Abstract
Osteoarthritis accounts for 0.6% of disability-adjusted life years globally. There is a paucity of research focused on cannabis-based medicinal products (CBMPs) for osteoarthritic chronic pain management. This study aims to assess changes in validated patient-reported outcome measures (PROMs) and CBMP clinical safety in patients with osteoarthritis. A prospective case series from the UK Medical Cannabis Registry was analyzed. Primary outcomes were changes in the Brief Pain Inventory (BPI), McGill Pain Questionnaire (MPQ2), EQ-5D-5L, Generalized Anxiety Disorder-7 (GAD-7) questionnaire, and Single-Item Sleep Quality Scale (SQS) at 1-, 3-, 6-, and 12-month follow-ups from baseline. Common Terminology Criteria for Adverse Events v.4.0 was used for adverse event (AE) analysis. Statistical significance was defined as p < 0.050. Seventy-seven patients met inclusion criteria. CBMP initiation correlated with BPI pain severity (p = 0.004), pain interference (p = 0.005), and MPQ2 (p = 0.017) improvements at all follow-ups compared to baseline. There were improvements in the EQ-5D-5L index (p = 0.026), SQS (p < 0.001), and GAD-7 (p = 0.038) up to 6 and 3 months, respectively. Seventeen participants (22.08%) recorded 76 mild AEs (34.86%), 104 moderate AEs (47.71%), and 38 severe AEs (17.43%). Though causality cannot be assumed in this observational study, results support development of randomized control trials for osteoarthritis pain management with CBMPs.
Introduction
Osteoarthritis is characterized by localized loss of cartilage, inflammation, and bone remodeling (Citation1) and poses a significant global burden (Citation2). It has gained priority status from the World Health Organization (Citation3), accounting for 0.6% of disability-adjusted life years and 2.2% of global years lived with disability (Citation4, Citation5). Between 1990 and 2010, the prevalence of osteoarthritis increased by 64%, and with the aging global population, the prevalence is set to rise (Citation6, Citation7).
Osteoarthritis-associated chronic pain can be disabling, affecting a patient’s quality of life through reduced sleep quality, mood disturbance, interference with social relations, and diminished cognitive function (Citation8, Citation9). This has a wider economic impact, with a microsimulation model suggesting that 61,000 productive life years will be lost in 2030 for people aged 45 to 64 owing to arthritis (Citation10).
Osteoarthritis pain management requires a multidisciplinary, biopsychosocial approach. Nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids are most commonly prescribed for osteoarthritis pain management (Citation11, Citation12). However, the use of NSAIDs and opioids may be contraindicated in certain patients with comorbidities or result in unwanted side effects (Citation13). NSAIDs are associated with increased risk of gastrointestinal bleeding, myocardial infarction, and stroke (Citation14). Opioids have a high side effect profile with particular concern for dependence (Citation15). Furthermore, depending on arthritis severity, elective surgery may be required for affected joints (Citation16). In the UK, joint replacement waiting lists can exceed 52 weeks (Citation17); hence, a need remains for better therapeutic options for chronic pain because many are not appropriate for long-term use or lack evidence for their effectiveness (Citation18). Therefore, patients with chronic pain secondary to osteoarthritis require a safe and effective form of analgesia (Citation19).
The endocannabinoid system (ECS) is a relatively novel physiological mechanism that has been implicated in the control of inflammatory and nociceptive signaling. It is now increasingly being investigated for the development of therapeutics for chronic pain (Citation20).
The major components of this system are cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2R), as well as their endogenous ligands, N-arachidonoyl-ethanolamine (AEA) and 2-arachindonoylglycerol (2-AG) (Citation21). Both receptors are G protein–coupled transmembrane domain receptors (Citation22). CB1R is mainly present on the presynaptic terminals of glutamatergic and γ-aminobutyric acidergic neurons (Citation23, Citation24) and on peripheral sympathetic nerves (Citation25). CB2R is predominantly expressed in immune system cells (Citation26–29). Both CB1R and CB2R are expressed on chondrocytes, osteocytes, osteoblasts, and osteoclasts (Citation30). AEA and 2-AG act as retrograde messengers that are rapidly inactivated by reuptake mechanisms (Citation22, Citation31). AEA is hydrolyzed by fatty acid amide hydrolase (FAAH) and 2-AG is hydrolyzed by both FAAH and mono-acyl hydrolases (Citation22, Citation32). There is growing evidence that cannabinoids interact with other receptors implicated in pain transmission and interpretation such as transient receptor potential vanilloid subtype 1 (TRPV1) channels and serotonin receptors (Citation22).
Research has implicated ECS dysregulation in the pathophysiology of osteoarthritis joint pain. Patients with osteoarthritis demonstrated higher plasma levels of 2-AG compared to healthy subjects (Citation33). Additionally, AEA and 2-AG have been detected in the joint synovial fluid of patients with osteoarthritis (Citation34, Citation35). These findings suggest the potential of ECS modulation to alleviate pain symptoms due to osteoarthritis.
Cannabis-based medicinal products (CBMPs), derived from the cannabis plant, have received growing interest as a therapeutic option for many conditions (Citation36) owing to their ability to modulate the ECS. The two most abundant phytocannabinoids are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) (Citation37). THC is a partial agonist of CB1R and CB2R (Citation38). CBD inhibits fatty acid binding proteins and FAAH (Citation39, Citation40), thereby inhibiting the degradation of AEA. CBD is also a negative allosteric modulator of CB1R (Citation41). Recent pharmacological studies revealed that nonselective CB1R and CB2R agonists induced antinociceptive effects (Citation42), whereas the acute blockade of CB1R or CB2R antagonism produced pronociceptive effects (Citation43). Casey et al. demonstrated that a THC:CBD combination alleviated allodynia in a mouse model of neuropathic pain (Citation44). In a monoiodoacetate-induced model of osteoarthritis, the local administration of a CB1R agonist into the knee joint reduced the hypersensitivity of nociceptors through CB1R and TRPV1 channels (Citation45). In a similar model, Philpott et al. revealed that prophylactic CBD treatment reduced later development of pain and nerve damage (Citation46).
A recent meta-analysis by Wang et al. indicated with moderate to high certainty evidence that noninhaled CBMP treatment was associated with increased likelihood of reduction in pain severity and sleep improvement (Citation47). A prospective, open-label study in patients with chronic pain administered CBMPs revealed significant improvements in pain symptoms, severity, and interference scores (Citation48). Furthermore, an exploratory cross-sectional study by Frane et al. reported a 44% improvement in arthritic pain after CBD use (Citation49), corroborated by another prospective, observational study in Australia where patients with arthritis reported reduced pain intensity scores (Citation50).
There is growing clinical evidence for the effects of CBMPs for chronic pain; however, most clinical analyses have a small sample size and subject to significant bias. Despite promising preclinical evidence for osteoarthritis, there is a paucity of supporting high-quality clinical studies (Citation47). Specific osteoarthritis-focused data for CBMP use remain scarce because it is often analyzed in a heterogeneous population of individuals with chronic pain. This study aimed to perform an analysis of outcomes for patients with osteoarthritis who were prescribed CBMPs and enrolled in the UK Medical Cannabis Registry (UKMCR). The UKMCR prospectively collects data with respect to clinical efficacy and safety. It is the largest registry of its kind in Europe. This study primarily aimed to evaluate pain-specific and general health-related quality of life (HRQoL) changes in patients with osteoarthritis using patient-reported outcome measures (PROMs). The secondary aim was to assess the incidence of adverse events to characterize the safety profile of CBMPs in this patient population.
Methods
Study design and participants
The UKMCR, managed by Curaleaf Clinic, began enrollment in December 2019. It is the first prospective registry collecting longitudinal pseudonymised data from patients prescribed CBMPs in the UK and Channel Islands.
This study investigated the effects of prescribed CBMPs in patients with chronic pain caused by osteoarthritis who visited Curaleaf Clinic. Individuals aged ≥18 with a primary indication for osteoarthritis-related chronic pain were eligible for inclusion in this formal, sequential clinical case series. CBMPs were prescribed by a specialist after approval by a multidisciplinary committee according to standards specified by the Medicines and Healthcare Products Regulatory Agency (Citation51). CBMPs prescribed complied with good manufacturing practice standards. Patients who had not completed baseline PROMs assessment and were not enrolled on the UKMCR for a minimum of 12 months were excluded. Data were extracted on January 9, 2022. This study followed STROBE reporting guidelines (Citation52). Ethics approval was provided by the Central Bristol Ethics Committee (22/SW/0145). All participants were enrolled consecutively and provided written informed consent.
Prescribed CBMPs included dried flower (flos or granulate) or oil-based formulations (isolate phytocannabinoids or full-spectrum products containing cannabinoids, terpenes, and flavonoids). Oils were administered orally or sublingually, and dried flowers were vaped.
Data collection
Data were collected remotely whereby patients completed PROMs and adverse event questionnaires electronically via an online web-based platform at 1, 3, 6, and 12 months.
During the initial consultation, baseline demographic data were collected: age, gender, occupation, and body mass index (kg/m2). The primary indication for treatment with CBMPs, other diagnoses where applicable, and comorbidities were recorded. The Charlson Comorbidity Index (a prognostic tool used to predict 10-year mortality) was calculated for each participant (Citation53).
Tobacco, alcohol, and cannabis status was also reported by clinicians at baseline, including smoking status, pack-year history, weekly alcohol consumption (units), cannabis use status, frequency of cannabis use, and current quantity of cannabis intake (grams). To quantify the individual history of prior cannabis use, a metric of “cannabis gram years” was used: Cannabis gram years = Mean cannabis consumption in grams per day × Years of use (Citation54). Patient medication data were documented, including drug names, medicine doses per 24 h, and prescription start/end dates. To ensure uniformity, medication names were mapped to SNOMED CT codes (Citation55). Oral morphine equivalents (OMEs) for opioid medicines were determined using conversion factors from the British National Formulary (Citation56). At baseline and follow-up intervals, the following CBMP prescription details were recorded: company, formulation, CBD dose per day (mg), THC dose per day (mg), other active ingredients, dose of other active ingredients per day (mg), and strain.
Patient-reported outcome measures
The following PROMs were recorded at baseline and each follow-up interval for all adult patients: General Anxiety Disorder Scale (GAD-7), Single-item Sleep Quality Scale (SQS), EQ-5D-5L, Brief Pain Inventory (BPI), the short-form McGill Pain Questionnaire (SF-MPQ-2), and the Patient Global Impression of Change.
Pain-specific PROMs
The BPI is a self-reported questionnaire measuring pain severity and interference based on 11 categories (Citation57, Citation58). Pain severity and interference are ranked on a scale of 0 to 10. Pain severity ranges from 0 = no pain to 10 = pain as awful as you can imagine and pain interference ranges from 0 = no interference to 10 = complete interference (Citation58). A minimal clinically important difference in BPI pain severity is a 1-point improvement (Citation59).
The SF-MPQ-2 is a self-reported questionnaire that evaluates both neuropathic and nonneuropathic pain (Citation60). It includes 22 categories to assess pain within four major domains: continuous, intermittent, neuropathic, and affective. Each category is rated on a scale of 0 to 10, where 0 = no pain and 10 = worst pain. The overall pain score is calculated as the mean score of all 22 categories (Citation60, Citation61).
HRQoL-specific PROMs
The GAD-7 is a self-reported questionnaire designed to screen and measure severity of symptoms of generalized anxiety disorder (GAD) (Citation62). Participants are asked how frequently they have been affected by the seven core symptoms of GAD over the last 2 weeks. The options are not at all, several days, more than half the days, and nearly every day, with each option receiving scores of 0, 1, 2, and 3, respectively. The score ranges from 0 to 21, with ≥5, ≥10, and ≥15 signifying mild, moderate, and severe anxiety symptoms, respectively (Citation62–64).
The SQS questionnaire is used to assess sleep quality. Participants rate overall sleep quality over the past 7 days (Citation65) using a scale of 0 to 10. The following sleep quality categories are formed: terrible (0), poor (1–3), fair (4–6), good (7–9), and excellent (Citation10, Citation65, Citation66).
The EQ-5D-5L is a self-report questionnaire that evaluates general HRQoL (Citation67). Patients rate their quality of life on a scale from 1 to 5 across five domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression (1 = no problems to 5 = extreme problems). The resulting health state is mapped to EQ-5D-5L index values validated for a UK population by van Hout et al. (Citation67, Citation68). Optimum health is assigned an index score of 1, and an index score <0 represents a health state worse than death (Citation67).
The Patient Global Impression of Change is a 7-point scale reflecting the patient’s rating of overall improvement (Citation69).
Adverse events
Adverse events (AEs) were collected at baseline and each follow-up interval through self-reporting, routine clinician follow-up, or direct questioning by the research team. These events and their severity were recorded following the Common Terminology Criteria for Adverse Events v4.0 (Citation70).
Missing data
Missing data during the follow-up period were handled using the baseline observation carried forward approach (Citation71).
Statistical methods
Descriptive statistics were used to assess clinicopathological, drug, and alcohol data. Demographic data were reported as the mean ± standard deviation (SD), median (interquartile range [IQR]), or frequency (%), as appropriate.
Longitudinal changes in PROMs were analyzed using repeated measures one-way analysis of variance. PROMs from 1-, 3-, 6-, and 12-month follow-ups were compared with baseline scores of participants included in each of the follow-up dates using post hoc pairwise comparisons with Bonferroni correction.
Changes in OMEs between baseline and 12 months were assessed using a paired t-test. The Statistical Package for Social Sciences v29.0.0.0 was used for statistical analysis of the data (Citation72). Statistical significance was defined as p < 0.050. Graphs were created using GraphPad Prism v9.5.1 (528) for macOS (Citation73).
Results
Patient data
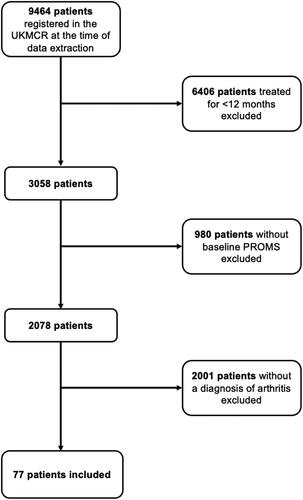
After application of inclusion and exclusion criteria, 77 patients were included in this study ().
Baseline demographic details for all patients included in the analysis are presented in . Though most patients were female (n = 40, 51.95%), the gender distribution of patients was relatively equal. The mean age of patients was 60.04 ± 14.27 years. The mean body mass index was 30.18 ± 6.22 kg/m2 and the most frequent occupation was “unemployed” (n = 45, 58.44%).
Table 1. Demographic details of patients at baseline assessment.
displays the baseline tobacco, alcohol, and cannabis status of patients. Baseline analysis revealed that half the patients were current cannabis consumers (n = 39, 50.65%), with the majority consuming cannabis daily (n = 34, 87.18%). The median daily quantity of cannabis consumed was 1.00 (IQR = 0.50–2.00) g/day. The median lifetime cannabis consumption of current cannabis users was 17.00 (IQR = 6.00–35.00) gram-years. Remaining patients were either ex-users (n = 9, 11.69%) or cannabis naïve (n = 29, 37.66%).
Table 2. Tobacco, alcohol, and cannabis status of study participants.
CBMP dosing
CBMP dosing is presented in . Most patients (n = 73; 94.81%) were prescribed both CBD and THC. Of the remaining patients, 3 were prescribed CBD only (3.90%) and 1 was prescribed THC only (1.30%). The median dose of CBD and THC was 25.50 (IQR = 20.00–47.50) mg/day and 105.00 (IQR = 10.00–222.00) mg/day, respectively. The most commonly prescribed treatments were Adven 20 and 50 sublingual oils and Adven EMT1 flos.
Table 3. Details of CBMPs prescribed for study participants (n = 159).
Patient-reported outcome measures
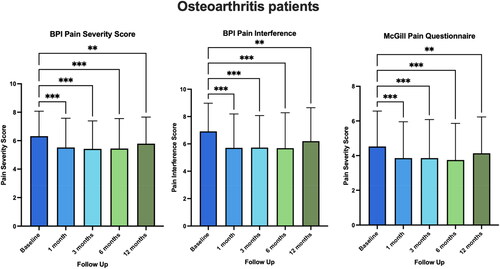
outlines the paired results comparing the pain-specific PROMs at baseline to 1, 3, 6, and 12 months. Improvements were observed in BPI pain severity and interference scores as well as the SF-MPQ-2 in all patients (p < 0.010). For specific p values across follow-up months, see Appendix A.
Figure 2. Paired baseline and follow-up scores for BPI and McGill Pain Questionnaire for patients with osteoarthritis at 1-, 3-, 6-, and 12-month follow-up. Scores presented as mean ± SD. **p < 0.01, ***p < 0.001.
displays HRQoL PROMs at all follow-up intervals (1, 3, 6, and 12 months). Significant improvements were observed in GAD-7, SQS, and the EQ-5D-5L index value. Patients reported statistically significant improvement in GAD-7 at 1- and 3-month follow-ups only (p < 0.050), and patients showed improvement in SQS up to 6-month follow-up (p < 0.001). There was improvement in EQ-5D-5L index up to 6-month follow-up (p < 0.050).
Table 4. Paired baseline and follow-up scores for HRQoL PROMs for patients with osteoarthritis at 1, 3, 6, and 12 months.
Oral morphine equivalents analysis
Thirty-four patients were regularly prescribed opioid medications at the time CBMP treatment was commenced (44.16%). There was no significant change in OME doses between baseline and 12-month follow-up after initiation of CBMP treatment (123.97 ± 490.04 mg vs. 116.35 ± 491.09 mg; p = 0.136).
Adverse events
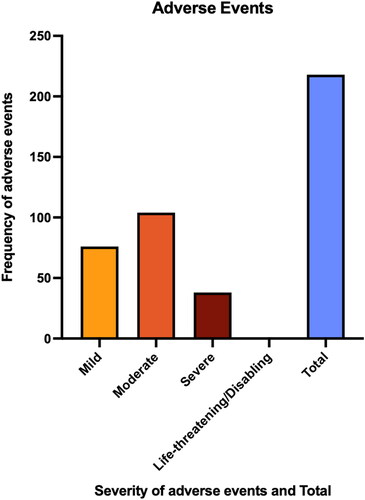
displays the incidence of AEs reported. A total of 218 adverse events (283.12%) were recorded by 17 patients (7.80%). The most common AE was fatigue (7.80%). There were 76 mild AEs (34.86%), 104 moderate AEs (47.71%), and 38 severe AEs (17.43%). No life-threatening AEs were reported by any of the study participants. For specific AEs reported, see Appendix B.
Discussion
In this prospective observational study utilizing UKMCR data, the reported outcome of osteoarthritis patients prescribed CBMPs was evaluated. Results demonstrate improvements in pain-specific PROMs in osteoarthritis patients at 1, 3, 6, and 12 months of follow-up. CBMPs were well tolerated during follow-up, with the majority of patients (77.92%) reporting no adverse events or experiencing mainly mild to moderate adverse events.
Patient-reported outcome measures
Commencement of CBMP treatment was associated with reductions in pain-specific PROMs at all time points in patients with osteoarthritis. Similar reductions in BPI were observed in a prospective observational study by Meng et al. (Citation74) that reported improvements in pain severity and interference at 12-month follow-up. Though study participants were prescribed both oils and flowers, the ratios of THC and CBD used were unknown and could be different from levels used in the present study. The results of this study were further corroborated by Frane et al. (Citation49), who demonstrated a 43.1% pain reduction in patients with osteoarthritis after CBD administration. Of note, Frane et al. only looked at the effects of CBD, whereas the present study investigates effects of both CBD and THC individually and combined.
CBMP treatment resulted in improvements in general HRQoL PROMs, including a significant improvement in GAD-7 for up to 3-month follow-up (p = 0.038), indicating an improvement in anxiety. The results of the present study did not corroborate the sustained improvement in anxiety as reported by Vickery et al. (Citation75), who demonstrated a decrease in anxiety at 24 months. This could be because the study conducted by Vickery et al. had a much larger sample than the present study. Furthermore, the GAD-7 used in this study is known to classify more individuals as having above-threshold symptoms of anxiety compared to Depression, Anxiety and Stress Scale (DASS)-Anxiety, which was used by Vickery et al (Citation76).
Significant improvements in SQS were observed up to 6-month follow-up (p < 0.001), indicating a general enhancement in sleep quality. These findings align with a prospective observational study by Moreno-Sanz et al. (Citation77) in which patients reported significant improvement in sleep quality up to 6 months. However, in the study conducted by Moreno-Sanz et al., a different scale was used for measuring sleep quality, preventing direct comparison of sleep improvement between studies.
There were improvements in the EQ-5D-5L index up to 6 months (p = 0.006), implying an overall increase in patients’ HRQoL. This improvement is likely attributable to reductions in pain and discomfort because the mean improvement in EQ-5D-5L pain and discomfort score was 0.33 (p = 0.002 at 12-month follow-up). However, EQ-5D-5L mobility and self-care scores showed no improvements over the follow-up period. These findings are similar to a prospective open-label study by Haroutounian et al. (Citation78), where no difference from baseline for improvements in upper and lower limb disability were observed but overall patient satisfaction was increased owing to pain improvement.
Overall, there was a general trend across the PROMs where greater improvements in pain and general HRQoL were seen in earlier months of follow-up compared to 12 months. This trend may be attributed to a considerable portion of the patient population being lost to follow-up at 12 months, and baseline observation carried forward analysis results in a trend toward baseline. Secondly, the development of tolerance to CBMP treatment effect could explain the plateau in pain alleviation after initiating CBMP treatment (Citation23, Citation79). Furthermore, osteoarthritis is primarily a degenerative condition (Citation80), and because CBMPs are prescribed for symptomatic relief rather than disease modification (Citation81), this could be indicative of the progressive nature of the condition.
Adverse events
AEs were mainly mild or moderate in severity (82.57%), aligning with findings from similar studies (Citation54, Citation82, Citation83). Fatigue was the most common AE in this study, which differed from other studies (Citation54, Citation82, Citation83), where dry mouth, drowsiness, and constipation were most common. Though these were common in the present study, variations in AE profile could be attributed to variations in formulations and administration of CBMPs between different studies, because the proportion of THC, CBD and other active phytocannabinoids in the CBMP prescription is known to influence the AE profile (Citation84). Furthermore, patients in this study had different baseline characteristics compared to participants in other studies, and given that certain formulations can exacerbate preexisting psychological factors (Citation85), this could explain this study’s AE profile.
Limitations
Several study limitations exist. The observational design prevents establishing causality, and it is uncertain whether improvements in pain and HRQoL were solely due to CBMPs and not confounding factors, such as interactions with other medications. The study was open-label and therefore susceptible to recall bias because patients might overstate CBMP treatment benefits. PROMs are subjective scores, and patients may interpret them differently (Citation86), reducing the study’s internal validity. The absence of a placebo control group hindered assessing CBMPs’ true effect given the potential placebo effect (Citation87). CBMPs are considered to have enhanced placebo effects secondary to their vasoactive and psychoactive effects (Citation88). There is an enhanced expectancy bias of people receiving CBMPs due to positive media attention on their potential effects (Citation89) and because medical care received during this study was self-funded. It has been demonstrated that the cost of medical products has been shown to increase their perceived effectiveness and quality (Citation90).
This study was subject to significant selection bias because patients received treatment from the same private clinic; hence, inclusion was limited to those who could afford treatment. However, the most commonly reported occupation was unemployed (58.44%), perhaps indicating that socioeconomic status may not affect access to treatment. Moreover, there was a high proportion of current cannabis users at baseline (50.65%), who could be more likely to report positive outcomes owing to a higher expectancy of CBMP treatment. However, current and ex-users of cannabis may have built up tolerance to CBD and THC (Citation79), which could reduce the impact of CBMP treatment.
There was considerable loss to follow-up, reasons for which were not collected; however, due to the clinic’s private nature, patients no longer being able to afford treatment may have been a significant factor. Though Bar-Lev Schleider et al. showed high patient compliance with CBMP treatment with 77.7% of patients remaining in active treatment at 6-month follow-up (Citation91), their study was conducted in Israel, where CBMPs are licensed for more conditions compared to the UK, so this study accounted for all uses of CBMPs rather than just chronic pain, which could have influenced the compliance percentage.
Conclusion
These results suggest an improvement in pain-related outcomes for patients with osteoarthritis following the initiation of CBMP treatment. Furthermore, there was an improvement in general HRQoL metrics across the follow-up period. CBMPs also appeared to be well-tolerated at 12-month follow-up. However, due to this study design, a causal effect cannot be established. Hence, this study supports the development of RCTs for CBMP use in osteoarthritis.
Ethics approval
Ethics approval provided by South West–Central Bristol Research Ethics Committee (Reference: 22/SW/0145).
Patient consent statement
All participants completed written, informed consent prior to enrollment in the registry.
Disclosure statement
Ann Francis is a medical student at Imperial College London. Ann Francis has no shareholdings in pharmaceutical companies. Simon Erridge is a junior doctor and head of research at Curaleaf Clinic. Simon Erridge is a research fellow at Imperial College London. Simon Erridge has no shareholdings in pharmaceutical companies. Carl Holvey is chief clinical pharmacist at Curaleaf Clinic. Carl Holvey has no shareholdings in pharmaceutical companies. Ross Coomber is a consultant orthopedic surgeon at St George’s Hospital, London, and operations director at Curaleaf Clinic. Ross Coomber has no shareholdings in pharmaceutical companies. Wendy Holden is a consultant rheumatologist and pain specialist at Curaleaf Clinic (London). Wendy Holden is a medical advisor to the Arthritis Action charity. Wendy Holden has no shareholdings in pharmaceutical companies. James Rucker is a consultant psychiatrist at Curaleaf Clinic (London), an honorary consultant psychiatrist at The South London & Maudsley NHS Foundation Trust, and an NIHR clinician scientist fellow at the Center for Affective Disorders at King’s College London. James Rucker is funded by a fellowship (CS-2017-17-007) from the National Institute for Health Research (NIHR). James Rucker leads the Psychedelic Trials Group at King’s College London. King’s College London receives grant funding from COMPASS Pathways PLC to undertake phase 1 and phase 2 trials with psilocybin. COMPASS Pathways PLC has paid for James Rucker to attend trial related meetings and conferences to present the results of research using psilocybin. James Rucker has undertaken paid consultancy work for Beckley PsyTech and Clerkenwell Health. Payments for consultancy work are received and managed by King’s College London and James Rucker does not benefit personally. James Rucker has no shareholdings in pharmaceutical companies. Michael Platt is a consultant in pain services at Curaleaf Clinic (London). Michael Platt has no shareholdings in pharmaceutical companies. Mikael Sodergren is a consultant hepatopancreatobiliary surgeon at Imperial College NHS Trust, London, a senior clinical lecturer at Imperial College London, and the chief medical officer of Curaleaf International. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Data availability statement
Data that support the findings of this study are available from the UK Medical Cannabis Registry. Restrictions apply to the availability of these data. Data specifications and applications are available from the corresponding author.
All authors contributed to and approved the final article.
All work was conducted at Curaleaf Clinic, London, UK.
Additional information
Funding
References
- Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage. 2015;23(8):1233–41. doi:10.1016/j.joca.2015.03.036.
- Caporali R, Cavagna L, Montecucco C. Pain in arthritis. Eur J Pain Supp. 2012;3(S2):123–7. doi:10.1016/j.eujps.2009.07.009.
- Priority medicines for Europe and the world. (Recent publications, information and events). WHO Drug Information. 2005;19(4):308. https://search.proquest.com/docview/215678671.
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. doi:10.1016/S0140-6736(12)61729-2.
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223.
- March L, Smith EUR, Hoy DG, Cross MJ, Sanchez-Riera L, Blyth F, Buchbinder R, Vos T, Woolf AD. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol. 2014;28(3):353–66. doi:10.1016/j.berh.2014.08.002.
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. doi:10.1016/S0140-6736(18)32279-7.
- Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain. 2019;20(2):146–60. doi:10.1016/j.jpain.2018.07.006.
- Fitzcharles M-A, Shir Y. New concepts in rheumatic pain. Rheum Dis Clin North Am. 2008;34(2):267–83. doi:10.1016/j.rdc.2008.03.005.
- Schofield D, Cunich M, Shrestha RN, Tanton R, Veerman L, Kelly S, Passey ME. The long-term economic impacts of arthritis through lost productive life years: results from an Australian microsimulation model. BMC Public Health. 2018;18(1):654. Doi:1186/s12889-018-5509-3.
- Osteoarthritis in over 16s: diagnosis and management. London (UK): National Institute for Health and Clinical Excellence (NICE); 2022.
- Ippolito M, Spurio G, Compagno V, Rizzo A, Di Simone M, Corsale AM, Mazzola G, Giarratano A, Meraviglia S, Cortegiani A, et al. Autologous conditioned serum for chronic pain in patients with osteoarthritis: a feasibility observational study. Br J Pain. 2023;17(1):103–11. doi:10.1177/20494637221134169.
- van Laar M, Pergolizzi JV, Mellinghoff H-U, Merchante IM, Nalamachu S, O’Brien J, Perrot S, Raffa RB. Pain treatment in arthritis-related pain: beyond NSAIDs. Open Rheumatol J. 2012;6(1):320–30. doi:10.2174/1874312901206010320.
- Davis A, Robson J. The dangers of NSAIDs: look both ways. Br J Gen Pract. 2016;66(645):172–3. doi:10.3399/bjgp16X684433.
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Phys. 2008;2s;11(3;2s):S105–S20. doi:10.36076/ppj.2008/11/S105.
- Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–78. doi:10.1001/jama.2020.22171.
- Divekar A, Divekar O, M Navaratnam D, Shrivastava R. Impact of the harm review service for patients awaiting elective hip and knee surgeries for more than 52 weeks. Curēus. 2022;14(4):e23805. doi:10.7759/cureus.23805.
- McCracken LM. Personalized pain management: is it time for process-based therapy for particular people with chronic pain? Eur J Pain. 2023;27(9):1044–55. doi:10.1002/ejp.2091.
- Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59(5-6):333–9. doi:10.1016/j.rehab.2016.07.004.
- La Porta C, Bura SA, Negrete R, Maldonado R. Involvement of the endocannabinoid system in osteoarthritis pain. Eur J Neurosci. 2014;39(3):485–500. doi:10.1111/ejn.12468.
- Costa B, Comelli F. Handbook of cannabis. Oxford (UK): Oxford University Press; 2014.
- Maldonado R, Baños JE, Cabañero D. The endocannabinoid system and neuropathic pain. Pain (Amsterdam). 2016;157 Suppl 1(Supplement 1):S23–S32. doi:10.1097/j.pain.0000000000000428.
- Compton DR, Dewey WL, Martin BR. Cannabis dependence and tolerance production. Adv Alcohol Subst Abuse. 1990;9(1-2):129–47. https://search.proquest.com/docview/232157757. doi:10.1300/J251v09n01_08.
- Rodríguez MJ, Robledo P, Andrade C, Mahy N. In vivo co‐ordinated interactions between inhibitory systems to control glutamate‐mediated hippocampal excitability. J Neurochem. 2005;95(3):651–61. doi:10.1111/j.1471-4159.2005.03394.x.
- Ishac EJN, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118(8):2023–8. doi:10.1111/j.1476-5381.1996.tb15639.x.
- Mbvundula EC, Bunning RAD, Rainsford KD. Arthritis and cannabinoids: HU-210 and Win-55,212-2 prevent IL-1alpha-induced matrix degradation in bovine articular chondrocytes in-vitro. J Pharm Pharmacol. 2006;58(3):351–8. https://www.ncbi.nlm.nih.gov/pubmed/16536902. doi:10.1211/jpp.58.3.0009.
- Whyte L, Ford L, Ridge S, Cameron G, Rogers M, Ross R. Cannabinoids and bone: endocannabinoids modulate human osteoclast function in vitro. Br J Pharmacol. 2012;165(8):2584–97. doi:10.1111/j.1476-5381.2011.01519.x.
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature (London). 1993;365(6441):61–5. doi:10.1038/365061a0.
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232(1):54–61. doi:10.1111/j.1432-1033.1995.tb20780.x.
- Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA. 2006;103(3):696–701. doi:10.1073/pnas.0504187103.
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, et al. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2011;15(1):64–9. https://escholarship.org/uc/item/3913r252
- Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, Cuomo V. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol. 2009;85:57–72. doi:10.1016/S0074-7742(09)85005-8.
- La Porta C, Bura SA, Llorente-Onaindia J, Pastor A, Navarrete F, García-Gutiérrez MS, De la Torre R, Manzanares J, Monfort J, Maldonado R, et al. Role of the endocannabinoid system in the emotional manifestations of osteoarthritis pain. Pain (Amsterdam). 2015;156(10):2001–12. doi:10.1097/j.pain.0000000000000260.
- Kwong FNK, Richardson SM, Evans CH. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res Ther. 2008;10(3):R65. doi:10.1186/ar2436.
- Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V, et al. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2008;10(2):R43. doi:10.1186/ar2401.
- Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105(1-2):1–25. doi:10.1016/j.jep.2006.02.001.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9‐tetrahydrocannabinol, cannabidiol and Δ9‐tetrahydrocannabivarin. Br J Pharmacol. 2007;153(2):199–215. doi:10.1038/sj.bjp.0707442.
- Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588–631.
- Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L, Sweeney JM, Miyauchi JT, Tsirka SE, Ojima I, et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem. 2015;290(14):8711–21. doi:10.1074/jbc.M114.618447.
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkötter J, Hellmich M, Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94. doi:10.1038/tp.2012.15.
- Laprairie RB, Bagher AM, Kelly MEM, Denovan‐Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–805. doi:10.1111/bph.13250.
- Nadal X, La Porta C, Andreea Bura S, Maldonado R. Involvement of the opioid and cannabinoid systems in pain control: new insights from knockout studies. Eur J Pharmacol. 2013;716(1-3):142–57. doi:10.1016/j.ejphar.2013.01.077.
- Simão da Silva KAB, Paszcuk AF, Passos GF, Silva ES, Bento AF, Meotti FC, Calixto JB. Activation of cannabinoid receptors by the pentacyclic triterpene α,β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain (Amsterdam). 2011;152(8):1872–87. Doi: pain.2011.04.005. doi:10.1016/j.pain.2011.04.005.
- Casey SL, Atwal N, Vaughan CW. Cannabis constituent synergy in a mouse neuropathic pain model. Pain (Amsterdam). 2017;158(12):2452–60. doi:10.1097/j.pain.0000000000001051.
- Schuelert N, McDougall JJ. Cannabinoid‐mediated antinociception is enhanced in rat osteoarthritic knees. Arthritis Rheum. 2008;58(1):145–53. doi:10.1002/art.23156.
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. 2017;158(12):2442–51. doi:10.1097/j.pain.0000000000001052.
- Wang L, Hong PJ, May C, Rehman Y, Oparin Y, Hong CJ, Hong BY, AminiLari M, Gallo L, Kaushal A, et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: a systematic review and meta-analysis of randomised clinical trials. BMJ. 2021;374:n1034. doi:10.1136/bmj.n1034.
- Bapir L, Erridge S, Nicholas M, Pillai M, Dalavaye N, Holvey C, Coomber R, Hoare J, Khan S, Weatherall MW, et al. Comparing the effects of medical cannabis for chronic pain patients with and without co-morbid anxiety: a cohort study. Expert Rev Neurother. 2023;23(3):281–95. doi:10.1080/14737175.2023.2181696.
- Frane N, Stapleton E, Iturriaga C, Ganz M, Rasquinha V, Duarte R. Cannabidiol as a treatment for arthritis and joint pain: an exploratory cross-sectional study. J Cannabis Res. 2022;4(1):47. doi:10.1186/s42238-022-00154-9.
- Schubert EA, Johnstone MT, Benson MJ, Alffenaar JC, Wheate NJ. Medicinal cannabis for Australian patients with chronic refractory pain including arthritis. Br J Pain. 2023;17(2):206–17. doi:10.1177/20494637221147115.
- Medicines and Healthcare Products Regulatory Agency. The supply, manufacture, importation and distribution of unlicensed cannabis-based products for medicinal use in humans. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/869284/Cannabis_Guidance__unlicensed_CBPMs__updated_2020.pdf.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. jclinepi.2007.11.008. doi:10.1016/j.jclinepi.2007.11.008.
- Brusselaers N, Lagergren J. The Charlson Comorbidity Index in Registry-based research. Methods Inf Med. 2017;56(5):401–6. doi:10.3414/ME17-01-0051.
- Erridge S, Salazar O, Kawka M, Holvey C, Coomber R, Usmani A, Sajad M, Beri S, Hoare J, Khan S, et al. An initial analysis of the UK Medical Cannabis Registry: outcomes analysis of first 129 patients. Neuropsychopharmacol Rep. 2021;41(3):362–70. doi:10.1002/npr2.12183.
- Lee D, de Keizer N, Lau F, Cornet R. Literature review of SNOMED CT use. J Am Med Inform Assoc. 2014;21(E1):E11–E19. doi:10.1136/amiajnl-2013-001636.
- British National Formulary. Medicines guidance: prescribing in palliative care [cited 2023 Apr 15]. Available from: https://bnf.nice.org.uk/medicines-guidance/prescribing-in-palliative-care/.
- Kapstad H, Rokne B, Stavem K. Psychometric properties of the Brief Pain Inventory among patients with osteoarthritis undergoing total hip replacement surgery. Health Qual Life Outcomes. 2010;8(1):148. doi:10.1186/1477-7525-8-148.
- Jumbo SU, MacDermid JC, Kalu ME, Packham TL, Athwal GS, Faber KJ. Measurement properties of the Brief Pain Inventory-Short Form (BPI-SF) and Revised Short McGill Pain Questionnaire Version-2 (SF-MPQ-2) in pain-related musculoskeletal conditions: a systematic review. Clin J Pain. 2021;37(6):454–74. doi:10.1097/AJP.0000000000000933.
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. doi:10.1016/j.jpain.2007.09.005.
- Dworkin RH, Turk DC, Revicki DA, Harding G, Coyne KS, Peirce-Sandner S, Bhagwat D, Everton D, Burke LB, Cowan P, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain (Amsterdam). 2009;144(1-2):35–42. pain.2009.02.007. doi:10.1016/j.pain.2009.02.007.
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63 Suppl 11(S11):S240–S252. doi:10.1002/acr.20543.
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. doi:10.1001/archinte.166.10.1092.
- Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, Herzberg PY. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266–74. doi:10.1097/MLR.0b013e318160d093.
- Plummer F, Manea L, Trepel D, McMillan D. Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry. 2016;39:24–31. doi:10.1016/j.genhosppsych.2015.11.005.
- Snyder E, Cai B, DeMuro C, Morrison MF, Ball W. A new single-item Sleep Quality Scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018;14(11):1849–57. doi:10.5664/jcsm.7478.
- Yi H, Shin K, Kim J, Kim J, Lee J, Shin C. Validity and reliability of Sleep Quality Scale in subjects with obstructive sleep apnea syndrome. J Psychosom Res. 2009;66(1):85–8. doi:10.1016/j.jpsychores.2008.07.008.
- van Hout B, Janssen MF, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, Lloyd A, Scalone L, Kind P, Pickard AS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15. doi:10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence. Position statement on the use of the EQ‐5D‐5L value set for England. [cited 2023 Apr 15]. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l.
- Ferguson L, Scheman J. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J Pain. 2009;10(4):S73. doi:10.1016/j.10jpain.2009.01.258.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009 [cited 2023 Apr 21].
- Liu-Seifert H, Zhang S, D’Souza D, Skljarevski V. A closer look at the baseline-observation-carried-forward (BOCF). Patient Prefer Adherence. 2010;4(default):11–6. doi:10.2147/PPA.S8135.
- IBM Corp. IBM SPSS Statistics for Macintosh, Version 29.0.2.0. Armonk (NY): IBM Corp; 2023.
- GraphPad Prism version 9.5.1 for Macintosh. Boston (MA): GraphPad Software. www.graphpad.com.
- Meng H, Page MG, Ajrawat P, Deshpande A, Samman B, Dominicis M, Ladha KS, Fiorellino J, Huang A, Kotteeswaran Y, et al. Patient-reported outcomes in those consuming medical cannabis: a prospective longitudinal observational study in chronic pain patients. Can J Anaesth. 2021;68(5):633–44. doi:10.1007/s12630-020-01903-1.
- Vickery AW, Roth S, Ernenwein T, Kennedy J, Washer P. A large Australian longitudinal cohort registry demonstrates sustained safety and efficacy of oral medicinal cannabis for at least two years. PLoS One. 2022;17(11):e0272241. doi:10.1371/journal.pone.0272241.
- Peters L, Peters A, Andreopoulos E, Pollock N, Pande RL, Mochari-Greenberger H. Comparison of DASS-21, PHQ-8, and GAD-7 in a virtual behavioral health care setting. Heliyon. 2021;7(3):e06473. doi:10.1016/j.heliyon.2021.e06473.
- Moreno-Sanz G, Madiedo A, Lynskey M, Brown MRD. “Flower power”: controlled inhalation of THC-predominant cannabis flos improves health-related quality of life and symptoms of chronic pain and anxiety in eligible UK patients. Biomedicines. 2022;10(10):2576. doi:10.3390/biomedicines10102576.
- Haroutounian S, Ratz Y, Ginosar Y, Furmanov K, Saifi F, Meidan R, Davidson E. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain. 2016;32(12):1036–43. doi:10.1097/AJP.0000000000000364.
- Colizzi M, Bhattacharyya S. Cannabis use and the development of tolerance: a systematic review of human evidence. Neurosci Biobehav Rev. 2018;93:1–25. doi:10.1016/j.neubiorev.2018.07.014.
- Nasiri N, Nateghi R, Zarei F, Hosseini S, Eslaminejad MB. Mesenchymal stem cell therapy for osteoarthritis: practice and possible promises. In: Advances in experimental medicine and biology. Cham (Switzerland): Springer International Publishing; 2022. p. 107–25.
- Heineman JT, Forster GL, Stephens KL, Cottler PS, Timko MP, DeGeorge BR. A randomized controlled trial of topical cannabidiol for the treatment of thumb basal joint arthritis. J Hand Surg Am. 2022;47(7):611–20. jhsa.2022.03.002. doi:10.1016/j.jhsa.2022.03.002.
- Cahill SP, Lunn SE, Diaz P, Page JE. Evaluation of patient reported safety and efficacy of cannabis from a survey of medical cannabis patients in Canada. Front Public Health. 2021;9:626853. doi:10.3389/fpubh.2021.626853.
- Ware MA, Wang T, Shapiro S, Collet J, COMPASS Study Team. Cannabis for the management of pain: assessment of safety study (COMPASS). J Pain. 2015;16(12):1233–42. doi:10.1016/j.jpain.2015.07.014.
- Wang T, Collet J, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008;178(13):1669–78. doi:10.1503/cmaj.071178.
- Hall W, Solowij N, Lemon J. Theœ health and psychological consequences of cannabis use. Canberra: Australian Government Publ. Service; 1995.
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, Gluud C, Martin RM, Wood AJG, Sterne JAC, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601–5. doi:10.1136/bmj.39465.451748.AD.
- Evers AWM, Colloca L, Blease C, Annoni M, Atlas LY, Benedetti F, Bingel U, Büchel C, Carvalho C, Colagiuri B, et al. Implications of placebo and nocebo effects for clinical practice. Psychother Psychosom. 2018;87(4):204–10. 10. 1159/000490354. doi:10.1159/000490354.
- Vase L, Petersen GL, Lund K. Placebo effects in idiopathic and neuropathic pain conditions. In: Handbook of experimental pharmacology. Berlin (Germany): Springer Berlin Heidelberg; 2014. p. 121–36.
- Gedin F, Blomé S, Pontén M, Lalouni M, Fust J, Raquette A, Vadenmark Lundquist V, Thompson WH, Jensen K. Placebo response and media attention in randomized clinical trials assessing cannabis-based therapies for pain: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(11):e2243848. doi:10.1001/jamanetworkopen.2022.43848.
- Díaz-Lago M, Blanco F, Matute H. Expensive seems better: the price of a non-effective drug modulates its perceived efficacy. Cogn Res Princ Implic. 2023;8(1):8. doi:10.1186/s41235-023-00463-4.
- Bar-Lev Schleider L, Mechoulam R, Sikorin I, Naftali T, Novack V. Adherence, safety, and effectiveness of medical cannabis and epidemiological characteristics of the patient population: a prospective study. Front Med (Lausanne). 2022;9:827849. doi:10.3389/fmed.2022.827849.
Appendix A.
Paired baseline and follow-up scores for pain-related PROMs in patients with osteoarthritis at 1-, 3-, 6-, and 12-month follow-up.
Appendix B.
Frequency of AEs, separated by severity.