Abstract
Objective: Recruiting into clinical trials on time and on target is a major challenge and yet often goes unreported. This study evaluated the adjustment to procedures, recruitment and screening methods in two multi-centre pharmaceutical randomised controlled trials (RCTs) for hearing-related problems in adults. Design: Recruitment monitoring and subsequent adjustment of various study procedures (e.g. eligibility criteria, increasing recruiting sites and recruitment methods) are reported. Participants were recruited through eight overarching methods: trial registration, posters/flyers, print publications, Internet, social media, radio, databases and referrals. The efficiency of the recruitment was measured by determining the number of people: (1) eligible for screening as a percentage of those who underwent telephone pre-screening and (2) randomised as a percentage of those screened. Study sample: A total of 584 participants completed the pre-screening steps, 491 screened and 169 participants were randomised. Results: Both RCTs completed adjustments to the participant eligibility, added new study sites and additional recruitment methods. No single recruitment method was efficient enough to serve as the only route to enrolment. Conclusion: A diverse portfolio of methods, continuous monitoring, mitigation strategy and adequate resourcing were essential for achieving our recruitment goals.
Key Words:
Introduction
As new pharmaceutical treatments are being developed for hearing-related disorders in the adult population (e.g. age-related hearing loss [ARHL], tinnitus), hearing healthcare professionals may be asked to participate in the recruitment of participants into a clinical trial. Participant recruitment is an essential component of conducting a successful clinical trial, yet it is one of the most difficult and least predictable elements. Investigators often overestimate the number of available potential participants who meet the inclusion criteria (Thoma et al. Citation2010) and many of those whom meet eligibility fail to be recruited (Allen et al. Citation1998). No matter what the clinical specialty, many trials fall short of their recruitment targets (see McDonald et al. Citation2006; Strasser, Cola, and Rosenblum Citation2013; Bower et al. Citation2014) and insufficient or untimely recruitment into randomised controlled trials (RCTs) has serious consequences. When the planned sample size is not achieved, the risk of making the erroneous conclusion that a drug is not effective is increased and external validity is diminished. When initial recruitment strategies are not successful, the trial period may need to be extended or the number of recruiting sites increased, all of which increases resources and costs. Alternatively, the trial may be abandoned, results may not be publishable or the findings may have little impact on patient health and wellbeing.
While we are not aware of any specific publications related to factors influencing recruitment into hearing-related pharmaceutical trials, Hong, Fiola, and Feld (Citation2013) found that building relationships with key stakeholders was an important factor in recruiting firefighters into an RCT of a hearing protection programme. This finding is perhaps not surprising as systematic reviews of strategies designed to improve recruitment into pharmaceutical trials also indicate that investigator and participant factors can influence recruitment rate (Fletcher et al. Citation2012; Treweek et al. Citation2013; Huynh et al. Citation2014). In general, the results of these systematic reviews highlight the benefits of making the trial open rather than blinded so that participants know what treatment they will receive; providing financial incentives to participants; reducing clinician’s workload; frequent contact between the trial coordinator and clinicians/trial site and continuous monitoring. The applicability of these approaches to increasing recruitment for hearing-related pharmaceutical intervention trials is unknown, particularly as none of the individual studies included in the systematic reviews related to hearing disorders.
Although we are not aware of any published reports specific to recruitment into pharmaceutical trials for hearing-related disorders, recent trials within hearing healthcare show recruitment challenges. For example, Piccirillo et al. (Citation2007) conducted an RCT of gabapentin for tinnitus funded by National Institute of Health in the United States (US). The recruitment goal was 160 participants (80 gabapentin and 80 placebo), but closed with 135 enrolled (59 gabapentin and 56 placebo). Considerable resources were likely expended on recruitment since 1028 participants were screened. The overall efficiency was low, with 669 (65%) of those screened not eligible and 224 (22%) of those eligible declining to participate. A majority of those excluded (n = 514) had only mild self-reported tinnitus severity. The high screen-failure rate is not isolated to this tinnitus RCT, as 48% of the 160 adults assessed for eligibility in a trial of an investigational medicinal product for the prevention of noise-induced hearing loss also failed to meet eligibility (Kil et al. Citation2017). In contrast, when normal hearing participants are being recruited a high efficiency can be achieved. For example Le Prell et al. (Citation2016) had a relatively low screen failure rate (26%) in a study focussed on a dietary supplement to prevent music-induced hearing loss and recruited university students (aged 18–31 years) who had normal hearing.
While challenges in participant recruitment are widely recognised in many areas of research, recruitment into pharmaceutical trials for hearing disorders may have some unique challenges. First is the relative complexity of current approaches to clinical management of hearing disorders, which can involve general practitioners (GPs), otologists (ENTs) and audiologists. Second is the relatively limited experience of the audiologists in participating in pharmaceutical RCTs. Increased understanding of the efficiency of methods which can be used to recruit into hearing-related pharmaceutical trials may therefore be beneficial.
The purpose of this report is to describe the recruitment monitoring and subsequent adjustment of various study procedures and describe the recruitment methods for two hearing-related phase IIa pharmaceutical RCTs: Quest In Eliminating Tinnitus (QUIET-1) in England and CLARITY-1 in the US. Each RCT tested the same novel drug compound for associated hearing-related problems. The sponsor for both trials was the same, Autifony Therapeutics Ltd, despite the populations recruited being different across the two RCTs with different regulatory requirements based on country having an influence on methodologies. Clinical trial support was provided in both trials by the same ISO-certified international Contract Research Organization (CRO), CROMSOURCE. Hearing expertise support was provided to the sites by two academic partners: the University of Nottingham for QUIET-1 and the University South Florida (USF) for the CLARITY-1. Using data from the academic partners, our aim was to evaluate the recruitment monitoring and subsequent adjustment of various study procedures (e.g. eligibility criteria, increasing recruiting sites and recruitment methods) and report the efficiency of recruitment and screening methods to achieve the planned sample size.
Overview of the RCTs
The QUIET-1 and CLARITY-1 trials had a number of common elements. Both compared the effect of repeat dosing of AUT00063 relative to a placebo control. AUT00063 is a synthetic molecule which modulates specific voltage-gated potassium ion channels present within the neurons of the central auditory system. Both QUIET-1 and CLARITY-1 were multi-centre, randomised, double-blind, parallel group, placebo-controlled trials with the primary endpoint at 28 days after the first drug dosing day. Consented participants attended between five and six in-clinic visits spread across a maximum of 10 weeks, with up to two monitoring telephone calls. Financial compensation was offered to all participants. Once randomised, the drug was taken orally, once daily, for 28 days. Relevant ethical and regulatory agencies approved all procedures, compensation and recruitment methods.
The two trials were open to recruitment in parallel. QUIET-1 targeted recruitment of up to 152 participants and enrolment was open from December 2014 until October 2015. CLARITY-1 initially sought to recruit 100 participants and recruitment was active from March 2015 until April 2016. For both RCTs, the CRO assigned staff that served as personal contact points, resolved queries, conducted monitoring visits and so on. Each site had a designated coordinator who weekly reported to the CRO the number of people pre-screened, the number of people attending a screening visit, the number of and reasons for screen failure and randomisation logs. Weekly recruitment reports for the trial as a whole were created by the CRO and monitored by the sponsor at a weekly teleconference.
Both RCTs utilised a structured telephone pre-screening interview asking questions about tinnitus/hearing loss, demographic factors and general physical health status. There was a major primary difference between the two trials for pre-screening. Specially, this related to use of a centralised versus decentralised pre-screening approach. For QUIET-1, the Nottingham site was the central contact point and two trial administrators conducted telephone pre-screening interviews. Those who were potentially eligible were advised to contact their local site to book a screening visit or ask their GP to write a letter. For CLARITY-1, pre-screening was decentralised, with each site independently responsible for telephone interviews.
Once the potential participants passed the telephone pre-screening they moved to on-site eligibility screening after completing the informed consent process. Measures to ensure general good health included physical examination, vital signs, urinalysis, haematology and biochemistry, medical history, electrocardiography and medication review. A number of eligibility criteria were common to both RCTs and these are presented in Supplementary File A. The RCT designs had a number of additional differences relating to eligibility criteria, number and type of recruiting sites and planned recruitment methods, as described below.
Participant eligibility
For QUIET-1, the therapeutic target was subjective tinnitus associated with ARHL and for CLARITY-1 it was difficulty understanding speech in noise associated with ARHL. For this reason, there were differences across the trials in terms of eligibility criteria. provides details specific to the QUIET-1 trial, according to the final version of the Clinical Trial Protocol (version 1.5), while provides details specific to CLARITY-1 according to the final protocol version (version 4.0). For QUIET-1, audiometric testing ensured sensorineural hearing loss and the score on the Tinnitus Functional Index (TFI; Meikle et al. Citation2012) identified those with moderate to severe tinnitus symptoms (). For CLARITY-1 (), audiometric testing ensured a strict hearing loss configuration and a significant difficulty with understanding speech in noise measured by the Quick Speech-in-Noise Test (QuickSIN; Killion et al. Citation2004). Further screening tests excluded individuals with any other relevant impairments (e.g. cognitive impairment).
Table 1. Eligibility criteria that were specific to QUIET-1, including a history of approved substantial changes to the Clinical Trial Protocol.
Table 2. Eligibility criteria that were specific to CLARITY-1, including a history of approved substantial changes to the Clinical Trial Protocol.
Recruiting sites
It was anticipated that 10 National Health Service (NHS) hospital sites would be sufficient to recruit the 152 target for QUIET-1. Two “backup” sites were identified as a mitigation for slow recruitment. Similarly, it was anticipated that 10 sites would be sufficient to reach the 100 target for CLARITY-1. All US sites were established ENT or Audiology practices or a research institute with a collaborating ENT/Audiology partner.
Recruitment methods
Across both trials there were eight recruitment methods. The methods utilised were primarily the result of the academic partner’s experiences with recruitment into non-pharmaceutical intervention studies for adults with ARHL and/or tinnitus in the two countries. Over the course of both trials, a small number of diverse methods to raise awareness of the study evolved. Both the initial planned methods and those that evolved over time are described below.
Trial registration: In an effort to increase transparency to all individuals potentially interested in participation in clinical research, trial registration is required. QUIET-1 and CLARITY-1 were registered on ClinicalTrials.gov (NCT02315508 and NCT02345031, respectively). Information about CLARITY-1 was also registered on a second trial website (www.centerwatch.com).
Posters/flyers: Both trials included the development and distribution of posters and flyers for display and dissemination. The four designs approved for QUIET-1 are shown in Supplementary File B and the three designs for CLARITY-1 in Supplementary File C. For QUIET-1, 12,800 posters were printed using all four designs and 3824 (31.6%) were distributed to GP centres, pharmacies and local hospitals by all trial sites. One of the sites requested electronic pdfs for display screens in waiting rooms. Electronic versions of the CLARITY-1 materials were made available to all study sites for local customisation. At USF, the posters were placed in multiple locations across the campus and electronic versions were posted on display screens in waiting rooms of the medical clinics.
Print publications: QUIET-1 budgeted for one planned paid feature article in a regional community magazine which targeted the post-60 year-old age group. In addition, free-of-charge short editorials appeared in the health section of several tabloid newspapers reporting on Autifony Therapeutics Ltd or reporting on tinnitus and QUIET-1. Feature articles were also published in major hearing-related national magazines in the United Kingdom, targeting both members of the public and hearing healthcare professionals (i.e. British Tinnitus Association, ENT and Audiology News, Hearing Times, Hearing Link). The Nottingham QUIET-1 site placed paid short feature articles in six community magazines across the region, including one which targeted the post-60 year’s age group, numerous unpaid methods were also introduced around Nottingham. These included: placing a short feature article in another community magazine distributing the four different poster to more diverse locations than initially planned (i.e. libraries, universities, GP centres and pharmacies across the surrounding regions) and electronic promotional activities by the lead academic site. While CLARITY-1 did not initially budget for any paid print recruitment methods, after nine months with less than expected participants enrolled in the study, Autifony Therapeutics Ltd placed a paid featured advertisement in “Audiology Today” (http://www.audiology.org/) with 12,000 professional members. In addition, two paid advertisements were placed in local newspapers (Tampa Bay Times and The Florida Healthcare News), with circulations of 35,000 and 20,000, respectively.
Internet: Autifony Therapeutics Ltd created “clinical trial” webpages (no longer active) on their website which directed viewers to contacts for each trial. QUIET-1 feature articles and updates were published on the webpages of Action on Hearing Loss, British Tinnitus Association and the lead academic site. CLARITY-1 feature articles and updates were published on USF’s webpages and press releases were submitted to several online science and technology forums that provided readers with general information about the trial.
Social media: QUIET-1 utilised social media platforms, but creating abbreviated recruitment announcements were posted into Facebook and Twitter by the local hospital (@nuhresearch ≈1200 followers) and lead academic site (@hearingnihr ≈600 followers). These announcements were forwarded to additional pages. Although not a part of the original recruitment plan, the USF site engaged in a social media campaign which focussed on posting and sharing posts on various university-affiliated Facebook accounts.
Referral: Referrals from a hearing healthcare professional primarily came from each principal investigator or from other ENTs and audiologists. E-mail dissemination, presentations at clinician meetings and word-of-mouth raised awareness and encouraged hearing health professionals to inform potential participants of the trial. Only done in CLARITY-1, other healthcare providers serving potential participants in the local area were sent a “Dear Doctor Letter” informing them about CLARITY-1. These routes were initially planned personal referrals in hopes of spreading the word about the study and encouraging individuals that knew about the study to share with others as a form of personal referrals. Besides patient referrals from healthcare professionals, both sites utilised methods to increase personal referrals. In the Nottinghamshire area, updates appeared in the quarterly newsletter for the lead academic site which had a distribution of over 1000 readers via post and email. For QUIET-1 ad hoc unplanned other personal referrals came from more informal channels, namely relevant charitable organisations with telephone helplines, Tinnitus Support Groups (including presentations to these groups), online discussion forums and personal recommendation by word-of-mouth. Similarly for CLARITY-1, USF has an “opt-in” listserv for current and previous faculty, staff, and students who are willing to receive a variety of announcements including current study alerts. A study-alert message was sent to all employees on the USF listserv. In a similar fashion, USF also maintains alumni e-mail distribution lists and an announcement was sent to these lists informing subscribers about the CLARITY-1 study.
Radio: Numerous local BBC and commercial radio stations were used to broadcast short unpaid interviews with local trial site staff about tinnitus and the launch of QUIET-1. This method of recruitment was not utilised for CLARITY-1.
Database review: For CLARITY-1 the primary planned recruitment method was through database review, either an electronic medical record or other custom local database, for identification of potential participants. While the National Health Services in the UK does have electronic medical records, information about tinnitus is not systematically captured, so database review was not a viable method for the identification of potential participants for QUIET-1 trial.
Recruitment monitoring
Recruitment tactics and other feedback to QUIET-1 sites occurred via email updates, six trial e-newsletters, an investigators kick-off meeting and four teleconferences attended by site representatives, sponsor and CRO. Feedback to CLARITY-1 sites with an initial investigators kick-off meeting then summary updates occurred via email updates, eight e-newsletters and two teleconferences.
Analyses
The data obtained were first examined descriptively in terms of the ability to meet target goals as a function of protocol modifications (e.g. participant eligibility criteria and recruiting sites) and then the recruitment strategies utilised. The efficiency of recruitment method was calculated, as defined by Lloyd, Dean, and Ada (Citation2010), as a function of the number of people that completed the telephone pre-screening and eligible for screening and also by determining the number of people randomised as a percentage of those screened. With respect to pre-screening efficiency calculation, the Nottingham site conducted centralised pre-screening for QUIET-1 with those passing referred to the closest study site for onsite screening. For the CLARITY-1 study, pre-screening data were only available for analysis from the primary academic partner site, USF. With respect to screening efficiency calculation, full records were kept at all sites on the number of people attending the screening visit and the number of screen failures; this allowed for the calculation of each site’s screening efficiency. The efficiency of the specific recruitment method leading to randomisation was only available from Nottingham and USF. Finally, since efficiency needed to be balanced by a recruitment method’s cost-effectiveness, relevant data from the two primary academic partners, Nottingham for QUIET-1 and the USF for CLARITY-1, were examined.
Results
Pre-specified target recruitment numbers were not met in either trial. For QUIET-1, 91 of the 152 target were consented, screened and randomised at which point a planned interim analysis was conducted. As is increasingly occurring in clinical trial practice, one purpose of the interim analysis was to determine whether or not the clinical trial, if continued, was likely to achieve its primary efficacy objective. For the QUIET-1 interim analysis, the futility criteria was met (p > 0.39), leading to a recommendation that the study be discontinued and the sponsor accepted.
Motivated by a slow recruitment rate into CLARITY-1, reconsideration of the statistical powering for the trial by the sponsor was conducted. Power was initially determined by a 3 dB improvement on the QuickSIN test, with an understanding that the initial target recruitment (n = 100) was greater than indicated through power analyses (n = 10), it was recommended that the recruitment goal decrease to 70 instead of 100 and the change was approved as part of Version 4.0 of the Clinical Trial Protocol on 24 February 2016. At the end of recruitment period (1 April 2016) a total of 79 individuals were consented, screened and randomised.
Participant eligibility criteria effect on efficiency
Active monitoring across sites occurred for both QUIET-1 and CLARITY-1. As a result of observing unexpectedly high screen fail rates, there were substantial amendments to both of the trials protocols.
Participants in QUIET-1 could fail eligibility on multiple criteria but the most common reason being failure to meet audiometric criteria, which accounted for 44 of the 106 exclusions or 41.5% (). Although audiometric criterion became a concern early in the trial based on feedback from site audiologists, only one participant failed the eligibility screening prior to the first change to the criterion in protocol amendment in November 2014. Hence, there was insufficient “before” data to explore the impact of this particular amendment. As shown in , a subsequent amendment in February 2015 relaxed the audiometric criterion again. Prior to this amendment, five of the nine excluded participants (55.6%) had failed on hearing status. Following amendment, this proportion was reduced to 40.6% (39 out of 96).
For CLARITY-1, the leading cause of the 277 screen failures or 68% of total failures, was the absence of a significant speech-in-noise deficit as measured by the QuickSIN (see ). The QuickSIN requirement could not be adjusted as the participants needed to demonstrate a deficit in speech-in-noise recognition and reducing the entry criteria would include clinically normal performance with no room for improvement. However, relaxing the audiometric criteria defining ARHL did improve recruitment ().
Recruiting sites
For both RCTs, more than the 10 planned sites were opened to support the slower than expected recruitment and mitigate for the delay in opening certain sites. illustrates the location of all sites that screened participants.
Figure 1. Recruiting sites for QUIET-1 in England (left-hand panel) and for CLARITY-1 in the US (right-hand panel).
and report the site timelines, screening and randomisation activity at each site. For QUIET-1, an additional eight sites including the two original backups were opened, while for CLARITY-1, three sites were added. Quick identification and qualification of new sites, as well as a focus on those familiar with delivering clinical trials were beneficial.
Table 3. Timeline for opening sites to QUIET-1 recruitment, with screening and randomisation listings.
Table 4. Timeline for opening sites to CLARITY-1 recruitment, with screening and randomisation listings.
Efficiency of recruitment
displays the data from the sites of the academic partners for the different recruitment methods at the pre-screening and then screening stage for academic sites for the QUIET-1 and CLARITY-1 trials, respectively. In the table, the specific recruitment methods are listed under larger categories and these larger categories were essentially used in both RCTs with the exception of “Radio” being solely used in QUIET-1 and “Database review” being a planned recruitment method for CLARITY-1. All recruitment methods are noted as either being planned/unplanned and paid/unpaid. Both RCTs added additional recruitment methods (unplanned) to increase recruitment along the course of the trials. While “Posters/flyers” generated the most pre-screens for QUIET-1 (n = 102), this was a smaller recruitment mode for CLARITY-1 (n = 14). Also interesting, the Internet was a much more popular route for Nottingham (n = 76) than at USF (n = 2). These data were used to determine the efficiency of the recruitment methods in terms of (1) pre-screening and (2) screening as discussed below.
Table 5. Efficiency of the different recruitment methods used in the QUIET-1 and CLARITY-1 RCTs.
Eligibility as a percentage of those who underwent telephone pre-screening: pre-screening efficiency
The numbers of telephone pre-screening calls completed and the individuals who passed the pre-screening are reported in and this information was also used to determine the pre-screening efficiency of the different recruitment methods (top panel, ). A higher pre-screening efficiency indicates that people responding to a particular method were more likely to pass pre-screening and invited to attend an in-person screening visit.
Figure 2. Efficiency of the different recruitment methods used in the QUIET-1 and CLARITY-1 RCTs. The top panel shows pre-screening efficiency measured by determining the number of people eligible for screening as a percentage of those who underwent telephone pre-screening. The bottom panel shows screening efficiency measured by the number of randomised participants as a percentage of those screened.
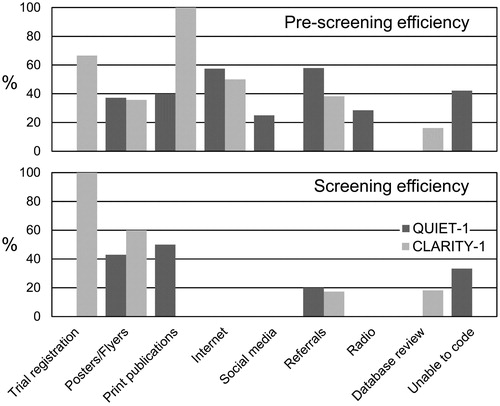
Nottingham was the pre-screening coordinating site for all QUIET-1 sites. The Nottingham staff conducted 425 structured pre-screening interviews between 2 December 2014 and 8 October 2015. Of these, 181 failed on a specific criterion for tinnitus (duration ≥6 and ≤18 months at enrolment), with the majority (n = 179) experiencing chronic tinnitus symptoms. A further 29 participants were excluded because they were taking concomitant medications that were not permitted in the Clinical Trial Protocol. As can be seen in , although the absolute number of planned referrals from a healthcare professional in QUIET-1 was small, the efficiency of this route at pre-screening was the highest (58%), certainly relative to the more costly printed publication methods (40%) or poster campaigns (37%). Overall and across all recruitment methods, 235 recruits did not pass the pre-screening and were not eligible for an on-site screening visit, while 190 individuals were invited to attend a screening visit at the closest QUIET-1 site (45.7% efficiency).
Unlike QUIET-1 where Nottingham coordinated all pre-screening activity, each CLARITY-1 site conducted their own pre-screening. CLARITY-1 pre-screening data were completed at USF (, ). Between 5 March 2015 and 1 April 2016 USF pre-screened 151 individuals, of whom 106 were excluded as not eligible, leading to an overall 29.8% pre-screening efficiency. Reasons for exclusion included: current or recent hearing aid use, demographics (age, English not the first/primary language and self-reported professional musicians), ineligible hearing loss (not age-related, caused by ototoxic medications and known asymmetry), use of prohibited medications, current enrolment in another study and self-report of severe tinnitus. The CLARITY-1 team anticipated that the main source of recruitment would be from database review but while the absolute numbers were high (68 pre-screens), efficiency was low (16%). Beyond the database recruitment, the planned methods of trial registration (67%), posters/flyer distribution (36%), Internet (50%) and referrals (37%) had a combined mean efficiency rate of 47.5%. The recruitment methods implemented later in the study (paid newspaper advertisements and social media) had a combined mean efficiency rate of 45.3%.
Eligibility as a percentage of those who underwent screening: screening efficiency
Data was recorded for all of the 214 screening visits conducted across the 15 QUIET-1 sites (). Of these, 91 participants were randomised (42.5% efficiency) and the median efficiency across sites was 44% (). Efficiency may be underestimated since, within the remainder, six individuals had a decision pending further medical investigation, one person was eligible but declined to participate and 10 individuals had been screened but were not taken further due to the study termination. In total, only six of the 123 participants (4.9%) identified as potential participants through telephone pre-screening strategy were excluded at the screening visit with the otologist consultant. Six were excluded for tinnitus duration, with the primary reason being duration. That is, the potential participants moved out of the eligible time window by the time of their screening appointment.
Across all 13 CLARITY-1 sites, 277 participants were screened (). Of these, 79 participants were randomised (28.5% efficiency) and the median efficiency across sites was 24% (). At the USF site, 34 of the 45 participants (76%) were still excluded at the screening visit and so we concluded that the pre-screening strategy was only moderately effective. The moderate efficiency was caused by lack of ability to predict speech-in-noise performance; thus, results of QuickSIN were a major stumbling block.
For both RCTs, reports the total number of subjects screened on-site and randomised with respect to recruitment methods utilised at Nottingham and USF and this information was also used to determine the screening efficiency of the different recruitment methods (bottom panel, ). As the numbers were small, we do not interpret observed trends. However, worthy of note is that the “Internet” channels were generally popular routes at the pre-screening stage, but did not yield any randomised participants at either site. In addition, both RCTs had acceptable efficiency from the “posters/flyers” (43% for Nottingham, 60% for USF) although the pre-screening efficiency was lower for this method (37% for Nottingham, 36% for USF). Although the absolute number of healthcare professional was small, these routes were efficient for both RCTs, certainly relative to the more expensive printed publications methods.
Resource implications
For QUIET-1, printed media were the most costly method of recruitment. A recruitment plan was developed at the start of the trial by a graduate in journalism and marketing at a cost of £3984. He prepared all of the features that were fed into the newspaper, poster and magazine advertisements. In addition to this, an estimate of the total cost for the poster campaigns was £3150, including design, printing, stationery and labour and £1050 for the magazine campaigns, including publication fees and labour. These figures are likely to be underestimated because labour costs for dealing with additional promotional opportunities were not recorded. Furthermore, labour costs for dealing with telephone and email queries, separate from the actual telephone pre-screening calls, is not accounted for.
For CLARITY-1, printed advertisements in local newspapers were the only recruitment method with a direct site cost ($4364). Again, this is an underestimate because labour costs of the study staff who designed the advertisements, obtained regulatory approval and negotiated the placement in the newspaper were not available. Although the paid advertisements only yielded three pre-screening interviews and none continued on to screening, the potential for good recruitment needs to be acknowledged. The two local advertisements were placed in March 2016 with recruitment and enrolment closed on 1 April 2016. The number of calls that could have potentially lead to successful recruitments were tallied to 34 individuals from April to June. Thus, the paid advertisements may have been more cost-effective if initiated earlier in the recruitment phase. Experience suggests that the database review method was unlikely to be cost-effective. As an example of the effectiveness of database review, the USF database included 478 adults which was narrowed to 68 who fit the age range, were known to have ARHL and did not report using hearing aids at time of last contact. Despite the substantial labour demands needed to search all database records and complete the 68 identified contacts, only 11 individuals were eligible for screening and only two were randomised.
The “posters/flyers” used to promote QUIET-1 turned out not to be so low-cost and low-resource, primarily because of the substantial labour required to manually enter the local contact details for each paper copy. The Nottingham site packaged and posted materials to other sites enabling us to at least track the demand across sites. For CLARITY-1, sites received electronic copies of posters/brochures and so greater responsibility for printing was locally delegated, with less opportunity for tracking poster-related promotional activities.
Both the telephone pre-screening and screening visits demanded considerable labour to sustain. For the Nottingham QUIET-1 team, the resource demand was two-fold. First, the number and duration of calls was greater than planned. The protocol planned for a short (15 min) call to assess duration of tinnitus, relevant medical history and concomitant medications. However, calls occasionally lasted up to one hour and some conversations were challenging because the trial staff had to manage the person’s anticipation to find a cure and disappointment at being excluded. Second, organising the screening visit was logistically challenging since it required appointments with multiple professionals who were based across two hospital campuses. For CLARITY-1, the labour demand was principally attributable to the high number of screen fails due to the QuickSIN scores. To conserve resources, study staff were recommended to start the screening visit with the hearing assessment, thus reducing the likelihood of completing unnecessary invasive procedures such as specimen collection for haematology and biochemistry.
Discussion
Multi-site randomised controlled clinical trials are relatively uncommon in ENT/Audiology clinics, but with high potential for novel interventions for ARHL and tinnitus there are likely to be more RCTs in the future. This is the first evaluation of recruitment methods in hearing-related, multi-site clinical trials in adults. The present work demonstrates the difficulties that may be encountered in ENT/Audiology recruitment. Based on our experiences, we have learned the importance of planning, budgeting, implementing and monitoring a recruitment plan that is relevant for all sites includes paid advertisements and has adequate risk mitigation. In this section, we reflect on the strengths and limitations of our different recruitment methods so that other study teams may take these into account when planning future multi-centre pharmaceutical trials in ENT/Audiology. The primary caveat to our reflections is that the systematic collection of such data was not planned into the studies. As a consequence, such information is available in a reliable form only from the primary academic sites.
Recruitment methods: strengths
Although telephone pre-screening was labour intensive, in general it contributed positively to the efficiency of the screening visits because participants with exclusions that were easily identifiable by verbal questioning could be ruled out. Nevertheless, in both RCTs a reasonably large proportion failed on audiological criteria that could only be assessed during an on-site visit. For QUIET-1, this was the audiometric threshold for identifying a sensorineural hearing loss and for CLARITY-1, it was performance threshold on the speech-in-noise recognition task. The sponsor’s and CRO’s experience in risk management promoted active monitoring. Regular communication with trial sites helped to identify the need for remedial action by substantive amendments to the eligibility criteria and by requesting each site to develop its own ad hoc recruitment plan. These steps contributed to the ultimate enrolment achievements.
For both RCTs, the internet avenues achieved the greatest outreach for low cost and low resource, especially given that the majority were externally maintained. In most cases, trial-specific details could be found by active searching for hearing-related research information. Evidence suggests that internet use by older adults makes this recruitment method worth considering. For example, about 59% of US adults aged >65 years use the Internet, with 24% going online multiple times per day (Perrin Citation2015; Anderson and Perrin Citation2016). Based on our QUIET-1 experience, it appeared that people with chronic tinnitus were likely to use the Internet to seek out information about clinical trials of tinnitus; in contrast, experience with CLARITY-1 indicated that people with ARHL were less likely to do so. We note that another tinnitus-related clinical study successfully recruited at least 26% of its participants using Internet recruitment methods (Handscomb et al. Citation2016), the mode of data collection which was Internet based , could have enhanced the success rate of this recruitment method (Rosa et al. Citation2015). Further research is warranted to determine whether or not internet recruitments can be successful for studies aimed at people with ARHL.
Unpaid newspaper and radio feature sessions were most effective at specific points in the trial cycle. For the QUIET-1 trial, media channels published and broadcasted promotional stories (1) when the trial opened for recruitment nationally, (2) when a new site opened for recruitment locally and (3) if there was specific newsworthy link to the story. An example of the latter case was a large-scale campaign planned around the UK Tinnitus Awareness Week (2–8 February 2015), with the resulting media coverage having a major (but transient) boost on telephone queries, such that Nottingham handled 90 telephone pre-screening interviews in February, compared to 14 in January and 57 in March. Although efficiency at pre-screening was around 30%, the campaign did have some drawbacks. There was a strong likelihood of attrition because many callers lived more than 20 miles travelling distance from a recruiting site and a large number of calls came from London area, exceeding the capacity of the London site.
Recruitment methods: limitations
It has been commented that recruitment strategies which work for some studies often do not work well for others and that it is important for sites to test out different recruitment methods to find out what works best (Kye et al. Citation2009). Nevertheless, our experiences can provide important lessons for ENT/Audiology. Across the two RCTs, recruitment proved to be more resource intensive and had a higher proportion of screen failures than initially anticipated during trial design and planning, requiring an extension to the recruitment period and the addition of sites. These recruitment struggles are similar to those reported for other disciplines too (Lloyd, Dean, and Ada Citation2010; Usadi et al. Citation2015).
Similarly a report by Usadi et al. (Citation2015), discussed that collaborative communication was positive and beneficial in an evaluation of recruitment strategies for randomised controlled trials involving infertile couples, both QUIET-1 and CLARITY-1 held regular teleconferences between recruiting sites and the trial’s data coordinating centre at which monthly enrolment data and effective recruitment strategies were shared and good performing sites were acknowledged. Such techniques seek to achieve “buy-in” from collaborators by developing a sense of personal ownership and commitment, techniques perhaps more common to business marketing than to clinical research. While both of the present trials did maintain contact through newsletters and teleconferences, feedback from sites suggests that there was room for improvement. For both trials, sites certainly welcomed the opportunity to discuss recruitment rates, highlight difficulties they were facing and successful recruitment outlets. However, each recruiting site could not always be represented at the teleconferences due to conflicting clinical priorities.
While physician referral can be more effective than advertising strategies for some intervention trials (Lloyd, Dean, and Ada Citation2010; Usadi et al. Citation2015) professional clinical referral was not as successful as had been anticipated at either site. Major eligibility criteria were not routinely assessed in-clinic, so a reasonably large proportion failed on those criteria at the screening visit. For the same reason, in CLARITY-1, database review also proved to be a rather inefficient and a large proportion of those contacted failed at pre-screening because they used hearing aids (see ). Like others (e.g. Usadi et al. Citation2015), professional referrals to CLARITY-1 were found to be most effective when there was an existing close relationship between specialties and the trial team. Similarly, Lloyd, Dean, and Ada (Citation2010) also commented that referral strategies are more successful and efficient than advertising strategies at recruiting community-dwelling stroke survivors. It is likely that referral was the most successful and efficient when the referring physicians were able to determine if stroke survivors initially fulfilled the inclusion criteria prior to contacting the investigators, resulting in higher efficiency in terms of eligibility as a proportion of those screened. Of the eight participants coming from professional referral who were screened by USF, seven came from the wider USF health network (one ENT and six audiologists) and just one came from outside the USF practice. This could be because the within-network referrals came from healthcare providers highly motivated to assist the primary coordinating site and so they actively looked out for potentially eligible participants. We also note that neither physician at the Nottingham or USF sites specialised in areas directly relevant to the target clinical population (i.e. paediatric and cochlear implant specialists); thus, the low referral from those physicians is perhaps not surprising.
Concluding remarks
Taking a business approach to trials has been shown to be beneficial in several multi-centre trials (McDonald et al. Citation2011). Adopting an explicit marketing plan, engaging charities or participants to act as champions, delivering effective messages to multiple audiences at multiple levels and achieving clinician and public buy-in are all known business components (Francis et al. Citation2007). Additionally, researchers need to ensure that they have sufficient budget to not only support staff to recruit participants, but to fund the evaluation of recruitment strategies in clinical trials. Our management of QUIET-1 and CLARITY-1 touched upon these factors, but such business approaches were not consistently planned and resourced at the outset. Further research is warranted to evaluate the effectiveness of recruitment strategies to ENT/Audiology clinical trials.
Declaration of interest
AS, JW, ST and PH were employees of Autifony Therapeutics Ltd at the time of the trials. There is no potential conflict of interest since there is no commercial or business interest in the research reported in this article.
QUIET-1 was funded by Innovate UK Ref 35370-247243 (June 2014–August 2016); Charles Large (Principle Investigator) and Deborah A. Hall (Academic Partner). CLARITY-1 was funded by Autifony Therapeutics Ltd.
Supplementary material available online
Victoria_A._Sanchez_et_al._Supplementary_files.zip
Download Zip (6.1 MB)Acknowledgements
We thank Theresa H. Chisolm and Matija Daniel for comments on earlier drafts of this manuscript. We thank all participants for their time and effort while enrolled in the studies. For QUIET-1, we thank the clinical and research staff at the following recruiting sites in England: Nottingham University Hospitals NHS Trust, Sheffield Teaching Hospitals NHS Foundation Trust, University Hospitals Birmingham NHS Foundation Trust, University College London Hospital NHS Trust, Shrewsbury and Telford Hospital NHS Trust, The Newcastle upon Tyne Freeman Hospital, The Newcastle upon Tyne Hospitals NHS Foundation Trust, Wigan and Leigh NHS Foundation Trust, Salford Royal NHS Foundation Trust, University Hospital of North Staffordshire NHS Trust, The Pennine Acute Hospitals NHS Trust, Frimley Health NHS Foundation Trust, Portsmouth Hospitals NHS Trust, The Norfolk and Norwich Hospitals NHS Foundation Trust, East and North Hertfordshire NHS Trust and Plymouth Hospitals NHS Trust. Thanks to the British Tinnitus Association for making introductions to Tinnitus Support Groups across England. For CLARITY-1, we thank the clinical and research staff at the following recruiting sites: University of South Florida; Jean Brown Research; Sacramento ENT; Colorado ENT and Allergy; ENT Associates of South Florida; QPS MRA (Miami Research Associates); University of Louisville; Mayo Clinic- Rochester; The University of Mississippi Medical Center; Long Island Jewish Medical Center; PMG Research Inc.; Piedmont Ear, Nose, & Throat Associates and Vanderbilt University Medical Center.
References
- Allen, P. J., A. Stojadinovic, C. D. Shriver, and D. P. Jaques. 1998. “Contributions from Surgeons to Clinical Trials and Research on the Management of Soft Tissue Sarcoma.” Annals of Surgical Oncology 5 (5): 437–441. doi:10.1007/BF02303862.
- Anderson, M., and A. Perrin. 2016. “13% of Americans Don’t Use the Internet. Who are they?” Pew Research Center, Internet and Tech, September 7. Accessed 12 October 2017. http://www.pewresearch.org/fact-tank/2016/09/07/some-americans-dont-use-the-internet-who-are-they/
- Bower, P., V. Brueton, C. Gamble, S. Treweek, C. T. Smith, B. Young, and P. Williamson. 2014. “Interventions to Improve Recruitment and Retention in Clinical Trials: A Survey and Workshop to Assess Current Practice and Future Priorities.” Trials 15: 399. doi:10.1186/1745-6215-15-399.
- Fletcher B., A. Gheorghe, D. Moore, S. Wilson and S. Damery. 2012. “Improving the Recruitment Activity of Clinicians in Randomised Controlled Trials: A Systematic Review.” BMJ Open 12: e000496. doi:10.1136/bmjopen-2011-000496.
- Francis, D., I. Roberts, D. R. Elbourne, H. Shakur, R. C. Knight, J. Garcia, C. Snowdon, et al. 2007. “Marketing and Clinical Trials: A Case Study.” Trials 8: 37. doi:10.1186/1745-6215-8-37.
- Handscomb, L., D. A. Hall, G. W. Shorter, and D. J. Hoare. 2016. “Online Data Collection to Evaluate a Theoretical Cognitive Model of Tinnitus.” American Journal of Audiology 25 (3S): 313–317. doi:10.1044/2016_AJA-16-0007.
- Hong, O., L. A. Fiola, and J. Feld. 2013. “Challenges and Successes in Recruiting Firefighters For Hearing Loss Prevention Research.” Workplace Health & Safety 61 (6): 257–263. doi:10.1177/216507991306100604.
- Huynh, L., B. Johns, S. -H. Liu, S. S. Vedula, T. Li, and M. A. Puhan. 2014. “Cost-Effectiveness of Health Research Study Participant Recruitment Strategies: A Systematic Review.” Clin Trials 11 (5): 576–583. doi:10.1177/1740774514540371.
- Kil J., E. Lobarinas, C. Spankovich, S. K. Griffiths, P. J. Antonelli, E. D. Lynch, and C. G. Le Prell. 2017. “Safety and Efficacy of Ebselen for the Prevention of Noise-Induced Hearing Loss: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial.” Lancet 390 (10098): 969–979. doi:10.1016/S0140-6736(17)31791-9.
- Killion, M. C., P. A. Niquette, G. I. Gudmundsen, L. J. Revit, and S. Banerjee. 2004. “Development of a Quick Speech-in-Noise Test for Measuring Signal-to-Noise Ratio Loss in Normal-Hearing and Hearing-Impaired Listeners.” The Journal of the Acoustical Society of America 116 (4 Pt 1): 2395–2405. doi:10.1121/1.1784440.
- Kye, S. H., D. P. Tashkin, M. D. Roth, B. Adams, W. -X. Nie, and J. T. Mao. 2009. “Recruitment Strategies for a Lung Cancer Chemoprevention Trial Involving Ex-Smokers.” Contemporary Clinical Trials 30 (5): 464–472. doi:10.1016/j.cct.2009.05.004.
- Le Prell, C. G., A. Fulbright, C. Spankovich, S. K. Griffiths, E. Lobarinas, K. C. Campbell, P. J. Antonelli, G. E. Green, K. Guire, and J. M. Miller. 2016. “Dietary Supplement Comprised of β-Carotene, Vitamin C, Vitamin E, and Magnesium: Failure to Prevent Music-Induced Temporary Threshold Shift.” Audiology and Neurotology Extra 6 (2): 20–39. doi:10.1159/000446600.
- Lloyd, G., C. M. Dean, and L. Ada. 2010. “Issues in Recruiting Community-Dwelling Stroke Survivors to Clinical Trials: The AMBULATE Trial.” Contemporary Clinical Trials 31 (4): 289–292. doi:10.1016/j.cct.2010.04.003.
- McDonald, A. M., R. C. Knight, M. K. Campbell, V. A. Entwistle, A. M. Grant, J. A. Cook, D. R. Elbourne, et al. 2006. “What Influences Recruitment to Randomised Controlled Trials? A Review of Trials Funded by Two UK Funding Agencies.” Trials 7: 9. doi:10.1186/1745-6215-7-9.
- McDonald, A. M., S. Treweek, H. Shakur, C. Free, R. Knight, C. Speed, and M. K. Campbell. 2011. “Using a Business Model Approach and Marketing Techniques for Recruitment to Clinical Trials.” Trials 12: 74. doi:10.1186/1745-6215-12-74.
- Meikle, M. B., J. A. Henry, S. E. Griest, B. J. Stewart, H. B. Abrams, R. McArdle, and P. J. Myers, et al. 2012. “The Tinnitus Functional Index: Development of a New Clinical Measure for Chronic, Intrusive Tinnitus.” Ear and Hearing 33 (2): 153–176. doi:10.1097/AUD.0b013e31822f67c0.
- Perrin, A. 2015. “One-Fifth of Americans Report Going Online ‘Almost Constantly’”, Pew Research Center, Internet and Tech, December 8. Accessed 17 October 2017. http://www.pewresearch.org/fact-tank/2015/12/08/one-fifth-of-americans-report-going-online-almost-constantly/
- Piccirillo, J. F., J. Finnell, A. Vlahiotis, R. A. Chole, and E. Spitznagel. 2007. “Relief of Idiopathic Subjective Tinnitus: Is Gabapentin Effective?” Archives of Otolaryngology–Head & Neck Surgery 133 (4):390–397. doi:10.1001/archotol.133.4.390.
- Rosa, C., A. N. Campbell, G. M. Miele, M. Brunner, and E. L. Winstanley. 2015. “Using E-Technologies in Clinical Trials.” Contemporary Clinical Trials 45 (Pt A): 41–54. doi:10.1016/j.cct.2015.07.007.
- Strasser, J. E., P. A. Cola, and D. Rosenblum. 2013. “Evaluating Various Areas of Process Improvement in an Effort to Improve Clinical Research: Discussions from the 2012 Clinical Translational Science Award (CTSA) Clinical Research Management Workshop." Clinical and Translational Science 6 (4): 317–320. doi:10.1111/cts.12051.
- Thoma, A., F. Farrokhyar, L. McKnight, and M. Bhandari. 2010. “Practical Tips for Surgical Research: How to Optimize Patient Recruitment.” Canadian Journal of Surgery 53 (3): 205–210.
- Treweek, S., P. Lockhart, M. Pitkethly, J. A. Cook, M. Kjeldstrøm, M. Johansen, T. K. Taskila, et al. 2013. “Methods to Improve Recruitment to Randomised Controlled Trials: Cochrane Systematic Review and Meta-Analysis.” BMJ Open 3: e002360. doi:10.1136/bmjopen-2012-002360.
- Usadi, R. S, M. P. Diamond, R. S. Legro, W. D. Schlaff, K. R. Hansen, P. Casson, G. Christman, et al. 2015. “Recruitment Strategies in Two Reproductive Medicine Network Infertility Trials.” Contemporary Clinical Trials 45 (Pt B): 196–200. doi:10.1016/j.cct.2015.09.010.
- Zigmond A. S. and R. P. Snaith. 1983. “The Hospital Anxiety and Depression Scale.” Acta Psychiatrica Scandinavica 67 (6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x.
