ABSTRACT
Background: The aim of our investigation was to estimate the stability of α-tocopheryl O-glycosides in relation to activity of exoglycosidases in selected rat tissues. Material and Methods: Acetylated glycosides were obtained in glucosidation of α-tocopherol using the Helferich method. The activity of exoglycosidases was determined by the Zwierz et al. method. Protein concentrations were determined by the biuret method. The concentration of released α-tocopherol was determined with the HPLC method. Results: The comparison of the amount of released α-tocopherol with the amount of released p-nitrophenol shows that glycoside bound in 2a–5a derivatives of α-tocopherol undergoes hydrolysis significantly harder than in appropriate 2b–5b p-nitrophenyl derivatives. Conclusion: The results indicate that tocopheryl O-glycosides are more resistant to enzymatic hydrolysis than appropriate p-nitrophenol O-glycosides 2a–5a. Among examined tocopheryl O-glycosides, galactoside 4 is the only compound that caused the significant increase in tocopherol concentration, as compared to its endogenic content.
INTRODUCTION
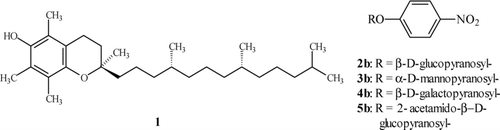
Vitamin E (α-tocopherol 1) () is regarded as the most important low-molecular lipophilic antioxidant that protects cellular membranes and other phospholipid structures against the destructive activity of oxygen-centered free radicals (Azzi and Stocker Citation2000; Wang and Quinn Citation1999). The investigations carried out for decades showed that administration of vitamin E helps in the treatment of such diseases as atherosclerosis and circulatory disorders. It inhibits prostate proliferation and delays progress of Alzheimer and Parkinson diseases and many other disorders connected with disturbances of free radical levels in the organism (Hasty et al. 2007; Negri et al. Citation1991; Vatassery et al. Citation1999). However, moderate absorption (10%–70% of content in diet) and long-lasting transport to cells limit the therapeutic use of vitamin E.
One of the possible ways of increasing the bioavailability of vitamin E is to convert free α-tocopherol into O-glycoside derivatives. O-glycosidic derivatives of vitamin E as prodrugs of higher amphiphilic character show better solubility in body fluids and better permeability across cellular membranes. Thus, O-glycosides of α-tocopherol would reach the target place faster and release active molecules of tocopherol as a result of the activity of appropriate exoglycosidases or acidic medium (under ischemic-reperfusion conditions) (Matsuoka et al. Citation1998; Mizuma et al. Citation1994, Citation1992; Veyhl et al. Citation1998).
Lahmann and Thiem (Citation1997) have reported that O-glycosides of α-tocopherol are resistant toward α- and β-glucosidases isolated from almonds and hen's eggs, respectively. On the other hand, our preliminary studies pointed to the fact that α-tocopheryl galactoside 4a underwent a slow decomposition in supernatant fluids from homogenates of selected rat tissues (ileum, kidney, etc.) (Witkowski et al. Citation2004). In another experiment, a prolonged photoprotective effect was observed for α-tocopheryl glucoside (2a) during UVB irradiation of naked mice (Niczyporuk et al. Citation2003). The present paper shows further data concerning the persistence of α-tocopheryl O-glycosides (2a–5a) () in the presence of exoglycosidases present in chosen rat tissues (ileum, brain, liver, and kidney).
MATERIAL AND METHODS
Synthesis of α-Tocopheryl Glycosides
α-Tocopheryl O-glycosides 2a–5a () were obtained according to the modified Helferich method (Sch. 1) (Lahmann and Thiem Citation1997; Witkowski and Walejko Citation2002). After deacetylation in anhydrous methanol in the presence of KCN, the crude glycosides were purified by column chromatography. The purity of the compounds 2a–5a was confirmed by 1H and 13C NMR as well as by HPLC.
Preparation of tissues
The ileum, brain, liver, and kidney tissues of Wistar rats were homogenized for 90 sec in four volumes of 0.2% of Triton X-100 in 0.15 M KCl. The homogenates were centrifuged at 12,000 × g, for 30 min at 4°C and the supernatants were used for further experiments. Antioxidants—8 mg of 2,6-di-tert-butyl-4-methylphenol (BHT) and 7 mg of ascorbic acid—were added to each sample containing 3.5 mL of the supernatant. Substrates for the activity determination of lysosomal exoglycosidases and Triton X-100 were purchased from Sigma (St. Louis, USA), the solvents for HPLC from Lab-Scan, and the rest of the reagents from the Polish Chemical Reagent Factory (POCh), Gliwice.
Methods of determination
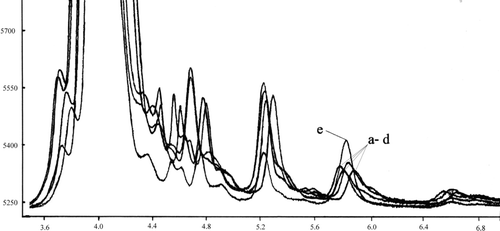
In order to determine the activity of exoglycosidases in a supernatant, we used the method of Zwierz et al. (Citation1981). Concentrations of proteins were determined by the biuret method, using serum bovine albumin as a standard (Dawson et al. Citation1969). The activity of lysosomal exoglycosidases N-acetyl-β-D-hexosaminidase, β-galactosidase, α-mannosidase, and β-glucosidase was determined on the basis of the amount of p-nitrophenol (in nmol) released after 60 min incubation at 37°C, of 50 μL of supernatant fluids with p-nitrophenyl O-glycosides 2b–5b (). Determination was conducted using a large excess of p-nitrophenyl glycosides and therefore the amount of released p-nitrophenyl is proportional to the activity of the appropriate enzyme. The release of α-tocopherol from α-tocopheryl O-glycosides (α-tocopheryl β-D-glucopyranoside [2a], α-tocopheryl β-D-mannopyranoside [3a], α-tocopheryl β-D-galactopyranoside [4a], α-tocopheryl 2-acetamido-β-D-glucopyranoside [5a]) in the presence of exoglycosidases was determined under identical conditions. The action of enzymes was stopped after 2 h of incubation by extraction with hexane (3 × 1 mL). The combined hexane extracts were stored at –10°C. The analyzed samples were evaporated to dryness immediately before the measurement and the residues were dissolved in of hexane (0.5 mL). The amount of released α-tocopherol (1) was determined by HPLC. The recovery of method was practically quantitative in the procedure used. The chromatograms obtained from the supernatant before and after addition of the known amount of α-tocopherol (10 μg) are presented in .
FIGURE 3 Chromatograms of the extracts from liver tissue (from 50 μL of supernatant): a–d: endogenic α-tocopherol; e: after addition 10 μg of α-tocopherol. 5.7–6.0 free α-tocopherol peak.
The HPLC system (LabAlliance) consisted of pumps (III Pump series), the detector UV-VIS (525 Dual-wavelength), and the device for sample introduction (a dosing valve Rheodyne Model 7725i and a 20-μL dosing loop). The column HPLC (25 cm × 4.6 mm, NH2-NP, 5 μm) (Supelco) was used. The 3% solution of isopropanol in hexane was a mobile phase. The analysis was conducted at the flow velocity of 1 mL/min, at room temperature, with the detection at 295 nm with the use of Data Ally Chromatography Manager for Windows 95.
The investigation has the approval of a Local Ethical Committee.
RESULTS
The activity of hepatic exoglycosidases was determined on the basis of the amount of p-nitrophenol released after 60 min incubation at 37°C of p-nitrophenyl glycosides 2b–5b in 50 μL of supernatant ().
Table 1 Amounts of p-nitrophenol (nmol) released after 60 min incubation of p-nitrophenyl glycosides 2b–5b in 50 μL of supernatant (n = 8)
The amounts of released α-tocopherol from glycosides 2a–5a after 2 h of incubation with 50 μL of supernatant from ileum, brain, liver, and kidney tissue supernatant are listed in . From the values of total released α-tocopherol in supernatants from the liver, kidneys, intestine, and brain were subtracted amounts of endogenic α-tocopherol: 0.154, 0.332, 0.307, and 0.385 nmol, respectively. The differences between total and endogenic content of α-tocopherol were shown as Δ parameter.
Due to the use of the marked excess of glycosides (1 mg), from each portion of glycosides 2a–5a the samples of 1 mg were taken, and then extracted with hexane (3 × 1 mL). The combined hexane extracts were evaporated to dryness and dissolved in 0.5 mL of hexane. In the combined hexane extracts we did not observe by HPLC (at the detection limit of 1 × 10–6 mg/mL) the presence of free α-tocopherol.
The stability of O-glycosides of α-tocopherol 2a–5a in the solution of citric buffer with an addition of BHT and ascorbic acid as antioxidants was also examined.
The samples of 2a–5a (1 mg) were incubated according to Zwierz et al. (Citation1981) using the same volume of citric buffer instead of tissue supernatant. The action of buffer solution was interrupted after 2, 4, 8, 12, and 24 h, respectively, by extraction with hexane (3 × 1 mL). The amounts of α-tocopherol released from the glycosides 2a–5a, due to hydrolysis in buffer, are presented in .
Table 2 Amounts of released -tocopherol (nmol) after incubation of glycosides 2a–5a with 50 μL of supernatant fluid (n = 8)
Table 3 Amounts of released α-tocopherol (nmol) after incubation of 2a–5a glycosides in citric buffer (n = 8)
The comparison of the amounts of α-tocopherol released from the glycosides 2a–5a incubated in tissue supernatants () and the amount of released p-nitrophenol from the glycosides 2b–5b () showed that O-glycosidic bonds in derivatives 2a–5a are more stable toward hydrolysis as compared with the appropriate p-nitrophenyl glycosides 2b–5b.
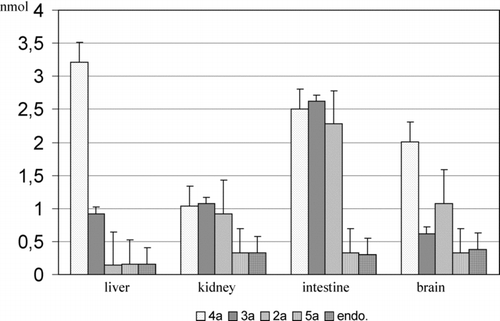
FIGURE 4 The comparison of released α-tocopherol after 2-h incubation of the glycosides 2a–5a with 50 μL of the specified supernatants and the endogenic content of α-tocopherol. The results are presented as the mean value ± standard deviation (n = 20).
A similar effect was observed previously for galactoside 4a in rat tissue supernatants (Witkowski et al. Citation2004). According to the literature, the 2,6-dimethylphenyl glycosides are more resistant to hydrolysis by exoglycosidases than the glycosides of p-substituted phenols (Hall et al. Citation1961; Nath and Rydon Citation1954).
A higher stability of glycosidic bonds in 2a–5a, as compared to 2b–5b, is a result of steric hindrance of the tocopheryl system, which hinders the effective contact of the active center of exoglycosidase with the substrate. However, the considerable amounts of free α-tocopherol (2–3 mmol) in the samples incubated with glycosides 3a and 4a in the respective supernatant of ileum and liver do not allow us to univocally conclude that the tested O-glycosides of α-tocopherol are completely passive toward endogenic glycosidases in supernatant fluids. It can be assumed that the presence of free α-tocopherol in the samples of 2a–5a is the effect of cleavage of the glycosides under incubation conditions. The comparison of the amount of released α-tocopherol from glycosides 2a–5a during a 2-h incubation with the endogenic content is presented in .
FIGURE 5 Amounts of α-tocopherol released from glycosides 2a–4a as a result of 2-h hydrolysis, diminished by the endogenic content α-tocopherol. The results (n = 10) are presented as the mean value ± standard deviation.
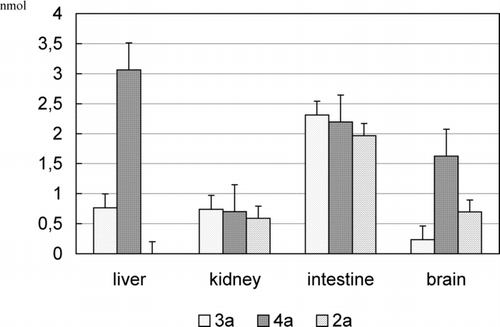
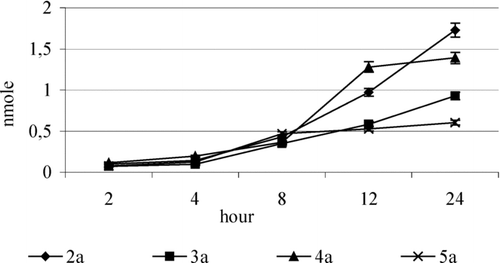
FIGURE 6 Amount of α-tocopherol released as a result of hydrolysis of the glycoside 2a–5a in buffer. The results (n = 10) were presented as the mean value ± standard deviation.
Among tested glycosides of α-tocopherol, the considerable amounts of α-tocopherol were released during the incubation of the glycosides 2a–4a in the supernatant of the ileum (1.967, 2.311, and 2.195 nmol, respectively) and galactoside 4a in the supernatant of the liver (3.063 nmol).
Unexpectedly, the most persistent glycosides seem to be 2a and 5a. The 2-acetamidoglucoside 5a does not practically undergo decomposition (). On the other hand, in case of p-nitrophenyl O-glycosides of 2b–5b, it can be pointed out that the amount of released p-nitrophenol during incubation () suggests that 2-acetamidoglucoside 5b undergoes enzymatic hydrolysis easiest.
In case of tested supernatant fluids, the relative activity of the enzymes, determined on the basis of the amount of released p-nitrophenol, decreases as follows: N-acetyl-β-hexosaminidase > β-glucosidase > β-galactosidase > α-mannosidase. However, the described order does not occur in the case of α-tocopheryl glycosides 2a–5a.
It can be suggested that α-tocopheryl glycosides undergo nonenzymatic hydrolysis due to environmental acidity (from citric buffer). Therefore, the stability of derivatives 2a–5a in citric buffer was examined.
The incubation was conducted in the mixture of identical composition as applied in the examination in supernatants, using 50 μL of citric buffer instead of the same amount of supernatant. The hydrolysis was stopped after 2, 4, 8, 12, and 24 h, and the amount of released α-tocopherol was determined by HPLC ().
All the tested compounds underwent nonenzymatic hydrolysis and the amount of released α-tocopherol varied from 0.07 to 0.166 nmol after 2 h of incubation.
The values were considerably lower than those obtained after incubation with tissue supernatants (). This suggests that α-tocopherol from O-glycosides is released in the way of enzymatic and nonenzymatic hydrolysis.
The amounts released from supernatants α-tocopherol after a 2-h incubation diminished by the endogenic content and the value obtained after hydrolysis in citric acid are presented in .
The above results point out that the tested glycosides 2a–4a most efficiently undergo enzymatic hydrolysis in the ileum supernatant.
According to the literature, the more axial hydroxyl groups there are in sugar residue, the higher the rate of nonenzymatic hydrolysis of O-glycosides (Helligso and Bols Citation2003).
Thus, the galactoside 4b is easily cleavable both enzymatically and nonenzymatically. Unexpectedly, 2-acetamidoglucosides of α-tocopherol 5a appeared to be the most stable among the tested glycosides. However, prolonged time of hydrolysis caused a slow hydrolysis of 2-acetamideglycoside ().
The obtained results show that the examined glycosidic derivatives 2a–5b, especially in the tissue of ileum, can undergo a prolonged cleavage with the release of free α-tocopherol.
CONCLUSION
The stability of glycosides of α-tocopherol 2a–5a in the presence of exoglycosidases that are present in supernatants of rat tissues (liver, ileum, kidney, and brain) was evaluated. The results show that O-glycosides of tocopherol 2a–5a are more resistant to enzymatic hydrolysis as compared with the respective p-nitrophenyl O-glycosides 2b–5b.
Among the tested derivatives, the galactoside 4a easily undergoes hydrolysis in supernatant fluid from ileum. The slow decomposition of α-tocopherol O-glycosides in tissues can be beneficial to obtain the vitamin E derivatives with prolonged action in the organism.
This article is not subject to United States Copyright Laws.
REFERENCES
- Azzi A., Stocker A. Vitamin E: non-antioxidat roles. Progr. Lipid Res. 2000; 39: 231–255
- Dawson R. M. C., Elliott D. C., Elliott W. H., Jones K. M. Data for Biochemical Research, , et al. Oxford University Press, Oxford 1969
- Hall A. N., Hollingshead S., Rydon H. N. The acid and alkaline hydrolysis of some substituted phenyl a-D-glucosides. J. Chem. Soc. 1961; 4290–4295
- Hasty A. H., Gruen M. L., Terry E. S., Surmi B. K., Atkinson R. D., Gao L., Morrow J. D. Effects of vitamin E on oxidative stress and atherosclerosis in an obese hyperlipidemic mouse model. J. Nutr. Biochem. 2007; 8(2)127–133
- Helligso J. H., Bols M. Steric effects are not the cause of the rate differences in hydrolysis of stereoisomeric glycosides. Org. Lett. 2003; 5: 3419–3421
- Lahmann M., Thiem J. Synthesis of alpha-tocopheryl oligosaccharides. Carbohydr. Res. 1997; 299: 23–31
- Matsuoka T., Nishizaki T., Kisby G. E. Na+-dependent and phlorizin-inhibitable transport of glucose and cycasin in brain endothelial cells. J. Neurochem. 1998; 70: 772–777
- Mizuma T., Ohta K., Awazu S. The beta-anomeric and glucose preferences of glucose transport carrier for intestinal active absorption of monosaccharide conjugates. Biochim. Biophys. Acta 1994; 1200: 117–122
- Mizuma T., Ohta K., Hayashi M., Awazu S. Intestinal active absorption of sugar-conjugated compounds by glucose transport system: implication of improvement of poorly absorbable drugs. Biochem. Pharmacol. 1992; 43: 2037–2039
- Nath R. L., Rydon H. N. The influence of structure on the hydrolysis of substituted phenyl b-D-glucosides by emulsin. Biochem. J. 1954; 57: 1–10
- Negri E., La Vecchia C., Franceschi S., D'Avanzo B., Parazzini F. Vegetable and fruit consumption and cancer risk. Int. J. Cancer 1991; 48: 350–354
- Niczyporuk W., Aleksiejczuk P., Witkowski S., Krajewska-Kułak E., Niczyporuk M., Wałejko P. In vivo studies of alfa-tocopheryl glucoside on mouse skin exposed to UVA irradiation. Farmacja Polska 2003; 59: 35–37
- Vatassery G. T., Bauer T., Dysken M. High doses of vitamin E in the treatment of disorders of the central nervous system in the aged. Am. J. Clin. Nutr. 1999; 70: 793–801
- Veyhl M., Wagner K., Volk C., Gorboulev V., Baumgarten K., Weber W. M., Schaper M., Bertram B., Wiessler M., Koepsell H. Transport of the new chemotherapeutic agent beta-D-glucosylisophosphoramide mustard (D-19575) into tumor cells is mediated by the Na+-D-glucose cotransporter SAAT1. Proc. Natl. Acad. Sci. U S A 1998; 95: 2914–2919
- Wang X., Quinn P. J. Vitamin E and its function in membranes. Progr. Lipid Res. 1999; 38: 309–336
- Witkowski S., Walejko P. Studies in the acid catalysed glycosylation of a-tocopherol. Z. Naturforsch 2002; 57b: 571–578
- Witkowski S., Walejko P., Knas M., Maj J., Dudzik D., Marciniak J., Wilczewska A. Z., Zwierz K. The cleavage of vitamin E galactoside in the rat tissue homogenates. Farmaco 2004; 59: 669–671
- Zwierz K., Gindzieński A., Głowacka D., Porowski T. The degradation of glycoconjugates in the human gastric mucosal membrane. Acta Med. Acad. Sci. Hung. 1981; 38: 145