Abstract
Formaldehyde (HCHO) generates reactive oxygen species (ROS) that induce DNA base modifications and DNA strand breaks and contributes to mutagenesis and other pathological processes. DNA non-homologous end-joining (NHEJ), a major mechanism for repairing DNA double-stranded breaks (DSB) in mammalian cells, involves the formation of a Ku protein heterodimer and recruitment of a DNA-dependent protein kinase catalytic subunit (DNA-PKcs) to the site of DNA damage. HCHO treatment induced DSB and decreased the protein expressions of Ku 70 and phosphorylated DNA-PKcs. Triphlorethol-A reduced DNA strand breaks and restored the expression of NHEJ-related proteins. In response to oxidative DNA base damage, 8-oxoguanine DNA glycosylase 1 (OGG1) plays a vital role in repair of 8-hydroxy-2′-deoxyguanosine (8-OhdG) via the base-excision repair (BER) process. In this study, HCHO significantly increased 8-OhdG levels, whereas triphlorethol-A lowered 8-OhdG levels. Suppression of 8-OhdG formation by triphlorethol-A was related to enhanced OGG1 protein expression. Triphlorethol-A also enhanced the expression of phosphorylated Akt (the active form of Akt), a regulator of OGG1, which was found to be decreased by HCHO treatment. The phosphoinositol 3-kinase (PI3K)-specific inhibitor LY294002 abolished the cytoprotective effects induced by triphlorethol-A, suggesting that OGG1 restoration by triphlorethol-A is involved in the PI3K/Akt pathway. These results suggest that triphlorethol-A may protect cells against HCHO-induced DNA damage via enhancement of NHEJ and BER capacity.
Formaldehyde (HCHO) is a common environmental contaminant found in tobacco smoke, paint, garments, diesel and gasoline exhaust, and medical and industrial products (CitationFlyvholm and Andersen 1993). Various animal studies showed that HCHO exposure generated reactive oxygen species (ROS) by disrupting the balance between oxidants and antioxidants in various tissues (CitationTeng et al. 2001; CitationGurel et al. 2005; CitationSaito et al. 2005; CitationZhou et al. 2006) and resulted in oxidative DNA damage (CitationSul et al. 2007). The formation of ROS leads to single- and double-strand breaks in DNA and modifications of purine and pyrimidine bases, as well as modification to 2′-deoxyribose (CitationPowell et al. 2005). Oxidative DNA modifications and mutagenic lesions are the most common threats to genomic stability and play important roles in numerous pathological conditions, including cancer (CitationKondo et al. 2000; CitationCaporaso 2003; CitationCooke et al. 2003; CitationGackowski et al. 2005). It was found that HCHO produced formation of DNA–protein cross-links and DNA strand breaks (CitationGrafström et al. 1984) and may result in genotoxicity by enhancing mutagenesis through inhibition of repair of DNA lesions (CitationGrafström et al. 1985).
The inability to repair DNA double-strand breaks (DSB) may lead to the accumulation of genomic rearrangements or mutations that contribute to tumorigenesis (Citationvan Gent et al. 2001). Homologous recombination (HR) and non-homologous end-joining (NHEJ) are the two major pathways to repair DNA DSB. NHEJ, which does not require the presence of a homologous template, is the predominant repair pathway for DSB. The DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and Ku 70/Ku 80 heterodimer play a prominent role in regulating the NHEJ process (CitationSmith and Jackson 1999). DNA-PKcs contains a DNA binding domain, a catalytic domain, and a Ku-binding domain. NHEJ is initiated when Ku 70/Ku 80 heterodimers recognize and bind to broken DNA ends, where they serve to recruit two DNA-PKcs molecules to the damaged site (CitationMeek et al. 2007). Once bound to DNA, DNA-PKcs is rapidly phosphorylated at several serine and threonine residues by itself and by other kinases. Phosphorylation destabilizes DNA-PKcs, which dissociates from DNA, enabling recruitment of several end-processing enzymes and consequently induction of the physical rejoining of DNA ends and repair of oxidative DNA damage (CitationChen and Nirodi 2007).
8-Hydroxy-2′-deoxyguanosine (8-OhdG) is an ROS-induced modification of a purine residue in DNA and a sensitive indication of oxidative DNA damage (CitationFortini et al. 2003). Large quantitites of 8-OhdG are produced in mammalian cells, either as a by-product of normal oxidative metabolism or as a result of exogenous sources of ROS (CitationZhang et al. 2009a; Citation2009b). During DNA replication, 8-OhdG mismatches with adenosine, giving rise to a G:C to T:A transversion mutation that damages active genes in cells, resulting in induction of cell loss (CitationHyun et al. 2000; Citation2003). Many DNA repair systems exist to preserve genomic integrity and the genetic function. The DNA base excision repair (BER) pathway represents a critical step in the maintenance of genomic stability. The BER process involves (1) recognition of damaged bases by specific DNA glycosylases, (2) hydrolysis of the glycosidic bonds between bases and deoxyribose, and (3) excision of the damaged DNA strand by an apurine/apyrimidine endonuclease at the resulting abasic site, thus creating a DNA single-strand break (CitationBoiteux et al. 1990). This pathway plays an important role in prevention of disease through the removal of oxidized bases. The BER enzyme 8-oxoguanine glycosylase (OGG1) repairs 8-OhdG in mammals, and thus preserves genomic integrity and genetic functions. CitationLan et al. (2004) suggested that OGG1 deficiency was associated with an increase in mutations and may participate in carcinogenesis.
Recently, antioxidant compounds such as luteolin, quercetin, rosmarinic acid, butin, and 7,8-dihydroxyflavone were shown to enhance oxidative DNA damage protection and repair (CitationSilva et al. 2008; CitationKang et al. 2009; CitationZhang et al. 2009a), suggesting these compounds may protect against oxidative stress-induced DNA damage, which occurs in several pathological conditions such as Alzheimer's disease, cancer, and hypertension (CitationZana et al. 2006; CitationThompson 2006; CitationSubash et al. 2010).
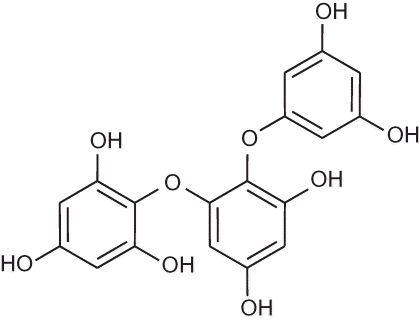
Triphlorethol-A (), an open-chain trimer of phloroglucinol, is a phlorotannin component isolated from Ecklonia cava. Ecklonia cava is a brown alga (Laminariaceae) abundant in the subtidal regions of the island of Jeju in Korea. Previously, CitationKang et al. (2005) reported that triphlorethol-A protects cells from hydrogen peroxide (H2O2)-induced oxidative stress via radical quenching and catalase activation. In addition, triphlorethol-A protected against cellular damage from ionizing radiation via inhibition of apoptosis (CitationKang et al. 2006). Triphlorethol-A induced the antioxidant enzyme heme oxygenase-1 via activation of the NF-E2-related factor 2 transcription factor (CitationKang et al. 2007). Furthermore, triphlorethol-A reduced matrix metalloproteinase-1 induction via inhibition of extracellular signal-regulated protein kinase and activator protein-1 (CitationKang et al. 2008). Recently, triphlorethol-A protected cells against formaldehyde-induced damage of cellular components including DNA (CitationZhang et al. 2010). The aim of the present study was to investigate whether triphlorethol-A was able to prevent oxidative DNA damage via the modulation of NHEJ and BER pathways, and to examine the possible protective mechanisms involved.
MATERIALS AND METHODS
Reagents
OGG1 antibody was purchased from A. G. Scientific Incorporation (San Diego, CA). Ku 70, phospho DNA-PKcs, phospho Akt, Akt, and β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The phospho histone H2A.X antibody was purchased from Upstate Biotechnology (Lake Placid, NY), and LY294002 was purchased from Calbiochem (San Diego, CA).
Cell Culture
Chinese hamster lung fibroblasts (V79-4 cells) from the American Type Culture Collection (Rockville, MD) were maintained at 37°C in an incubator with a humidified atmosphere of 5% CO2 and were cultured in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 U/ml).
Western Blot
Cells were seeded in a plate at a concentration of 1 × 105 cells/ml. Sixteen hours after plating, the cells were treated with triphlorethol-A (10 μg/ml), and 1 h later, 150 μM HCHO was added to the plate and incubated for an additional 24 h at 37°C. The harvested cells were then lysed on ice for 30 min in 100 μl of lysis buffer [120 mM NaCl, 40 mM Tris (pH 8), 0.1% NP 40] and centrifuged at 13,000 × g for 15 min. Supernatants were collected from the lysates and protein concentrations were determined. Aliquots of the lysates (40 μg protein) were boiled for 5 min and electrophoresed on 10% sodium dodecyl sulfate (SDS) polyacrylamide gels. Gels were transferred onto nitrocellulose membranes for blotting (Bio-Rad, CA), and membranes were then incubated with primary antibodies. The membranes were further incubated with secondary immunoglobulin G–horseradish peroxidase conjugates and then exposed to x-ray film. Protein bands were detected using an enhanced chemiluminescence Western blotting detection kit (Amersham, Buckinghamshire, UK).
Comet Assay
A comet assay was performed to assess DNA strand breakage (CitationSingh 2000). The cell pellet (1.5 × 105 cells) was mixed with 100 μl of 0.5% low-melting agarose (LMA) at 39°C and was spread on a fully frosted microscopic slide that was precoated with 200 μl 1% normal melting agarose (NMA). After solidification of the agarose, slides were covered with another 75 μl 0.5% LMA and then immersed in lysis solution (2.5 M NaCl, 100 mM Na-EDTA, 10 mM Tris, 1% Triton X-100, and 10% dimethyl sulfoxide [DMSO], pH 10) for 1 h at 4°C. The slides were then placed in a gel electrophoresis apparatus containing 300 mM NaOH and 10 mM Na-EDTA (pH 13) for 40 min to allow DNA unwinding and expression of the alkali labile damage. An electrical field was applied (300 mA, 25 V) for 20 min at 4°C to draw negatively charged DNA toward the anode. After electrophoresis, the slides were washed thrice for 5 min at 4°C in a neutralizing buffer (0.4 M Tris, pH 7.5) and then stained with 75 μl of ethidium bromide (20 μg/ml). The slides were observed using a fluorescence microscope and image analysis software (Kinetic Imaging, Komet 5.5, UK). The percent total fluorescence in the tail and the tail length of the 50 cells/slide were then recorded.
Detection of 8-Hydroxy-2′- deoxyguanosine (8-OhdG)
Cellular DNA was isolated using DNAzol reagent (Life Technologies, Grand Island, NY) and quantified using a spectrophotometer. The quantity of 8-OhdG in the DNA was determined using the Bioxytech 8-OhdG enzyme-linked immunosorbent assay (ELISA) kit from OXIS Health Products (Portland, OR) according to the manufacturer's instructions. The 8-OhdG levels were also determined by a fluorescent binding assay (CitationStruthers et al. 1998). Cells were fixed and permeabilized with ice-cold methanol for 15 min. 8-OhdG was visualized using avidin-conjugated TRITC under a fluorescence microscope.
Cell Viability
Cells were seeded in a 96-well plate at a concentration of 1 × 105 cells/ml, and were pretreated with 50 μM of LY294002 (a specific PI3K inhibitor) for 1 h, followed by treatment with triphlorethol-A (10 μg/ml) for 1 h, and finally 150 μM HCHO for 24 h. Fifty microliters of MTT stock solution (2 mg/ml) was then added to each well for a total reaction volume of 200 μl. After being incubated for 4 h, the plate was centrifuged at 800 × g for 5 min and the supernatants were aspirated. The formazan crystals in each well were dissolved in 150 μl dimethyl sulfoxide and absorbance A 540 was read with a scanning multi-well spectrophotometer (CitationCarmichael et al. 1987).
Statistical Analysis
All measurements were conducted in triplicate (n = 3), and all values are represented as means ± standard error (SE). Data were analyzed by analysis of variance (ANOVA) using the Tukey test. The criterion for significance was set at p < .05.
RESULTS
Triphlorethol-A Inhibits DNA Strands Break Induced by HCHO
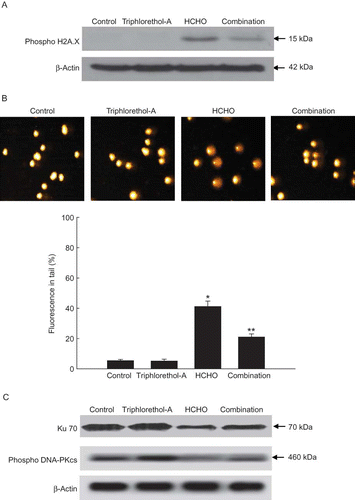
HCHO treatment increased the expression of phosphorylated histone H2A.X, a protein marker of DSB; however, triphlorethol-A treatment significantly decreased the expression of phosphorylated histone H2A.X (). Comet data demonstrated that HCHO treatment produced a significant increase in tail length and DNA damage. Triphlorethol-A treatment was able to lower this to 21% (). Ku 70/Ku 80 proteins are two key regulatory subunits of the DNA-dependent protein kinase (DNA-PK), which plays an essential role in repairing DSB (CitationSmith and Jackson 1999). DNA-PKcs undergoes a series of phosphorylations at several serine and threonine residues by itself and by other, as yet unknown, kinases. This phosphorylation is postulated to induce the repair of oxidative DNA damage (CitationChen and Nirodi 2007). In our study, HCHO treatment decreased the expression of Ku 70 and phophorylation of DNA-PKcs, whereas triphlorethol-A restored these processes ().
FIGURE 2. Effect of triphlorethol-A on HCHO-induced DNA strand breaks. (A) The cell lysates were electrophoresed and the phosphohistone H2A.X was detected using a specific antibody. (B) Representative images and % cellular DNA damage detected by comet assay. Asterisk indicates significantly different from control (p < .05); **significantly different from HCHO-treated cells (p < .05). (C) Ku 70 and phospho DNA-PKcs proteins were detected using specific antibodies (color figure available online).
Triphlorethol-A Inhibits Base Modification Induced by HCHO Treatment
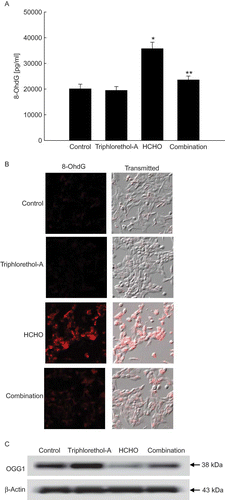
The levels of 8-OhdG, a hallmark of DNA base damage produced by oxidative stress, were measured using an 8-OhdG detection kit and verified by immunochemistry. The levels of 8-OhdG in DNA were significantly higher in HCHO-treated cells than in untreated cells or in cells treated with triphlorethol-A. In addition, triphlorethol-A reduced the levels of 8-OhdG induced by HCHO treatment (). Condensed staining intensity of 8-OhdG was observed in HCHO-treated cells but was significantly diminished when treated with triphlorethol-A (). These results show that triphlorethol-A decreased the elevated levels of 8-OhdG induced by HCHO. To explain the observed inhibition of HCHO-induced 8-OhdG formation by triphlorethol-A, the expression of OGG1 was determined. HCHO treatment decreased OGG1 protein levels, whereas triphlorethol-A was able to prevent this fall ().
FIGURE 3. Effect of triphlorethol-A on HCHO-induced 8-OHdG base modification. (A) The amount of 8-OHdG in DNA was determined using the Bioxytech 8-OHdG-ELISA kit. Asterisk indicates significantly different from control (p < .05); **significantly different from HCHO- treated cells (p < .05). (B) 8-OHdG levels reflected by the binding of avidin-TRITC were visualized with a fluorescence microscope. (C) OGG1 protein was detected using a specific antibody (color figure available online).
Cytoprotective Effect of Triphlorethol-A via Activation PI3K/Akt Signaling Pathway
Protein kinase B (PKB) or Akt is a major signaling enzyme involved in cell survival against oxidative stress, and recently it has been reported that the phosphoinositol 3-kinase (PI3K)/Akt pathway regulates OGG1 expression (CitationUeta et al. 2008; CitationKang et al. 2009).
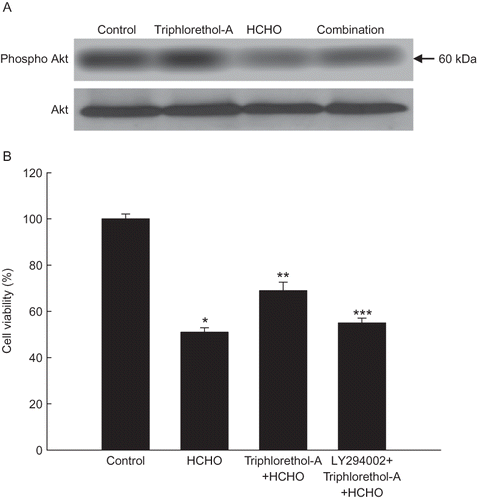
Triphlorethol-A pretreatment in HCHO-treated cells restored the expression of phosphorylated Akt (the active form of Akt), which was decreased only in HCHO-treated cells (). Furthermore, LY294002 (a specific PI3K inhibitor) attenuated the cytoprotective effect of triphlorethol-A against HCHO-induced cell death, suggesting the involvement of PI3K/Akt signaling in the cytoprotective effect of triphlorethol-A against HCHO-induced cell death.
FIGURE 4. Effect of triphlorethol-A on the the phosphorylation of Akt and cell viability. (A) Cell lysates were electrophoresed, and phospho Akt was detected by a specific antibody. (B) After treatment with LY294002 for 1 h, followed by treatment with triphlorethol-A for 1 h, and finally 150 μM of HCHO for 24 h, cell viability was assessed using the MTT assay. Asterisk indicates significantly different from control (p < .05); **significantly different from HCHO-treated cells (p < .05); ***significantly different from triphlorethol-A plus HCHO-treated cells (p < .05).
DISCUSSION
Double-strand breaks are repaired by homologous recombination or by NHEJ. Homologous recombination, a type of genetic recombination common to all forms of life involves nucleotide sequences that are exchanged between two similar or identical strands of DNA, is the predominant DSB repair mechanism in prokaryotes, whereas in eukaryotes it plays a prominent role during the S and G2 phases of the cell cycle. NHEJ, referred to as non-homologous because the break ends are directly ligated without the need for a homologous template, functions in all phases of the cell cycle (CitationBurma et al. 2006). Furthermore, NHEJ is evolutionarily conserved throughout the animal kingdom and is the predominant double-strand break repair pathway in higher eukaryotes such as mammals. NHEJ is a versatile mechanism using the Ku heterodimer (Ku 70/80 subunits), DNA-PKcs, ligase IV/XRCC4, and a multitude of other proteins that juxtapose two broken-off DNA strands for ligation (CitationRiha et al. 2006).
Among the many modified DNA bases generated by oxidative stress, 8-oxoguanine is the most abundant product thought to play a major role in mutagenesis and carcinogenesis (CitationFortini et al. 2003). Recently, CitationHyun et al. (2000) demonstrated that OGG1-deficient human leukemia cells might be required to undergo apoptosis by 8-OhdG. Conversely, expression of OGG1 suppressed oxidative stress derived DNA damage and enhanced cell survival (CitationKannan et al. 2006).
In our study, triphlorethol-A suppressed DNA breakage induced by HCHO via induction of Ku 70 and DNA-PKcs, indicating triphlorethol-A induced DSB repair. In addition, triphlorethol-A was shown to repair oxidized DNA bases by restoring OGG1 levels in response to the damaging effect of HCHO treatment. The PI3K/Akt pathway plays a crucial role in controlling transcription, cell cycle progression, and apoptosis (CitationBrazil and Hemmings 2001). DNA-PKcs co-localizes and associates with Akt at the plasma membrane (CitationFeng et al. 2004). In vitro analysis using purified DNA-PK and recombinant Akt proteins revealed that DNA-PK directly induces the phosphorylation and activation of Akt (CitationDragoi et al. 2005). In a previous study, CitationKang et al. (2009) demonstrated that OGG1 induced this via the activation of the PI3K/Akt pathway. In contrast, CitationSimone et al. (2008) found that inhibition of PI3K/Akt reversed the OGG1 expression downregulated by high glucose treatment in the renal cortex of diabetic rats. Therefore, the relationships of DNA-PKcs, Ku heterodimer, and OGG1 to PI3K/Akt need to be elucidated in future. In conclusion, triphlorethol-A improved the BER and NHEJ capacity impaired by HCHO and reduced the levels of DNA damage. This protective effect of triphlorethol-A may contribute to the prevention from carcinogenesis and mutagenesis development.
Acknowledgments
This work was supported by the Ministry of Education, Science and Technology of Korea (2010-0027722).
REFERENCES
- Boiteux , S. , O'Connor , T. R. , Lederer , F. , Gouyette , A. and Laval , J. 1990 . Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites . J. Biol. Chem. , 265 : 3916 – 3922 .
- Brazil , D. P. and Hemmings , B. A. 2001 . Ten years of protein kinase B signalling: A hard Akt to follow . Trends Biochem. Sci. , 26 : 657 – 664 .
- Burma , S. , Chen , B. and Chen , D. 2006 . Role of non-homologous end joining (NHEJ) in maintaining genomic integrity . DNA Repair (Amst.) , 5 : 1042 – 1048 .
- Caporaso , N. 2003 . The molecular epidemiology of oxidative damage to DNA and cancer . JNCI , 95 : 1263 – 1265 .
- Carmichael , J. W. , DeGraff , W. G. , Gazdar , A. F. , Minna , J. D. and Mitchell , J. B. 1987 . Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing . Cancer Res. , 47 : 936 – 941 .
- Chen , D. J. and Nirodi , C. S. 2007 . The epidermal growth factor receptor: A role in repair of radiation-induced DNA damage . Clin. Cancer Res. , 13 : 6555 – 6560 .
- Cooke , M. S. , Evans , M. D. , Dizdaroglu , M. and Lunec , J. 2003 . Oxidative DNA damage: Mechanisms, mutation and disease . FASEB J , 17 : 1195 – 1214 .
- Dragoi , A. M. , Fu , X. , Ivanov , S. , Zhang , P. , Sheng , L. , Wu , D. , Li , G. C. and Chu , W. M. 2005 . DNA-PKcs, but not TLR9, is required for activation of Akt by CpG-DNA . EMBO J. , 24 : 779 – 789 .
- Feng , J. , Park , J. , Cron , P. , Hess , D. and Hemmings , B. A. 2004 . Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase . J. Biol. Chem. , 279 : 41189 – 41196 .
- Flyvholm , M. A. and Andersen , P. 1993 . Identification of formaldehyde releasers and occurrence of formaldehyde and formaldehyde releasers in registered chemical products . Am. J. Ind. Med. , 24 : 533 – 552 .
- Fortini , P. , Pascucci , B. , Parlanti , E. , D'Errico , M. , Simonelli , V. and Dogliotti , E. 2003 . 8-Oxoguanine DNA damage: At the crossroad of alternative repair pathways . Mutat. Res. , 531 : 127 – 139 .
- Gackowski , D. , Kowalewski , J. , Siomek , A. and Olinski , R. 2005 . Oxidative DNA damage and antioxidant vitamin level: Comparison among lung cancer patients, healthy smokers and nonsmokers . Int. J. Cancer , 114 : 153 – 156 .
- Grafström , R. C. , Curren , R. D. , Yang , L. L. and Harris , C. C. 1985 . Genotoxicity of formaldehyde in cultured bronchial fibroblasts . Science , 228 : 89 – 91 .
- Grafström , R. C. , Fornace , A. Jr. and Harris , C. C. 1984 . Repair and DNA damage caused by formaldehyde in human cells . Cancer Res. , 44 : 4323 – 4327 .
- Gurel , A. , Coskun , O. , Armutcu , F. , Kanter , M. and Ozen , O. A. 2005 . Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: Biochemical and histological studies . J. Chem. Neuroanat. , 29 : 173 – 178 .
- Hyun , J. W. , Choi , J. Y. , Zeng , H. H. , Lee , Y. S. , Kim , H. S. , Yoon , S. H. and Chung , M. H. 2000 . Leukemic cell line, KG-1 has a functional loss of hOGG1 enzyme due to a point mutation and 8-hydroxydeoxyguanosine can kill KG-1 . Oncogene , 19 : 4476 – 4479 .
- Hyun , J. W. , Jung , Y. C. , Kim , H. S. , Choi , E. Y. , Kim , J. E. , Yoon , B. H. , Yoon , S. H. , Lee , Y. S. , Choi , J. , You , H. J. and Chung , M. H. 2003 . 8-Hydroxydeoxyguanosine causes death of human leukemia cells deficient in 8-oxoguanine glycosylase 1 activity by inducing apoptosis . Mol. Cancer Res. , 1 : 290 – 299 .
- Kang , K. A. , Lee , K. H. , Chae , S. , Koh , Y. S. , Yoo , B. S. , Kim , J. H. , Ham , Y. M. , Baik , J. S. , Lee , N. H. and Hyun , J. W. 2005 . Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage . Free Radical Res. , 39 : 883 – 892 .
- Kang , K. A. , Lee , J. H. , Chae , S. , Zhang , R. , Piao , M. J. , Kim , H. S. , You , H. J. and Hyun , J. W. 2009 . Butin decreases oxidative stress-induced 8-hydroxy-2′-deoxyguanosine levels via activation of oxoguanine glycosylase 1 . Chem. Biol. Interact. , 181 : 338 – 342 .
- Kang , K. A. , Lee , K. H. , Park , J. W. , Lee , N. H. , Na , H. K. , Surh , Y. J. , You , H. J. , Chung , M. H. and Hyun , J. W. 2007 . Triphlorethol-A induces heme oxygenase-1 via activation of ERK and NF-E2 related factor 2 transcription factor . FEBS Lett. , 581 : 2000 – 2008 .
- Kang , K. A. , Zhang , R. , Lee , K. H. , Chae , S. , Kim , B. J. , Kwak , Y. S. , Park , J. W. , Lee , N. H. and Hyun , J. W. 2006 . Protective effect of triphlorethol-A from . Ecklonia cava against ionizing radiation in vitro. J. Radiat. Res. , 47 : 61 – 68 .
- Kang , K. A. , Zhang , R. , Piao , M. J. , Ko , D. O. , Wang , Z. H. , Lee , K. , Kim , B. J. , Shin , T. , Park , J. W. , Lee , N. H. , Yoo , B. S. and Hyun , J. W. 2008 . Inhibitory effects of triphlorethol-A on MMP-1 induced by oxidative stress in human keratinocytes via ERK and AP-1 Inhibition . J. Toxicol. Environ. Health A , 71 : 992 – 999 .
- Kannan , S. , Pang , H. , Foster , D. C. , Rao , Z. and Wu , M. 2006 . Human 8-oxoguanine DNA glycosylase increases resistance to hyperoxic cytotoxicity in lung epithelial cells and involvement with altered MAPK activity . Cell Death Different. , 13 : 311 – 323 .
- Kondo , S. , Toyokuni , S. , Tanada , T. , Hiai , H. , Onodera , H. , Kasai , H. and Immamura , M. 2000 . Overexpression of the hOGG1 gene and high 8-hydroxy-2′-deoxyguanosine (8-OHdG) lyase activity in human colorectal carcinoma: regulation mechanism of the 8-OHdG level in DNA . Clin. Cancer Res. , 6 : 1394 – 1400 .
- Lan , Q. , Mumford , J. L. , Shen , M. , Demarini , D. M. , Bonner , M. R. , He , X. , Yeager , M. , Welch , R. , Chanock , S. , Tian , L. , Chapman , R. S. , Zheng , T. , Keohavong , P. , Caporaso , N. and Rothman , N. 2004 . Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions . Carcinogenesis , 25 : 2177 – 2181 .
- Meek , K. , Douglas , P. , Cui , X. , Ding , Q. and Lees-Miller , S. P. 2007 . trans Autophosphorylation at DNA-dependent protein kinase's two major autophosphorylation site clusters facilitates end processing but not end joining . Mol. Cell. Biol. , 27 : 3881 – 3890 .
- Powell , C. L. , Swenberg , J. A. and Rusyn , I. 2005 . Expression of base excision DNA repair genes as a biomarker of oxidative DNA damage . Cancer Lett. , 229 : 1 – 11 .
- Riha , K. , Heacock , M. L. and Shippen , D. E. 2006 . The role of the nonhomologous end-joining DNA double-strand break repair pathway in telomere biology . Annu. Rev. Genet. , 40 : 237 – 277 .
- Saito , Y. , Nishio , K. , Yoshida , Y. and Niki , E. 2005 . Cytotoxic effect of formaldehyde with free radicals via increment of cellular reactive oxygen species . Toxicology , 210 : 235 – 245 .
- Silva , J. P. , Gomes , A. C. and Coutinho , O. P. 2008 . Oxidative DNA damage protection and repair by polyphenolic compounds in PC12 cells . Eur. J. Pharmacol. , 601 : 50 – 60 .
- Simone , S. , Gorin , Y. , Velagapudi , C. , Abboud , H. E. and Habib , S. L. 2008 . Mechanism of oxidative DNA damage in diabetes: Tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2′-deoxyguanosine-DNA glycosylase . Diabetes , 57 : 2626 – 2636 .
- Singh , N. P. 2000 . Microgels for estimation of DNA strand breaks, DNA protein cross links and apoptosis . Mutat. Res. , 455 : 111 – 127 .
- Smith , G. C. and Jackson , S. P. 1999 . The DNA-dependent protein kinase . Genes Dev. , 13 : 916 – 934 .
- Struthers , L. , Patel , R. , Clark , J. and Thomas , S. 1998 . Direct detection of oxodeoxyguanosine and 8-oxoguanine by avidin and its analogues . Anal. Biochem. , 255 : 20 – 31 .
- Subash , P. , Premagurumurthy , K. , Sarasabharathi , A. and Cherian , K. M. 2010 . Total antioxidant status and oxidative DNA damage in a South Indian population of essential hypertensives . J. Human Hypertens. , 24 : 475 – 482 .
- Sul , D. , Kim , H. , Oh , E. , Phark , S. , Cho , E. , Choi , S. , Kang , H. S. , Kim , E. M. , Hwang , K. W. and Jung , W. W. 2007 . Gene expression profiling in lung tissues from rats exposed to formaldehyde . Arch. Toxicol. , 81 : 589 – 597 .
- Teng , S. , Beard , K. , Pourahmad , J. , Moridani , M. , Easson , E. , Poon , R. and O'Brien , P. J. 2001 . The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxicmechanismin isolated rat hepatocytes . Chem. Biol. Interact. , 130-132 : 285 – 296 .
- Thompson , H. J. 2006 . Oxidative DNA damage and cancer risk assessment . J. Nutr. , 136 : 2693 – 2694 .
- Ueta , E. , Sasabe , E. , Yang , Z. , Osaki , T. and Yamamoto , T. 2008 . Enhancement of apoptotic damage of squamous cell carcinoma cells by inhibition of the mitochondrial DNA repairing system . Cancer Sci. , 99 : 2230 – 2237 .
- van Gent , D. , Hoeijmakers , J. and Kanaar , R. 2001 . Chromosomal stability and DNA double-strand break connection . Nat. Rev. Genet. , 2 : 196 – 206 .
- Zana , M. , Szecscenyi , A. , Czibula , A. , Bjelik , A. , Juhasz , A. , Rimanoczy , A. , Szabo , K. , Vetro , A. , Szucs , P. , Varkonyi , A. , Pakaski , M. , Boda , K. , Rasko , I. , Janka , Z. and Kalman , J. 2006 . Age-dependent oxidative stress-induced DNA damage in Down's lymphocytes . Biochem. Biophys. Res. Commun. , 345 : 726 – 733 .
- Zhang , R. , Kang , K. A. , Piao , M. J. , Ko , D. O. , Wang , Z. H. , Chang , W. Y. , You , H. J. , Lee , I. K. , Kim , B. J. , Kang , S. S. and Hyun , J. W. 2009a . Preventive effect of 7,8-dihydroxyflavone against oxidative stress induced genotoxicity . Biol. Pharm. Bull. , 32 : 166 – 171 .
- Zhang , R. , Kang , K. A. , Piao , M. J. , Maeng , Y. H. , Lee , K. H. , Chang , W. Y. , You , H. J. , Kim , J. S. , Kang , S. S. and Hyun , J. W. 2009b . Cellular protection of morin against the oxidative stress induced by hydrogen peroxide . Chem. Biol. Interact. , 177 : 21 – 27 .
- Zhang , R. , Lee , I. K. , Kang , K. A. , Piao , M. J. , Kim , K. C. , Kim , B. J. , Lee , N. H. , Choi , J. Y. , Choi , J. and Hyun , J. W. 2010 . Cytoprotective effects of triphlorethol-A against formaldehyde-induced oxidative damage and apoptosis: role of mitochondria-mediated caspase-dependent pathway . J. Toxicol. Environ. Health A , 73 : 1477 – 1489 .
- Zhou , D. X. , Qiu , S. D. , Zhang , J. , Tian , H. and Wang , H. X. 2006 . The protective effect of vitamin E against oxidative damage caused by formaldehyde in the testes of adult rats . Asian J. Androl. , 8 : 584 – 588 .