formation of CaM/Munc13 complexes [Citation106]. According to these data, the contact to the C-lobe occurs first already at Ca2+ levels just above resting concentration, followed by the contact to the N-lobe at higher Ca2+ concentrations. This sequential binding mode may enable CaM/Munc13 complexes to sense transient increases in Ca2+ concentrations over a broad concentration range, which may be an essential feature to fulfill their role in regulating SV priming. Of note, Munc13s are large (~200 kDa) multidomain proteins (see [Citation51] for domain structure) and structural information is only available for some domains of Munc13-1, i.e. for the C2A domain [Citation108], C1 domain [Citation109], C2B domain [Citation80], and MUN domain [Citation76]. Structural information is still missing for the region containing the CaM-binding domain, and it is thus still unclear how the activation of Munc13s by CaM works mechanistically. Intriguingly, bMunc13-2 and Munc13-3 contain neither the 1–5–8–26 CaM-binding motif of Munc13-1/ubMunc13-2, nor a related motif with comparable spacing of hydrophobic anchor residues. Nevertheless, as electrophysiological data indicate a conserved regulation by CaM binding, it is possible that some type of sequential interaction does occur during the CaM binding of all Munc13 isoforms [Citation51]. Hydrophobic cluster that could serve as additional contact sites may be present up- or down-stream of the motif in bMunc13-2/Munc13-3, and the existence of three-dimensional interaction surfaces (i.e. contact sites that are spatially close, but remote in the primary structure) can also not be excluded. To test this, structural characterization of CaM complexes with longer peptides or protein fragments under near-physiological conditions is necessary.
2.4. Is the 1–5–8–26 calmodulin-binding motif unique?
The discovery of a novel 1–5–8–26 CaM-binding motif in Munc13-1 and ubMunc13-2 raised several questions. Are longer CaM-binding motifs a unique feature of Munc13s? Or were such motifs just overlooked so far, because shorter peptides were mainly used in structural investigations of CaM/target interactions? What are the determinants of a longer CaM-binding motif that lead to an extended CaM conformation? To address these questions, we used the CaM-binding peptide of skeletal muscle MLCK (skMLCK) as the prototype for the 1–5–8–14 motif and gradually transformed it into a Munc13-1-like 1–5–8–26 CaM-binding motif by C-terminal elongation, removal of the hydrophobic anchor residue in motif position 14 (Phe of skMLCK replaced by Glu of Munc13-1) and by introduction of a more bulky, hydrophobic residue in motif position 26 (Leu of skMLCK replaced by Trp of Munc13-1). Our structural proteomic data on the respective CaM complexes [Citation110] were only compatible with compact structure models, indicating that CaM-binding motifs like that of Munc13-1 likely exhibit unique structural features that promote the extended conformation of CaM, and depend not only on the spacing of the hydrophobic anchor residues. This study and others highlight that the structure of CaM/target complexes is nonpredictable based on the CaM-binding motif involved, and indicate that a simple search for additional hydrophobic clusters up- or down-stream of an established CaM-binding site is not sufficient to predict if a CaM/peptide complex adopts an extended conformation. Thus, straightforward experimental approaches for the identification of extended CaM/target complex structures are needed. As classical high-resolution techniques and even cross-linking approaches are elaborate and time-consuming, we see great potential in the direct mass spectrometric analysis of protein assemblies and will thus dedicate the next section to present our concept of using ion-mobility MS (IM-MS) for conformational screening.
3. Investigation of CaM/peptide complexes using ion mobility-mass spectrometry
The investigation of proteins and protein complexes in the gas phase of a mass spectrometer while preserving folded structure and ligand-binding properties – often referred to as native MS – has developed into an important tool in structural biology [Citation111]. Native MS was also applied to CaM and some of its noncovalent peptide complexes [Citation112–Citation117], providing valuable information on the metal and target binding properties of CaM, as well as the stoichiometry of CaM/peptide complexes. The powerful combination of ion mobility spectrometry and mass spectrometry (IM-MS), allows obtaining structural insights into proteins and their assemblies [Citation118–Citation123]. In IM-MS, ions are guided by a weak electric field through a cell, which is filled with an inert buffer gas such as helium or nitrogen. During this process, which can be considered as ‘gas-phase electrophoresis’, ions with a compact shape undergo fewer collisions with the buffer gas and therefore drift faster through the cell than ions with more extended conformations. As a result, the investigated species are not only separated according to their mass-to-charge ratio (m/z), but also according to their size and shape. The drift time of a particular ion can be further converted into a rotationally averaged collision cross section (CCS), which can also be determined theoretically and compared universally [Citation124]. The instrument platforms most commonly used for such IM-MS experiments are either of the drift tube (DT)-type, allowing direct CCS determination, or of the traveling wave (TW)-type, requiring the use of calibrants for CCS estimation (see [Citation122,Citation123] and references therein for details). With respect to ion polarity, measurements in negative ion mode have the advantage that nonspecific metal ion attachment is suppressed [Citation125] and are thus often preferred for the analysis of acidic (pI ~4) Ca2+-binding proteins like CaM.
In a pioneer IM-MS study on metalloproteins, an analysis of CaM and its Ca2+-binding behavior was reported [Citation126]. Using a home-built DT instrument with helium as drift gas, the authors observed two conformers of apo-CaM for charge states 7+ to 9+ (most likely indicating potential energy minima that proteins are able to access in the gas phase), but only a single conformational species for charge states 10+ and higher. When the experimental CCSs were compared to theoretical CCS of apo-CaM derived from its solution structure (PDB 1CFD [Citation127]), it was found that the theoretical value of 1870 Å2 was best matched by the CCS of the two apo-CaM conformers of the 8+ (1655 and 2068 Å2) and 9+ (1660 and 2022 Å2) species, indicating that CaM ions of these lower charge states are of comparable size to structures obtained under native solution conditions. Moreover, the CCSs of different CaM/nCa2+ complexes revealed a trend toward more compact structures with the addition of calcium.
Shortly after, DT and TW IM-MS were used to systematically investigate the conformation of CaM during sequential binding of Ca2+ and the impact of binding to CaMKII-derived target peptides or melittin, a 26-amino acid peptide from bee venom. In order to classify the obtained gas-phase structures, experimental CCSs were compared to theoretical data derived from NMR (PDB 1CFD [Citation127]; 1SW8 [Citation128]) and crystal (3CLN [Citation129]; 1PRW [Citation130]) structures. In agreement with an earlier study [Citation126], two conformational species were observed for CaM in several charge states with CCSs of approximately ~2000 Å2 for the extended species and 15–20% smaller values for the more compact species [Citation131]. These two conformers were assigned to dumbbell-extended and canonical-compact structures, which, using X-ray crystallography, have previously been shown to coexist under certain conditions [Citation129,Citation130]. In addition, the authors observed a collapse of the dumbbell structure of CaM with further calcium ions attached to it. In case of the noncovalent peptide/CaM complexes, IM-MS revealed a preferred stoichiometry of 4:1:1 for Ca2+/CaM/melittin and for Ca2+/CaM/CaMKII290-309. A shorter, C-terminal melittin fragment (Melittin13-26), on the other hand, showed two distinct stoichiometries: a 4:1:1 binding and a 4:1:2 Ca2+/CaM/peptide complex. Interestingly, only in case of the 1:1 CaM/melittin13-26 complex a small amount of the more extended dumbbell conformation was detected for the charge state z = 8 of the Ca2+-free complex. All other complexes showed predominantly compact conformations. This finding led to the conclusion that CaM in the dumbbell-like conformation is able to complex only with very small and weakly binding peptides with only one lobe being involved in binding. For larger peptides on the other hand, it is likely that both lobes are involved in binding and that the resulting compact globular structure is the prerequisite for formation of particularly stable CaM/peptide complexes [Citation131]. In general, the experimentally obtained IM-MS data of this study agreed better with theoretical data obtained from X-ray rather than with NMR structures.
In 2012, the first application of TW IM-MS in negative ion mode for CCS determination of CaM was reported [Citation132]. To accurately estimate the CCS of CaM and its complexes, a calibration procedure for negatively charged protein ions using the CCS of myoglobin and cytochrome c as reference was developed (Clemmer Group Cross Section Database, www.indiana.edu/~clemmer). CCSs were determined for several charge states of unbound CaM and CaM in complex with melittin or with the plasma membrane Ca2+ pump peptide C20W, while exploring different metalation states. CaM/melittin complexes were found to be of the compact type with CaM being wrapped around the peptide, as previously reported [Citation131]. The complex with C20W, however, typifies a structure in which the first 12 residues of the peptide are embraced by only the C-terminal lobe of CaM, leading to an unusually extended structure [Citation133] that was indeed confirmed by IM-MS. More recently, the same group investigated CaM in complex with four amphibian peptides that are involved in defense mechanisms by preventing nitric oxide (NO) production [Citation134]. IM-MS revealed a clear preference for a 4:1:1 stoichiometry with narrow charge state and arrival time distributions (ATDs), both indicative for a well-defined structure. In the absence of any high-resolution structures of CaM in complex with amphibian peptides, experimental CCSs were compared to theoretically determined CCS derived from prototypic canonical (PDB 2BBM [Citation21]) or extended (1CFF [Citation133]) structures. The authors concluded that the experimentally determined CCSs for the CaM/amphibian peptide complexes agree well with those predicted as compact structures.
Stimulated by the studies summarized above, we consider IM-MS as a screening tool to distinguish between different CaM/target structures. We use a Synapt G2-S quadrupole time-of-flight (QTOF) hybrid instrument (Waters Corporation), which was modified in a way that the original TW ion mobility cell was replaced by a radio frequency (RF)-confining DT cell [Citation135]. As a result, absolute CCSs can be determined without using external calibrants. This is especially beneficial in negative ion mode, where a TW IM-MS-based CCS-estimation on the basis of standard proteins remains challenging. The idea of this approach is to experimentally determine the CCSs for CaM and CaM/peptide complexes, and to compare these values as a function of molecular mass. (a) shows such a theoretical plot with helium DT CCSs (DTCCSsHe) of the compact and dumbbell conformation of apo-CaM. The estimated mass-to-CCS correlation for compact conformations is assumed to behave like an idealized spherical particle (CCS ~ M2/3 [Citation136]), whereas extended conformations are estimated to grow in cylindrical-like (dumbbell) shape (CCS ~ M). Thus, individual CaM/peptide-complexes can be distinguished on the basis of the trend line they follow ((a)).
Figure 2. Investigation of CaM/peptide complexes using ion mobility-mass spectrometry.
(a.) Collision cross sections (CCSs) of CaM and CaM/peptide complexes as a function of their protein mass. CCSs of the compact and dumbbell conformation of CaM are shown as open squares. The estimated mass-to-CCS correlation of idealized spherical particles and idealized cylindrical particles are shown as black and grey lines, respectively. The trendlines provide an indication if the investigated complexes formed by CaM and different peptides (P1, P2, P3) are of the compact or extended type.(b.) Amino acid sequences of the investigated peptides skMLCK577-602 (M13) and skMLCK575-607 (skMLCK). Hydrophobic anchor residues of the 1–5–8–14 CaM-binding motif position are marked in red, while a potential hydrophobic contact site in motif position 26 of skMLCK is marked in green.(c, d.) Negative ion mass spectra of CaM/peptide complexes. Spectra were derived from 10 µM CaM without external addition of Ca2+ and in presence of 20 µM M13 (c) and 20 µM skMLCK (d), respectively. Charge states of unliganded CaM are shown in black, whereas the charge states of the CaM/M13 and the CaM/skMLCK complex are highlighted in red and blue, respectively. In all cases the ratio of bound complex is Ca2+/CaM/peptide 4:1:1.(e.) Arrival time distributions (ATDs) and experimental CCSs. Shown are helium CCSs (DTCCSHe) of CaM and the corresponding CaM/peptide complexes with four Ca2+ in charge state 8- and 9-. Note that in both charge states, the experimental DTCCSHe of the CaM/skMLCK complex does not differ significantly from that of the CaM/M13 complex, identifying both CaM/peptide complexes as compact type.
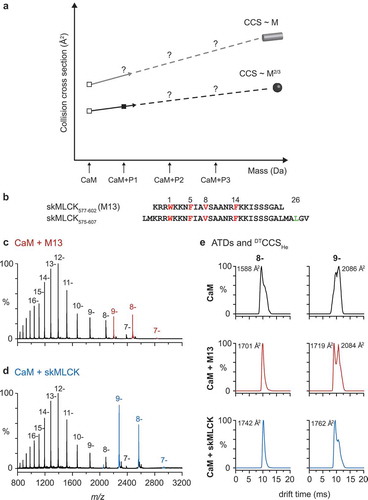
In a pilot experiment, we analyzed a complex between CaM and two different peptides derived from the CaM-binding region of skMLCK. The shorter peptide skMLCK577-602 (referred to as M13) contains a prototypic 1–5–8–14 binding motif and leads to a compact complex structure [Citation21]. The longer, C-terminally elongated peptide skMLCK575-607 contains an additional hydrophobic amino acid in motif position 26 (Leu-605; (b)), somewhat reminiscent of the 1–5–8–26 motif of Munc13-1. We tested whether the elongation of the peptide would lead to a CaM conformation that is compact (as indicated by our previous cross-linking experiments [Citation110]) or extended. Mass spectra of both complexes ((c,d)) reveal a clear preference for a 4:1:1 Ca2+/CaM/peptide stoichiometry and a narrow charge state distribution with the most abundant complex intensities in charge state 8- and 9-. (e) shows ATDs of these two charge states for free CaM (i.e. with four Ca2+ bound, but without peptidic ligand) and for the two complexes with M13 and skMLCK. In general, ATDs for both complexes appear narrower and more defined than those for free CaM. The experimentally determined CCSs are similar and thus identify both complexes as compact type, despite the elongated amino acid sequence and the related ~20% mass gain of skMLCK575-607 in comparison with M13. Interestingly, for the charge state 9-, two distinct conformations are clearly observed for CaM and both CaM/peptide complexes. In particular the ATD of the M13 complex exhibits two distinct features, which are roughly similar in intensity, whereas in the ATD of the skMLCK575-607 complex, the feature corresponding to the species with the larger CCS is much less abundant. This result is in good agreement with our previous cross-linking experiments [Citation110] and indicates that the CaM/skMLCK575-607 complex is even more compact than the CaM/M13 complex.
Although further evaluation of this experimental approach with respect to the description of extended conformations is still pending, we expect IM-MS to provide both, the throughput and the resolving power for straightforward conformational screening of CaM/peptide complexes. Other approaches with screening potential include single-molecule force spectroscopy methods [Citation137] and electron paramagnetic resonance (EPR; sometimes referred to as electron spin resonance, ESR) spectroscopy [Citation138–Citation140]. However, although proven to be powerful tools for the investigation of CaM structural dynamics and CaM/peptide complexes [Citation141–Citation145], these approaches are less direct and likely only accessible for highly specialized laboratories. Application of IM-MS to elongated CaM-binding peptides derived from all Munc13 isoforms and other CaM targets from the SV cycle is expected to shed light on the question whether extended CaM conformations and sequential CaM binding are unique to Munc13s or a rather general feature of (presynaptic) CaM targets.
4. Towards calmodulin-protein cross-linking
The structures of CaM complexes currently available in the PDB can be considered as biased toward short peptide targets employing canonical binding modes. Short peptides are typically used for conceptual reasons as the definition of the minimal CaM-binding motif is often desired (with the potential risk of picking up incomplete binding modes) and/or for technical reasons as standard solid-phase peptide synthesis typically restricts the peptide size to a maximum of ~50 residues. Short peptides are also highly useful in cases where recombinant expression of the target protein, or of a defined domain of that protein are not possible, like in the case of Munc13s. However, such peptides can be poor mimetics of the intact protein and may lead to a compact CaM conformation in the complex that is otherwise prevented in the full-length context [Citation146]. Short peptides may also be unable to make contacts to both CaM lobes [Citation147]. Based on these caveats described by others and by ourselves, we stress that it is of general importance to build structural characterization of CaM complexes on elongated peptides and/or protein fragments in addition to minimally short model peptides. Here, we review technical advances in protein–protein cross-linking with an emphasis on the development of novel chemical cross-linking reagents and photoreactive proteins, which we are expecting to have great impact for the structural analysis of protein–protein complexes.
Chemical cross-linking combined with MS is an auspicious tool for the structural characterization of protein–protein interactions [Citation91,Citation94,Citation96,Citation97]. The cross-linking/MS approach has rapidly developed since the year 2000 and has now matured into an alternative technique for structural biology studies. Basically, cross-linking introduces a covalent bond between the functional groups of two amino acids that are in spatial proximity within a protein or a protein complex. Due to the specific spacer length of the cross-linking reagent, the distance between the covalently connected amino acids is derived, providing three-dimensional structural information on the protein complex. Analysis of cross-links is commonly performed in a ‘bottom-up’ strategy, involving the proteolytic cleavage of proteins followed by MS analysis of the resulting peptides. This allows identifying cross-linked amino acids at the peptide level. Identification of the covalently connected peptides from a complex dataset is facilitated by the application of specific software tools to identify mass spectra of cross-linked peptides using specified search algorithms [Citation148–Citation150]. Advantages of the cross-linking/MS approach include high throughput, applicability at near-physiological protein concentration and buffer conditions, high accessibility, and relatively low technical efforts.
A large variety of cross-linking reagents have been designed with the aim of targeting different functionalities of amino acid side chains and bridging various distances within the protein complexes (see for example Cross-linking Technical Handbook, Thermo Scientific, www.thermoscientific.com/pierce). One exciting novel development is the design of MS-cleavable cross-linkers creating characteristic fragment ion signatures in MS/MS spectra. Such an MS-cleavable linker is BuUrBu (spacer length 12.5 Å), containing a central urea moiety that will fragment preferably under CID conditions applied during mass spectrometric peptide sequencing ((a)). Thereby, specific fragment ions are generated, which facilitate the automated assignment of cross-links [Citation76]. In our initial structural studies on CaM/Munc13 peptide complexes [Citation90], we have used the homobifunctional reagents bissulfosuccinimidyl suberate (BS3, spacer length 11.4 Å) and sulfosuccinimidyl glutarate (BS2G, spacer length 7.7 Å), which are commonly used amine-reactive cross-linkers containing two identical functional groups. Complementary data are obtained by combining different reactive groups in one heterobifunctional cross-linker, such as amine-reactive, sulfhydryl-reactive or photoreactive moieties. A combination of amine- and sulfhydryl-reactive groups has been realized for the cross-linker N-[γ-maleimidobutyryloxy]succinimide ester (GMBS, spacer length 7.3 Å) that connects Lys and Cys residues. More recently, we have developed the amine/photoreactive cross-linker N-succinimidyl-p-benzoyldihydrocinnamate (SBC, spacer length 10.2 Å, (b)) [Citation117] and used it to cross-link Munc13 peptides to CaM [Citation51]. SBC contains a photophore that reacts with a rather broad spectrum of amino acid side chains and does not require the proximity of two Lys residues or two other specific functionalities for cross-link formation. Thus, the key advantage of incorporating photoreactive groups, such as benzophenones or diazirines, into cross-linking reagents is their ability to insert into C-H and N-H bonds of amino acid side chains. Of note, benzophenones exhibit a slight preference for C-H bonds adjacent to heteroatoms and in particular for the Met side chain, while diazirines react nonspecifically with all amino acids.
Figure 3. Reagents and strategies for protein-protein cross-linking.
(a.) Homobifunctional, MS-cleavable BuUrBu-linker containing two amine-reactive N-hydroxysuccinimid (NHS) esters. The linker reacts with primary amine groups in Lys residues creating an amide bond. Under CID conditions the central urea moiety is cleaved generating a characteristic fragment ion signature.(b.) Heterobifunctional, amine/photo-reactive cross-linker N-succinimidyl-p-benzoyldihydrocinnamate (SBC). Cross-linking reactions with SBC are typically conducted in a two-step fashion. First, the amine-reactive site of SBC (NHS ester) is allowed to react with protein 1 and non-reacted reagent can be quenched and removed. Second, SBC-labeled protein one is incubated with protein 2 and activation of the photo-reactive site of SBC (benzophenone moiety) by UV irradiation leads to the formation of covalent bonds via a diradicalic mechanism.(c.) Photo-Met contains a diazirine that is activated by UV irradiation. Consequently, a carbene is created that forms a covalent bond with N-H and C-H groups of an amino acid in spatial proximity. Note the structural similarity of Met (left) and photo-Met (right), the latter containing a diazirine group instead of the sulfur atom.(d.) Incorporation strategy of photo-Met into proteins in E. coli cells. Cells are grown in mineral salts medium before photo-Met is directly added to the medium. Photo-Met is incorporated into proteins during protein synthesis.(e.) In vivo and in vitro approach for protein interaction studies using photo-Met.
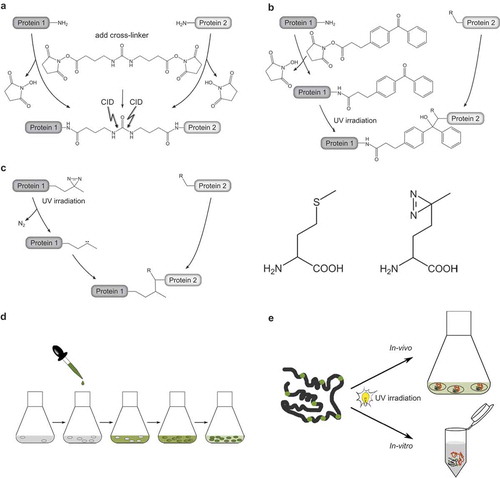
Photophores are also incorporated into artificial photo-amino acids which can be introduced into peptides during solid phase synthesis to produce photoreactive peptides or into proteins during translation to produce photoreactive proteins. The Schultz group provided a method for directed incorporation of unnatural amino acids into proteins in living cells by utilizing an amber stop codon, a specific aminoacyl-tRNA transferase, and a specific tRNA recognizing the unnatural amino acid [Citation151]. An alternative that does not require extensive genetic engineering is to incorporate a photoreactive amino acid in a nondirected fashion [Citation152]. In the photo-amino acid photo-Met ((c)), the sulfur atom of Met is replaced by a diazirine group. As the slight structural difference between Met and photo-Met is not recognized by the cellular translation machinery, the position of the photo-Met does not have to be defined by a special codon. UV irradiation of diazirines induces the loss of nitrogen (N2), forming a reactive carbene that will form a covalent bond with an adjacent amino acid.
The nondirected incorporation of photo-Met has been developed for human (HeLa, HEK 293) and bacterial (Escherichia coli) cells [Citation152–Citation154]. We have adapted the protocol for optimum incorporation in E. coli cells using mineral salts medium as illustrated in (d), and demonstrated the efficiency of our incorporation strategy for the expression of recombinant CaM. A relatively small amount (30 mg/L) of photo-Met, added to the medium, proved sufficient to obtain incorporation rates of photo-Met as high as 30% into CaM [Citation155]. All nine Met residues of CaM were found to be replaced by their photoreactive counterparts, allowing all parts of CaM (N-lobe, C-lobe, and linker region) to form covalent bonds. The diazirine group of photo-Met was found to be remarkably stable during the purification process and storage of CaM, and can easily be activated by irradiation with UV light. In a proof-of-principle study, we identified a number of intramolecular cross-links in CaM after photo-Met incorporation [Citation155].
In summary, the incorporation of photo-Met in E. coli cells is a simple and straightforward approach that is applicable in any molecular biology laboratory. Such strategy overcomes the limitations of solid phase peptide synthesis and allows protein–protein photo-cross-linking for the structural characterization of protein complexes, both in vitro and in vivo ((e)). Specifically, we expect that cross-linking studies with photo-Met-labeled CaM and larger fragments of Munc13 proteins will deepen our structural understanding of CaM-Munc13 interactions, particularly in bMunc13-2 and Munc13-3, where it is still unclear if elongated/alternative CaM-binding motifs exist.
5. Expert commentary
Over the past decade, the combination of protein chemistry approaches (like cross-linking, covalent modification, limited proteolysis, affinity labeling, and hydrogen-deuterium-exchange, HDX) with modern bioanalytical MS has shaped the field of structural proteomics, giving rise to powerful techniques for the structural characterization of proteins and protein assemblies. It is now well accepted that structural proteomics can effectively complement classical high-resolution techniques like X-ray crystallography and NMR spectroscopy, in particular when protein complexes are large, dynamic, and heterogeneous.
In this article, we have outlined cross-linking/MS and native MS combined with ion mobility separation (IM-MS) as alternative structural biology techniques that can be applied with considerable throughput to protein mixtures under near-physiological conditions. Our main conclusion on cross-linking/MS approaches is that experimental distance constraints are applicable best when obtained by combining different cross-linking strategies, i.e. by using cross-linkers with different spacer length and reactivity, and by using the incorporation of unnatural photoreactive amino acids into peptides and proteins. This results in a complementary set of constraints that covers multiple structural features of the protein complex under study (i.e. from overall domain orientation to direct contact sites at the amino acid level) and guides computational molecular modeling toward meaningful protein structures. For the design of photo-reactive proteins, we typically prefer nonspecific incorporation of photoreactive amino acids due to its technical simplicity and availability in bacterial as well as mammalian expression systems, and consider the site-specific incorporation via genetic engineering as a powerful, but more sophisticated tool to target specific questions.
IM-MS as a more recent addition to the portfolio of MS-based structural biology techniques has rapidly matured into a powerful tool for the structural characterization of biomolecules and their assemblies. We conclude that IM-MS, with its capability of determining rotationally averaged collision cross sections (CCSs), provides important additional information about the overall size and shape of the investigated protein complexes, and the possibility to compare experimental CCSs to theoretical results derived from high-resolution X-ray or NMR structures [Citation156,Citation157]. We reason that IM-MS can be applied for straightforward conformational screening of biomolecular assemblies (as proposed here for CaM/peptide complexes), and for monitoring of protein folding/unfolding, aggregation events, and ligand-induced conformational changes. In line with this high potential, more and more mass spectrometers with IM options are becoming commercially available, making this technique accessible to a larger scientific community.
6. Five-year view
Cross-linking/MS techniques traditionally resided in specialized laboratories, but with the advent of MS as a routine tool in biochemistry, we anticipate a more common use of these tools to structurally complement classical molecular biology and biochemical approaches for the characterization of protein–protein interactions. Chemical cross-linking can be performed and photoreactive proteins can be produced in laboratories with molecular biology expertise, and more proteomics facilities are hardware- and software-wise able to analyze cross-linked peptides from complex mixtures. Therefore, basic structural questions like mapping of protein binding sites can be readily addressed, eliminating the need for strenuous fragmentation/deletion or site-directed mutagenesis experiments. However, the subsequent step toward structural information, namely computational approaches that integrate experimental distance constraints into coherent structural models, still requires expert knowledge typically not available to the standard biochemistry laboratory. Thus, in the coming years, we see the need to intensify the development of intuitive computational biology tools for the nonexpert user, which will eventually lead to a more common use of cross-linking/MS-based structural biology approaches by a broader scientific community.
While the majority of structural proteomics literature deals with the characterization of isolated (often bipartite) protein complexes in defined systems, exciting developments in the cross-linking/MS field are aimed at characterizing protein interaction networks in highly complex mixtures. We expect that applications of cross-linking to cell lysates or even in vivo will considerably increase the physiological relevance of the data generated. Among the recent technical advances enabling such studies, MS-cleavable cross-linkers currently appear to be the most promising option as gas-phase cleavage of the linker gives rise to signature pattern, which can be automatically identified by data analysis algorithms. Initial studies demonstrate technical feasibility at the proteome level [Citation158], but major challenges of proteome-wide cross-linking/MS approaches, such as the complexity of the sample and the low abundance of the cross-linked peptides, remain to be addressed, for example by prefractionation of protein assemblies and enrichment of interpeptide cross-links, respectively. The same holds true for in vivo cross-linking approaches, but with the additional challenge that cross-linking reagents must enter the cell. We expect that future compound development will center on multifunctional cross-linkers that are not only chemically reactive, but also MS-cleavable, enrichable through affinity-tagging, and membrane permeable.
The fact that inter-peptide cross-links can be imagined as branched needles in a stack of unbranched hay argues for their separation according to size and shape. Thus, it is obvious to use IM-MS as a tool for directing mass spectrometric peptide sequencing events toward the branched peptides of interest, which can be considered as H-shaped in case of inter-peptide cross-links. With its additional dimension of separation and its potential for high-throughput, IM-MS is increasingly being applied for the routine analysis of complex mixtures in all kinds of ‘omics’ experiments. We expect to see more IM-driven approaches not only for conformational screening in native MS applications (see above), but also in cross-linking/MS workflows.
What can structural proteomics teach us on presynaptic CaM complexes in the near future? Efforts to identify molecular targets of CaM and the effect of CaM binding on these targets are crucial for dissecting the multitude of Ca2+-dependent processes of the synaptic vesicle (SV) cycle and their effects on speed, accuracy, and fidelity of synaptic transmission. Screening approaches provide us with long lists of potential presynaptic CaM interactors (see ), and the task remains to (i) establish their interaction with CaM, (ii) map the CaM-binding site, (iii) identify the physiological effect of the interaction, and (iv) study the relationship between the physiological significance of the interaction and the mode of CaM binding. Based on our experience from characterizing the interactions of CaM with members of the Munc13 protein family, we propose a workflow of structural proteomic methods for the characterization of such interactions, which we believe can facilitate comparable projects in the future. Photoaffinity labeling experiments facilitate the mapping of CaM-binding sites and the identification of anchor residues involved in CaM binding, a prerequisite for the detailed structural and functional characterization of the interaction. Photoaffinity labeling, chemical cross-linking and IM-MS are then used in combination to create a structural model for protein complex and to understand the structural dynamics of the interaction. These insights from structural proteomics can be used to generate CaM-insensitive mutants of CaM targets for functional studies in vitro or ideally in vivo.
In view of the growing evidence that CaM interactions occur with target sequences longer than the canonical CaM-binding sites, we suggest that the diversity of CaM-binding modes may still be underestimated and particularly CaM/target complex structures of the compact type may be overrepresented, as they are often established using minimal peptides as protein mimetics. Ongoing and future studies of CaM targets will likely resolve whether extended CaM complex structures exist in the presence of CaM-binding sites from elongated peptides or protein fragments, whether Ca2+-dependent binding of CaM to a target protein occurs in a sequential manner, and whether this is relevant for the protein’s function as proposed for the presynaptic Munc13 proteins.
Key issues
Calmodulin (CaM) is exceptionally abundant in the brain and serves as a Ca2+ signaling hub for the regulation of synaptic transmission.
CaM interactor screens revealed hundreds of candidate binding partners, for most of which biochemical and functional validation are pending. Focusing on the presynaptic compartment of neurons, a list of CaM targets with established or putative roles in the synaptic vesicle cycle is presented.
The presynaptic Munc13 proteins are essential for neurotransmitter release and Ca2+/CaM-dependent regulation of their activity is structurally and functionally conserved in all four neuronal isoforms
Cross-linking/mass spectrometry (MS) approaches are alternative tools for the structural characterization of proteins and protein complexes, which can be applied under near-physiological conditions. The most meaningful information is achieved when complementary cross-linking strategies are applied, i.e. by using cross-linkers with variable reactivity and spacer length, and by using the incorporation of unnatural photo-reactive amino acids
Given the limited throughput of high-resolution structural biology and cross-linking/MS approaches, we propose native MS with ion mobility (IM) separation as a straightforward tool for conformational screening of CaM/peptide complexes
The structural characterization of CaM complexes should be based on elongated peptides and/or protein fragments as short CaM-binding peptides might be poor mimetics of the intact protein. We consider photo-reactive CaM as an important tool facilitating CaM-protein cross-linking
Declaration of interest
M. Göth received funding for a PhD fellowship by Bayer Pharma AG. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgements
The authors would like to thank Nils Brose (MPI Experimental Medicine) for his valuable input and his continuous support.
Additional information
Funding
References
- Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 2007;8(3):182–193.
- Kreutz MR, Naranjo JR, Koch KW, et al. The neuronal functions of EF-hand Ca2+-binding proteins. Front Mol Neurosci. 2012;5:92.
- Mikhaylova M, Hradsky J, Kreutz MR. Between promiscuity and specificity: novel roles of EF-hand calcium sensors in neuronal Ca2+ signalling. J Neurochem. 2011;118(5):695–713.
- Davis TN, Thorner J. Vertebrate and yeast calmodulin, despite significant sequence divergence, are functionally interchangeable. Proc Natl Acad Sci U S A. 1989;86(20):7909–7913.
- Biber A, Schmid G, Hempel K. Calmodulin content in specific brain areas. Exp Brain Res. 1984;56(2):323–326.
- Cimler BM, Andreasen TJ, Andreasen KI, et al. P-57 is a neural specific calmodulin-binding protein. J Biol Chem. 1985;260(19):10784–10788.
- Kakiuchi S, Yasuda S, Yamazaki R, et al. Quantitative determinations of calmodulin in the supernatant and particulate fractions of mammalian tissues. J Biochem. 1982;92(4):1041–1048.
- Berchtold MW, Villalobo A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochim Biophys Acta. 2014;1843(2):398–435.
- Chou JJ, Li S, Klee CB, et al. Solution structure of Ca(2+)-calmodulin reveals flexible hand-like properties of its domains. Nat Struct Biol. 2001;8(11):990–997.
- Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10(8):322–328.
- Tidow H, Nissen P. Structural diversity of calmodulin binding to its target sites. Febs J. 2013;280(21):5551–5565.
- Kursula P. The many structural faces of calmodulin: a multitasking molecular jackknife. Amino Acids. 2014;46(10):2295–2304.
- Villarroel A, Taglialatela M, Bernardo-Seisdedos G, et al. The ever changing moods of calmodulin: how structural plasticity entails transductional adaptability. J Mol Biol. 2014;426(15):2717–2735.
- Radivojac P, Vucetic S, O’Connor TR, et al. Calmodulin signaling: analysis and prediction of a disorder-dependent molecular recognition. Proteins. 2006;63(2):398–410.
- Yap KL, Kim J, Truong K, et al. Calmodulin target database. J Struct Funct Genomics. 2000;1(1):8–14.
- Chou KC. Pseudo amino acid composition and its applications in bioinformatics, proteomics and system biology. Curr Proteomics. 2009;6(4):262–274.
- Liu B, Liu F, Wang X, et al. Pse-in-one: a web server for generating various modes of pseudo components of DNA, RNA, and protein sequences. Nucleic Acids Res. 2015;43(W1):W65–71.
- Dunlap TB, Guo HF, Cook EC, et al. Stoichiometry of the calcineurin regulatory domain-calmodulin complex. Biochemistry. 2014;53(36):5779–5790.
- Rellos P, Pike AC, Niesen FH, et al. Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. Plos Biol. 2010;8(7):e1000426.
- Taylor CW, Tovey SC. IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol. 2010;2(12):a004010.
- Ikura M, Clore GM, Gronenborn AM, et al. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science. 1992;256(5057):632–638.
- Meador WE, Means AR, Quiocho FA. Target enzyme recognition by calmodulin: 2.4 Å structure of a calmodulin-peptide complex. Science. 1992;257(5074):1251–1255.
- Meador WE, Means AR, Quiocho FA. Modulation of calmodulin plasticity in molecular recognition on the basis of x-ray structures. Science. 1993;262(5140):1718–1721.
- Tripathi S, Waxham MN, Cheung MS, et al. Lessons in protein design from combined evolution and conformational dynamics. Sci Rep. 2015;5:14259.
- Xu XZ, Wes PD, Chen H, et al. Retinal targets for calmodulin include proteins implicated in synaptic transmission. J Biol Chem. 1998;273(47):31297–31307.
- Shen X, Valencia CA, Szostak JW, et al. Scanning the human proteome for calmodulin-binding proteins. Proc Natl Acad Sci U S A. 2005;102(17):5969–5974.
- Berggard T, Arrigoni G, Olsson O, et al. 140 mouse brain proteins identified by Ca2+-calmodulin affinity chromatography and tandem mass spectrometry. J Proteome Res. 2006;5(3):669–687.
- O’Connell DJ, Bauer MC, O’Brien J, et al. Integrated protein array screening and high throughput validation of 70 novel neural calmodulin-binding proteins. Mol Cell Proteomics. 2010;9(6):1118–1132.
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547.
- Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435(7041):497–501.
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59(6):861–872.
- Wojcik SM, Brose N. Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron. 2007;55(1):11–24.
- Faas GC, Raghavachari S, Lisman JE, et al. Calmodulin as a direct detector of Ca2+ signals. Nat Neurosci. 2011;14(3):301–304.
- Timofeeva Y, Volynski KE. Calmodulin as a major calcium buffer shaping vesicular release and short-term synaptic plasticity: facilitation through buffer dislocation. Front Cell Neurosci. 2015;9:239.
- Pang ZP, Cao P, Xu W, et al. Calmodulin controls synaptic strength via presynaptic activation of calmodulin kinase II. J Neurosci. 2010;30(11):4132–4142.
- Pang ZP, Xu W, Cao P, et al. Calmodulin suppresses synaptotagmin-2 transcription in cortical neurons. J Biol Chem. 2010;285(44):33930–33939.
- Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6(4):267–276.
- Hilfiker S, Pieribone VA, Nordstedt C, et al. Regulation of synaptotagmin I phosphorylation by multiple protein kinases. J Neurochem. 1999;73(3):921–932.
- Popoli M. Synaptotagmin is endogenously phosphorylated by Ca2+/calmodulin protein kinase II in synaptic vesicles. FEBS Lett. 1993;317(1–2):85–88.
- De Jong AP, Meijer M, Saarloos I, et al. Phosphorylation of synaptotagmin-1 controls a post-priming step in PKC-dependent presynaptic plasticity. Proc Natl Acad Sci U S A. 2016;113(18):5095–5100.
- Liu H, Bai H, Hui E, et al. Synaptotagmin 7 functions as a Ca2+-sensor for synaptic vesicle replenishment. Elife. 2014;3:e01524.
- Igarashi M, Watanabe M. Roles of calmodulin and calmodulin-binding proteins in synaptic vesicle recycling during regulated exocytosis at submicromolar Ca2+ concentrations. Neurosci Res. 2007;58(3):226–233.
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490(7419):201–207.
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3(8):a003947.
- Sun T, Wu XS, Xu J, et al. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci. 2010;30(35):11838–11847.
- Yao L, Sakaba T. Activity-dependent modulation of endocytosis by calmodulin at a large central synapse. Proc Natl Acad Sci U S A. 2012;109(1):291–296.
- Yue HY, Xu J. Myosin light chain kinase accelerates vesicle endocytosis at the calyx of Held synapse. J Neurosci. 2014;34(1):295–304.
- Okamoto Y, Lipstein N, Hua Y, et al. Distinct modes of endocytotic presynaptic membrane and protein uptake at the calyx of held terminal of rats and mice. Elife. 2016;5:e14643.
- Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32(6):1119–1131.
- Junge HJ, Rhee J-S, Jahn O, et al. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118(3):389–401.
- Lipstein N, Schaks S, Dimova K, et al. Nonconserved Ca(2+)/calmodulin binding sites in Munc13s differentially control synaptic short-term plasticity. Mol Cell Biol. 2012;32(22):4628–4641.
- Lipstein N, Sakaba T, Cooper BH, et al. Dynamic control of synaptic vesicle replenishment and short-term plasticity by Ca(2+)-calmodulin-Munc13-1 signaling. Neuron. 2013;79(1):82–96.
- Adelman JP. SK channels and calmodulin. Channels. 2016;10(1):1–6.
- Hammer JA 3rd, Wagner W. Functions of class V myosins in neurons. J Biol Chem. 2013;288(40):28428–28434.
- Watanabe M, Nomura K, Ohyama A, et al. Myosin-va regulates exocytosis through the submicromolar Ca2+-dependent binding of syntaxin-1A. Mol Biol Cell. 2005;16(10):4519–4530.
- Schluter OM, Basu J, Sudhof TC, et al. Rab3 superprimes synaptic vesicles for release: implications for short-term synaptic plasticity. J Neurosci. 2006;26(4):1239–1246.
- Park JB, Farnsworth CC, Glomset JA. Ca2+/calmodulin causes Rab3A to dissociate from synaptic membranes. J Biol Chem. 1997;272(33):20857–20865.
- Schluter OM, Khvotchev M, Jahn R, et al. Localization versus function of Rab3 proteins. Evidence for a common regulatory role in controlling fusion. J Biol Chem. 2002;277(43):40919–40929.
- Coppola T, Perret-Menoud V, Luthi S, et al. Disruption of Rab3-calmodulin interaction, but not other effector interactions, prevents Rab3 inhibition of exocytosis. Embo J. 1999;18(21):5885–5891.
- De Haro L, Ferracci G, Opi S, et al. Ca2+/calmodulin transfers the membrane-proximal lipid-binding domain of the v-SNARE synaptobrevin from cis to trans bilayers. Proc Natl Acad Sci U S A. 2004;101(6):1578–1583.
- Quetglas S, Iborra C, Sasakawa N, et al. Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. Embo J. 2002;21(15):3970–3979.
- Siddiqui TJ, Vites O, Stein A, et al. Determinants of synaptobrevin regulation in membranes. Mol Biol Cell. 2007;18(6):2037–2046.
- Di Giovanni J, Iborra C, Maulet Y, et al. Calcium-dependent regulation of SNARE-mediated membrane fusion by calmodulin. J Biol Chem. 2010;285(31):23665–23675.
- Wang D, Epstein D, Khalaf O, et al. Ca2+-Calmodulin regulates SNARE assembly and spontaneous neurotransmitter release via v-ATPase subunit V0a1. J Cell Biol. 2014;205(1):21–31.
- Zhang W, Wang D, Volk E, et al. V-ATPase V0 sector subunit a1 in neurons is a target of calmodulin. J Biol Chem. 2008;283(1):294–300.
- Korber C, Horstmann H, Venkataramani V, et al. Modulation of presynaptic release probability by the vertebrate-specific protein mover. Neuron. 2015;87(3):521–533.
- Bacaj T, Wu D, Yang X, et al. Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release. Neuron. 2013;80(4):947–959.
- Jackman SL, Turecek J, Belinsky JE, et al. The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature. 2016;529(7584):88–91.
- Brose N, Hofmann K, Hata Y, et al. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995;270(42):25273–25280.
- Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem J. 2000;349(Pt 1):247–253.
- Cooper B, Hemmerlein M, Ammermuller J, et al. Munc13-independent vesicle priming at mouse photoreceptor ribbon synapses. J Neurosci. 2012;32(23):8040–8052.
- Vogl C, Cooper BH, Neef J, et al. Unconventional molecular regulation of synaptic vesicle replenishment in cochlear inner hair cells. J Cell Sci. 2015;128(4):638–644.
- Imig C, Min SW, Krinner S, et al. The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron. 2014;84(2):416–431.
- Ma C, Su L, Seven AB, et al. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 2013;339(6118):421–425.
- Siksou L, Varoqueaux F, Pascual O, et al. A common molecular basis for membrane docking and functional priming of synaptic vesicles. Eur J Neurosci. 2009;30(1):49–56.
- Yang X, Wang S, Sheng Y, et al. Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming. Nat Struct Mol Biol. 2015;22(7):547–554.
- Varoqueaux F, Sigler A, Rhee J-S, et al. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99(13):9037–9042.
- Betz A, Ashery U, Rickmann M, et al. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21(1):123–136.
- Rhee J-S, Betz A, Pyott S, et al. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108(1):121–133.
- Shin OH, Lu J, Rhee JS, et al. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol. 2010;17(3):280–288.
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol. 2011;21(2):269–274.
- Regehr WG. Short-term presynaptic plasticity. Cold Spring Harb Perspect Biol. 2012;4(7):a005702.
- Hennig MH. Theoretical models of synaptic short term plasticity. Front Comput Neurosci. 2013;7:45.
- Rosenmund C, Sigler A, Augustin I, et al. Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron. 2002;33(3):411–424.
- Lipstein N, Verhoeven-Duif NM, Michelassi FE, et al. Synaptic UNC13A protein variant causes increased neurotransmission and dyskinetic movement disorder. J Clin Invest. 2017. doi:10.1172/JCI90259.
- Engel AG, Selcen D, Shen XM, et al. Loss of MUNC13-1 function causes microcephaly, cortical hyperexcitability, and fatal myasthenia. Neurol Genet. 2016;2(5):e105.
- Dorman G, Prestwich GD. Benzophenone photophores in biochemistry. Biochemistry. 1994;33:5661–5673.
- Jahn O, Eckart K, Brauns O, et al. The binding protein of corticotropin-releasing factor: ligand-binding site and subunit structure. Proc Natl Acad Sci U S A. 2002;99(19):12055–12060.
- Dimova K, Kawabe H, Betz A, et al. Characterization of the Munc13-calmodulin interaction by photoaffinity labeling. Biochim Biophys Acta. 2006;1763(11):1256–1265.
- Dimova K, Kalkhof S, Pottratz I, et al. Structural insights into the calmodulin-Munc13 interaction obtained by cross-linking and mass spectrometry. Biochemistry. 2009;48(25):5908–5921.
- Leitner A, Walzthoeni T, Kahraman A, et al. Probing native protein structures by chemical cross-linking, mass spectrometry, and bioinformatics. Mol Cell Proteomics. 2010;9(8):1634–1649.
- Liu F, Heck AJ. Interrogating the architecture of protein assemblies and protein interaction networks by cross-linking mass spectrometry. Curr Opin Struct Biol. 2015;35:100–108.
- Petrotchenko EV, Borchers CH. Crosslinking combined with mass spectrometry for structural proteomics. Mass Spectrom Rev. 2010;29(6):862–876.
- Rappsilber J. The beginning of a beautiful friendship: cross-linking/mass spectrometry and modelling of proteins and multi-protein complexes. J Struct Biol. 2011;173(3):530–540.
- Robinette D, Neamati N, Tomer KB, et al. Photoaffinity labeling combined with mass spectrometric approaches as a tool for structural proteomics. Expert Rev Proteomics. 2006;3(4):399–408.
- Sinz A. Chemical cross-linking and mass spectrometry to map three-dimensional protein structures and protein-protein interactions. Mass Spectrom Rev. 2006;25(4):663–682.
- Sinz A. The advancement of chemical cross-linking and mass spectrometry for structural proteomics: from single proteins to protein interaction networks. Expert Rev Proteomics. 2014;11(6):733–743.
- Vodovozova E. Photoaffinity labeling and its application in structural biology. Biochemistry. 2007;72(1):1–20.
- Müller DR, Schindler P, Towbin H, et al. Isotope-tagged cross-linking reagents. A new tool in mass spectrometric protein interaction analysis. Anal Chem. 2001;73(9):1927–1934.
- Pettelkau J, Ihling CH, Frohberg P, et al. Reliable identification of cross-linked products in protein interaction studies by 13C-labeled p-benzoylphenylalanine. J Am Soc Mass Spectrom. 2014;25(9):1628–1641.
- Arlt C, Götze M, Ihling CH, et al. Integrated workflow for structural proteomics studies based on cross-linking/mass spectrometry with an MS/MS cleavable cross-linker. Anal Chem. 2016;88(16):7930–7937.
- Clavier S, Bolbach G, Sachon E. Photocross-linked peptide-protein complexes analysis: a comparative study of CID and ETD fragmentation modes. J Am Soc Mass Spectrom. 2015;26(6):1014–1026.
- Giese SH, Belsom A, Rappsilber J. Optimized fragmentation regime for diazirine photo-cross-linked peptides. Anal Chem. 2016;88(16):8239–8247.
- Kage R, Leeman SE, Krause JE, et al. Identification of methionine as the site of covalent attachment of a p-benzoyl-phenylalanine-containing analogue of substance P on the substance P (NK-1) receptor. J Biol Chem. 1996;271(42):25797–25800.
- O’Neil KT, DeGrado WF. How calmodulin binds its targets: sequence independent recognition of amphiphilic [alpha]-helices. Trends Biochem Sci. 1990;15(2):59–64.
- Rodriguez-Castaneda F, Maestre-Martinez M, Coudevylle N, et al. Modular architecture of Munc13/calmodulin complexes: dual regulation by Ca2+ and possible function in short-term synaptic plasticity. Embo J. 2010;29(3):680–691.
- Hu Z, Tong XJ, Kaplan JM. UNC-13L, UNC-13S, and tomosyn form a protein code for fast and slow neurotransmitter release in Caenorhabditis elegans. Elife. 2013;2:e00967.
- Lu J, Machius M, Dulubova I, et al. Structural basis for a Munc13-1 homodimer to Munc13-1/RIM heterodimer switch. Plos Biol. 2006;4(7):e192.
- Shen N, Guryev O, Rizo J. Intramolecular occlusion of the diacylglycerol-binding site in the C1 domain of munc13-1. Biochemistry. 2005;44(4):1089–1096.
- Herbst S, Maucher D, Schneider M, et al. Munc13-like skMLCK variants cannot mimic the unique calmodulin binding mode of Munc13 as evidenced by chemical cross-linking and mass spectrometry. Plos One. 2013;8(10):e75119.
- Mehmood S, Allison TM, Robinson CV. Mass spectrometry of protein complexes: from origins to applications. Annu Rev Phys Chem. 2015;66:453–474.
- Ly T, Julian RR. Protein–metal interactions of Calmodulin and α-Synuclein monitored by selective noncovalent adduct protein probing mass spectrometry. J Am Soc Mass Spectrom. 2008;19(11):1663–1672.
- Nemirovskiy OV, Ramanathan R, Gross ML. Investigation of calcium-induced, noncovalent association of calmodulin with melittin by electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 1997;8(8):809–812.
- Pan J, Konermann L. Calcium-induced structural transitions of the Calmodulin−Melittin system studied by electrospray mass spectrometry: conformational subpopulations and metal-unsaturated intermediates. Biochemistry. 2010;49(16):3477–3486.
- Pukala TL, Urathamakul T, Watt SJ, et al. Binding studies of nNOS-active amphibian peptides and Ca2+ calmodulin, using negative ion electrospray ionisation mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3501–3509.
- Shirran S, Garnaud P, Daff S, et al. The formation of a complex between calmodulin and neuronal nitric oxide synthase is determined by ESI-MS. J R Soc Interface. 2005;2(5):465–476.
- Watt SJ, Oakley A, Sheil MM, et al. Comparison of negative and positive ion electrospray ionization mass spectra of calmodulin and its complex with trifluoperazine. Rapid Commun Mass Spectrom. 2005;19(15):2123–2130.
- Bleiholder C, Dupuis NF, Wyttenbach T, et al. Ion mobility–mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation. Nat Chem. 2011;3(2):172–177.
- Bohrer BC, Merenbloom SI, Koeniger SL, et al. Biomolecule analysis by ion mobility spectrometry. Annu Rev Anal Chem (Palo Alto Calif). 2008;1:293–327.
- Pagel K, Natan E, Hall Z, et al. Intrinsically disordered p53 and its complexes populate compact conformations in the gas phase. Angew Chem Int Ed Engl. 2013;52(1):361–365.
- Ruotolo BT, Giles K, Campuzano I, et al. Evidence for macromolecular protein rings in the absence of bulk water. Science. 2005;310(5754):1658–1661.
- Uetrecht C, Rose RJ, van Duijn E, et al. Ion mobility mass spectrometry of proteins and protein assemblies. Chem Soc Rev. 2010;39(5):1633–1655.
- Zhong Y, Hyung SJ, Ruotolo BT. Ion mobility-mass spectrometry for structural proteomics. Expert Rev Proteomics. 2012;9(1):47–58.
- Bush MF, Hall Z, Giles K, et al. Collision cross sections of proteins and their complexes: a calibration framework and database for gas-phase structural biology. Anal Chem. 2010;82(22):9557–9565.
- Hu P, Ye Q-Z, Loo JA. Calcium stoichiometry determination for calcium binding proteins by electrospray ionization mass spectrometry. Anal Chem. 1994;66(23):4190–4194.
- Faull PA, Korkeila KE, Kalapothakis JM, et al. Gas-phase metalloprotein complexes interrogated by ion mobility-mass spectrometry. Int J Mass Spectrom. 2009;283(1–3):140–148.
- Kuboniwa H, Tjandra N, Grzesiek S, et al. Solution structure of calcium-free calmodulin. Nat Struct Mol Biol. 1995;2(9):768–776.
- Bertini I, Del Bianco C, Gelis I, et al. Experimentally exploring the conformational space sampled by domain reorientation in calmodulin. Proc Natl Acad Sci U S A. 2004;101(18):6841–6846.
- Babu YS, Bugg CE, Cook WJ. Structure of calmodulin refined at 2.2 Å resolution. J Mol Biol. 1988;204(1):191–204.
- Fallon JL, Quiocho FA. A closed compact structure of native Ca2+-Calmodulin. Structure. 2003;11(10):1303–1307.
- Wyttenbach T, Grabenauer M, Thalassinos K, et al. The effect of calcium ions and peptide ligands on the relative stabilities of the Calmodulin dumbbell and compact structures. J Phys Chem B. 2010;114(1):437–447.
- Calabrese AN, Speechley LA, Pukala TL. Characterisation of Calmodulin structural transitions by ion mobility mass spectrometry. Aust J Chem. 2012;65(5):504–511.
- Elshorst B, Hennig M, Försterling H, et al. NMR solution structure of a complex of Calmodulin with a binding peptide of the Ca2+ pump. Biochemistry. 1999;38(38):12320–12332.
- Calabrese AN, Bowie JH, Pukala TL. Structural analysis of Calmodulin binding by nNOS inhibitory amphibian peptides. Biochemistry. 2015;54(2):567–576.
- Allen SJ, Giles K, Gilbert T, et al. Ion mobility mass spectrometry of peptide, protein, and protein complex ions using a radio-frequency confining drift cell. Analyst. 2016;141(3):884–891.
- Ruotolo BT, Benesch JL, Sandercock AM, et al. Ion mobility-mass spectrometry analysis of large protein complexes. Nat Protoc. 2008;3(7):1139–1152.
- Zoldak G, Rief M. Force as a single molecule probe of multidimensional protein energy landscapes. Curr Opin Struct Biol. 2013;23(1):48–57.
- Hubbell WL, Lopez CJ, Altenbach C, et al. Technological advances in site-directed spin labeling of proteins. Curr Opin Struct Biol. 2013;23(5):725–733.
- Ji M, Ruthstein S, Saxena S. Paramagnetic metal ions in pulsed ESR distance distribution measurements. Acc Chem Res. 2014;47(2):688–695.
- Sahu ID, McCarrick RM, Lorigan GA. Use of electron paramagnetic resonance to solve biochemical problems. Biochemistry. 2013;52(35):5967–5984.
- Astashkin AV, Chen L, Zhou X, et al. Pulsed electron paramagnetic resonance study of domain docking in neuronal nitric oxide synthase: the calmodulin and output state perspective. J Phys Chem A. 2014;118(34):6864–6872.
- Bowman PB, Puett D. Electron paramagnetic resonance spectroscopy of nitroxide-labeled calmodulin. Protein J. 2014;33(3):267–277.
- Junker JP, Rief M. Single-molecule force spectroscopy distinguishes target binding modes of calmodulin. Proc Natl Acad Sci U S A. 2009;106(34):14361–14366.
- Junker JP, Ziegler F, Rief M. Ligand-dependent equilibrium fluctuations of single calmodulin molecules. Science. 2009;323(5914):633–637.
- McCarthy MR, Thompson AR, Nitu F, et al. Impact of methionine oxidation on calmodulin structural dynamics. Biochem Biophys Res Commun. 2015;456(2):567–572.
- de Diego I, Kuper J, Bakalova N, et al. Molecular basis of the death-associated protein kinase-calcium/calmodulin regulator complex. Sci Signal. 2010;3(106):ra6.
- Hoffman L, Chandrasekar A, Wang X, et al. Neurogranin alters the structure and calcium binding properties of calmodulin. J Biol Chem. 2014;289(21):14644–14655.
- Gotze M, Pettelkau J, Schaks S, et al. StavroX–a software for analyzing crosslinked products in protein interaction studies. J Am Soc Mass Spectrom. 2012;23(1):76–87.
- Yang B, Wu YJ, Zhu M, et al. Identification of cross-linked peptides from complex samples. Nat Methods. 2012;9(9):904–906.
- Rinner O, Seebacher J, Walzthoeni T, et al. Identification of cross-linked peptides from large sequence databases. Nat Methods. 2008;5(4):315–318.
- Xie J, Schultz PG. A chemical toolkit for proteins–an expanded genetic code. Nat Rev Mol Cell Biol. 2006;7(10):775–782.
- Suchanek M, Radzikowska A, Thiele C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat Methods. 2005;2(4):261–267.
- Koberova M, Jecmen T, Sulc M, et al. Photo-cytochrome b(5) - A new tool to study the cytochrome P450 electron-transport chain. Int J Electrochem Sci. 2013;8(1):125–134.
- Lossl P, Sinz A. Combining amine-reactive cross-linkers and photo-reactive amino acids for 3D-structure analysis of proteins and protein complexes. Methods Mol Biol. 2016;1394:109–127.
- Piotrowski C, Ihling CH, Sinz A. Extending the cross-linking/mass spectrometry strategy: facile incorporation of photo-activatable amino acids into the model protein calmodulin in Escherichia coli cells. Methods. 2015;89:121–127.
- Jurneczko E, Barran PE. How useful is ion mobility mass spectrometry for structural biology? The relationship between protein crystal structures and their collision cross sections in the gas phase. Analyst. 2011;136(1):20–28.
- Konijnenberg A, Butterer A, Sobott F. Native ion mobility-mass spectrometry and related methods in structural biology. Biochim Biophys Acta. 2013;1834(6):1239–1256.
- Liu F, Rijkers DT, Post H, et al. Proteome-wide profiling of protein assemblies by cross-linking mass spectrometry. Nat Methods. 2015;12(12):1179–1184.
