ABSTRACT
Objective: There is a need to explore multi-discipline general treatment modes to improve the survival period of patients with SCLC and brain metastases undergoing standard radiotherapy treatment.
Methods: A total of 101 patients with SCLC and brain metastases were included into this study. These patients were classified into 4 groups, based on different treatment modes: chemotherapy group, brain radiotherapy group, brain radiotherapy combined with sequential chemotherapy, and chemotherapy combined with sequential brain radiotherapy. Recent and long-term curative effects were compared among the 4 groups.
Results: A RR of 42.57% was determined for all 4 groups, and median PFS and OS was 11.56 and 17.32 months, respectively. After SCLC with brain metastases manifested in the limited stage, the difference in median survival period was not statistically significant among the 4 treatment groups (P = 0.29). At the extensive stage of SCLC, survival period was superior in the brain radiotherapy combined with sequential chemotherapy group, compared with other groups (P<0.05). Furthermore, median survival period in the brain radiotherapy combined with sequential chemotherapy group was 15.5 ± 1.03 months. This was followed by 12.0 ± 3.06 months in the chemotherapy combined with sequential brain radiotherapy group, 8.0 ± 1.49 months in the chemotherapy group, and 8.0 ± 0.43 months in the brain radiotherapy group.
Conclusion: Combining chemotherapy with brain radiotherapy is a better treatment mode compared with single therapy for treating SCLC with brain metastases. Furthermore, it is recommended for patients in the extensive stage to initially receive brain radiotherapy.
Introduction
Small cell lung cancer approximately accounts for 10–15% of all lung cancer cases, and has a high invasiveness.Citation1 It often concurs with metastases in the early period, and approximately 10–18% of patients are complicated with brain metastases at onset. In the development of this disease, approximately 40–50% of patients finally appear with brain metastases.Citation2 Patients with brain metastases have a bad prognosis, and the mean survival period is 2 months. For patients with small cell lung cancer (SCLC) in the limited stage and extensive stage, evidence-based medicine revealed that relieved patients were administered with prophylactic cranial irradiation (PCI) Citation3 to reduce the incidence of brain metastases. For patients with brain metastases, whole brain radiation therapy (WBRT) is used as the standard treatment. However, single radiotherapy can effectively extend the survival period of patients.Citation4
Part of chemotherapy drugs can pass through the blood-brain barrier (BBB). Due to the biologic characteristics of SCLC, which is different from non-small cell lung cancer (NSCLC), chemotherapy is useful for patients with SCLC and brain metastases.Citation5 However, there is no evidence-based medicine to prove whether chemotherapy combined with radiotherapy can further extend the survival period.Citation6,Citation7 In addition, no relevant studies have compared radiotherapy followed by sequential chemotherapy with systematic chemotherapy for controlling symptoms followed by brain radiotherapy after the co-occurrence of brain metastases.Citation8,Citation9 Therefore, there is a need to further investigate how optimal treatment can be adopted in clinic, to further extend the survival period and improve the quality of life of patients with SCLC and brain metastases. This study retrospectively analyzed the clinically curative effects and prognosis-related factors of the combined treatment of chemotherapy and radiotherapy for 101 patients with SCLC and brain metastases, who were admitted to our department from January 2008 to May 2013. Results of this study are described as follows.
Materials and methods
General information
A total of 101 patients with SCLC, who were diagnosed at the beginning and middle of treatment based on histology and cytology, were included into this study. The diagnosis of brain metastases was mainly verified using CT or MRI examinations. The clinical manifestations of these patients are shown in .
Table 1. Characteristics o f 101 SCLC patients with brain metastases.
Treatment methods
All patients received surgery, chemotherapy, radiotherapy, or combined therapies. Patients who have not been treated were excluded from this study. At the beginning of the diagnosis, patients in the limited stage (n = 55) and extensive stage (n = 46) were included; and all patients were treated with first-line chemotherapy solutions of EP, EC, EL, BCNU+EP, and amrubicin combined with cisplatin. The proportion of second-line and third-line chemotherapy was 62.37% (63/101) and 29.7% (71/101), respectively Chest radiotherapy was mainly applied to patients in the limited stage with a proportion of 70.9% (39/55). Then, patients were classified into 4 groups, based on the therapies for brain metastases: chemotherapy group, brain radiotherapy group, brain radiotherapy combined with sequential chemotherapy group, and chemotherapy combined with sequential brain radiotherapy group. Chemotherapy group (n = 21): patients did not receive brain radiotherapy, but chemotherapy that could pass through the BBB for at least 2 cycles was given, as required; brain radiotherapy group (n = 32): patients only received brain radiotherapy after brain metastases co-occurred; brain radiotherapy combined with sequential chemotherapy group (n = 32): brain radiotherapy followed by chemotherapy regimens specific for brain metastases were given after brain metastases co-occurred; chemotherapy combined with sequential brain radiotherapy group (n = 16): chemotherapy that could pass through the BBB for at least 2 cycles followed by brain radiotherapy was given after brain metastases co-occurred. The chemotherapy regimens used that were specific for brain metastases include carmustine, temozolomide, teniposide, bevacizumab, irinotecan, topotecan and other single or combined platinum regimens. For brain radiotherapy, patients received WBRT with a dosage of DT 25–30 Gy/15 times/20 d. For patients not diagnosed with brain metastases in the preliminary diagnosis, the proportion of PCI was small. Among the 55 patients who had such situations, only 4 patients received PCI, including 3 patients in the limited stage and one patient in the extensive stage.
Curative effect evaluation and follow-up visits
For the recent curative effect, response rate (RR) was calculated by CR+PR based on RECIST 1.0 solid tumor curative effect evaluation standards; that is, complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). Progression-free survival (PFS) refers to the duration from the start of treatment to disease progression. In this study, PFS included PFS in first-line treatment and PFS in lesion progression in the brain after brain metastases treatment; and the sum was the total PFS indicator of patients. Overall survival time refers to the duration from the start of treatment to death or last follow-up.
Statistical analysis
Data analysis was performed using SPSS 13.0 statistical software. Enumeration data were detected using the X2–test, and survival analysis was performed using the Kaplan-Meier method. Significant variables in the single factor analysis were included into the COX regression model. P<0.05 was considered statistically significant.
Results
Comparison of recent curative effects under different treatment modes in patients with SCLC and brain metastases
All 101 patients received chemotherapy, radiotherapy, or combined therapy; and underwent 755 chemotherapy cycles. describes the recent and long-term curative effects for the treatment of brain metastasis lesions in the chemotherapy group, brain radiotherapy group, brain radiotherapy combined with sequential chemotherapy group, and chemotherapy combined with sequential brain radiotherapy group. Among these 4 groups, patients in the brain radiotherapy combined with sequential chemotherapy group presented with the highest RR, and the number of patients with symptoms accounted for 68.75% (22/32). However, most patients with brain metastases in the chemotherapy combined with sequential radiotherapy group had no symptoms, while patients with symptoms accounted for 37.5% (6/16). The best recent curative effect was observed in patients in the brain radiotherapy combined with sequential chemotherapy group, and its difference between other 3 groups was not statistically significant ().
Table 2. Recent and long-term curative effects for the treatment of brain metastasis lesions ineach group.
Comparison of survival periods under different treatment modes for patients with SCLC and brain metastases
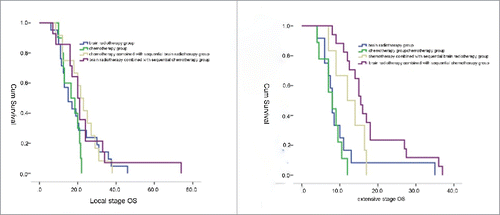
For all groups, median PFS and OS was 11.56 and 17.32 months, respectively. For patients with SCLC under the 4 treatment modes, there were no significant differences in gender, age, PS scores, clinical staging at the preliminary diagnosis, the number of patients with brain metastases, the presence of complications with brain metastasis symptoms, and organ metastasis among all groups. However, staging was an independent prognosis factor for patients with SCLC. Therefore, the survival period of patients summarized based on clinical staging was the stratification factor. Furthermore, the OS of patients with brain metastases in the limited stage and extensive stage in all 4 treatment modes were compared. The difference in median survival period under the 4 treatment modes in patients with brain metastases in the limited stage was not statistically significant (P = 0.29). The median survival period of patients in the brain radiotherapy group, chemotherapy group, chemotherapy combined with sequential brain radiotherapy group, and brain radiotherapy combined with sequential chemotherapy group was 15.0 ± 3.05 months, 16.5 ± 4.35 months, 21.5 ± 2.60 months and 20.0 ± 1.87 months, respectively. In the extensive stage, for patients with brain metastases, the survival period of patients in the brain radiotherapy combined with sequential chemotherapy group was superior to that of other groups (P<0.05). The median survival period of patients in the brain radiotherapy combined with sequential chemotherapy group was 15.5 ± 1.03 months, which was followed by 12.0 ± 3.06 months in the chemotherapy combined with sequential brain radiotherapy group, 8.0 ± 1.49 months in the chemotherapy group, and 8.0 ± 0.43 months in the brain radiotherapy group. The survival curves of these 4 treatment modes for patients with brain metastases in the limited stage and extensive stage are shown in .
Analysis of single factors that impact the survival period
Prognosis-related factors that impact the survival period were compared among the 101 patients with SCLC. The single factor analysis indicated that the main factors that impact the prognosis of patients included staging, PS scores, baseline NSE, different treatment modes, the presence of brain metastasis in the initial treatment, and the decision to receive chemotherapy. The difference in correlation with the prognosis for gender, age, baseline blood sodium and the number of brain metastasis was not statistically significant ().
Table 3. Univariate analysis of overall survival to 101cases of SCLC patients with brain metastases.
Analysis of multiple factors that impact the OS of patients with SCLC and brain metastases
Cox analysis indicated that staging, whether complicated with brain metastasis at the beginning of treatment, with or without brain metastasis symptoms, baseline NSE, first-line chemotherapy PFS, and PFS after the treatment of brain metastasis are independent prognosis factors that impact the total survival period of patients with brain metastasis ().
Table 4. Multivariate analysis of prognosis in patients with SCLC brain metastases.
Discussion
The incidence rate of brain metastasis at the initial diagnosis of SCLC was approximately 10%, and the development of this disease co-occurred with brain metastasis in 40–50% of patients.Citation10 Brain metastasis is one of the factors that result in the bad prognosis of SCLC, and is also one of the main factors of treatment failure. Brain metastasis from SCLC is different from metastases in other sites or organs.Citation11 Hence, its treatment method is also different from conventional treatments. To date, the treatment methods for SCLC with brain metastases include local treatment (surgery, radiotherapy, etc.), systematic chemotherapy, and symptomatic and supportive treatment. Although there are various treatment methods, the curative effect remains unsatisfactory with poor prognosis. Chest radiotherapy has become the standard treatment of patients with SCLC and brain metastasis. However, a single radiotherapy cannot significantly improve the prognosis of patients, and mean survival period is only 3–6 months.Citation12 Furthermore, there is not enough evidence to prove whether previous chemotherapy drugs that pass through the BBB can relieve brain metastasis in patients with SCLC and brain metastasis, and this is also not recommended in NCCN guidelines. However, it was clinically determined that there are significant differences in the prognosis of patients with SCLC and brain metastasis, and even part of these patients could survive for more than 3 to 5 y. Therefore, it is necessary to further determine whether different treatment methods and prognosis-related factors have an influence on the prognosis of patients with SCLC and brain metastasis.
To date, brain radiotherapy is one of the effective treatment methods for patients with SCLC and brain metastasis. Wong et al.Citation13 performed WBRT for 129 patients with brain metastasis, and 47% of these patients presented with relieved symptoms and improved quality of life. A common treatment method is to perform forward and backward radiation to the bilateral fields of the whole brain with a dosage of 30–40 Gy/10–20 times. A very high dosage may result in radiation brain injury. Pathogenic characteristics of patients with SCLC and brain metastasis include multiple central nervous system metastases. WBRT treatment is more effective than single stereotactic radiosurgery (SRS), especially for patients with brain metastasis who previously received PCI treatment.Citation14,Citation15 To date, there are debates on the application of chemotherapy drugs for brain metastasis in patients with SCLC with brain metastasis. A meta-analysis included 3 randomized and controlled clinical trials.Citation16 In one clinical trial, all 33 patients received whole brain radiotherapy or whole brain radiotherapy combined with topotecan chemotherapy. Results revealed that the difference in survival period between these 2 methods was not statistically significant. Another study included 120 patients, and divided these patients into 2 groups: teniposide group, and teniposide combined with whole brain radiotherapy group; and median PFS was 3.5 months and 3.2 months, respectively. The difference between both groups was not statistically significant. However, CR rate was higher in the combined group. In another clinical trial, sequential chemotherapy and radiotherapy was compared with concurrent chemotherapy and radiotherapy (teniposide combined with cisplatin), and the difference between these 2 methods was not statistically significant. The results of the meta-analysis revealed that there is not enough evidence to evaluate the treatment of radiotherapy and chemotherapy in patients with SCLC and brain metastasis. Few studies have demonstrated that chemotherapy cannot improve brain PFS and OS in patients with SCLC and brain metastasis. This retrospective study revealed that the best recent curative effect on brain metastasis lesions was observed in patients in the brain radiotherapy combined with sequential chemotherapy group, compared with the other 3 groups’ and the RR rate was 56.25%. The worst recent curative effect was observed in patients in the chemotherapy group, with an RR rate of 28.5%. In comparing the survival period under different treatment modes for patients with SCLC and brain metastasis, median OS in the brain radiotherapy combined with sequential chemotherapy group, chemotherapy combined with sequential brain radiotherapy group, brain radiotherapy group and chemotherapy group was 21.03 months, 19.69 months, 15.86 months and 12.06 months, respectively. The difference in survival period between the brain radiotherapy combined with sequential chemotherapy group and chemotherapy combined with sequential brain radiotherapy group was statistically significant (P = 0.003), and that between the chemotherapy combined with sequential brain radiotherapy group and chemotherapy group was also statistically significant (P = 0.028). However, the difference in survival period between the brain radiotherapy combined with sequential chemotherapy group and chemotherapy combined with sequential radiotherapy group was not statistically significant. This indicates the survival period was extended in patients with SCLC and brain metastasis who received active brain radiotherapy and chemotherapy drug treatment specific for brain metastasis, compared with single brain radiotherapy and chemotherapy. For patients with mild brain symptoms, they can first be given chemotherapy, followed by brain radiotherapy, after such symptoms are controlled. It is possible that controlling extracranial lesions is one of factors to extend the survival period. For patients with SCLC and brain metastasis, good PS scores and previously receiving combined therapies, active radiotherapies and multi-line chemotherapies may be a good treatment mode to extend the survival period of patients. However, no clinical evidence has been reported by randomized and controlled trials.
Some studies have shown that SCLC with brain metastasis is sensitive to chemotherapy, and that the sensitivity of brain metastasis lesions to chemotherapy was similar to extracranial lesions.Citation17 However, some scholars have found that its effectiveness for brain metastasis lesions was lower.Citation18 Better curative effects for patients with SCLC and brain metastasis could be achieved when using teniposide (VM-26), carmustine (BCNU), lomustine (CCNU), temozolomide (TMZ) and topotecan. Temozolomide can pass through the BBB, and has gradually become an alternative drug for brain metastasis chemotherapy. In stage II clinical trials, using temozolomide combined with WBRT relieve symptoms and improve the effect of radiotherapy. However, in this study, few patients presented with SCLC. In this retrospective analysis, carmustine was the most frequently used among the chemotherapy drugs applied for brain metastasis, which accounted for approximately 70% of the whole chemotherapy cycle; and this was followed by topotecan, teniposide and temozolomide. In addition, 4 patients were given chemotherapy combined with bevacizumab.
Patients with SCLC in the limited stage and extensive stage can benefit from PCI, which has been proven by multiple clinical, randomized and controlled studies.Citation19 In this study, among the 55 patients with SCLC in the limited stage, only 3 patients underwent PCI, while only one patient received PCI among the 16 patients with SCLC in the extensive stage without brain metastasis. It was considered that the small proportion of patients who received PCI was related with their resistance to the toxicity of PCI. In addition, guidelines recommend that only patients with SCLC in the limited stage who reach CR, and patients with SCLC in the extensive stage who reach PR or CR, can receive PCI. In clinic, these patients cannot reach such curative effects. In this study, most of the patients with brain metastasis did not previously receive PCI. Hence, there is a need to carry out prospective studies to determine whether patients who did not reach PR and CR can also benefit from PCI.
Patients with SCLC and brain metastasis have poor prognosis. In most studies, staging, gender and PS scores have been considered independent prognosis factors for patients with SCLC.Citation20 In this study, a comparison was performed on prognosis-related factors in patients with SCLC. The single-factor analysis indicated that staging, PS score, baseline NSE, different treatment modes, the presence of complications with brain metastasis at the beginning of treatment, and the determination of whether to perform chemotherapy for brain metastasis, were main factors that impact the prognosis of patients; while the prognostic relevance of gender, age, baseline serum sodium and the number of brain metastasis lesions was not statistically significant. Different treatment methods result in different prognosis in patients. The single factor analysis indicated that chemotherapy can improve the survival period of patients with brain metastasis, considering that chemotherapy drugs can control brain metastasis lesions, as well as extracranial lesions. In this study, the survival period reached up to 74 months, and the survival period after the occurrence of brain metastasis reached up to 55 months, in one patient who received brain radiotherapy combined with sequential chemotherapy, with 18 chemotherapy cycles and etoposide capsules via intermittent p.o. to keep the treatment. Cox analysis indicated that staging, whether complicated with brain metastasis at the beginning of treatment, with or without brain metastasis symptoms, baseline NSE, first-line chemotherapy PFS, and PFS after brain metastasis treatment were independent prognosis factors that impact the total survival period of patients with brain metastasis. For patients with SCLC and brain metastasis in the extensive period, who present with symptoms, a short PFS of first-line chemotherapy may be caused by the resistance of tumor cells to drugs and the rapid progression of tumors. Therefore, this presents patients with SCLC and brain metastasis have a late initial staging but with severe symptoms. Bad curative effect after first-line chemotherapy and brain metastasis may be an independent prognosis factor that impacts the survival period. For such patients with good PS scores, active concurrent chemoradiotherapy and improvements in recent RR and PFS may further improve their prognosis. This is to further compare the curative effect of chemotherapy with best supportive treatment of patients with bad PS scores and poor prognosis factors.
The results of this study indicate that patients with SCLC and brain metastasis should be given active combined treatment modes. The combination of chemotherapy and brain radiotherapy is a good treatment method for patients with SCLC and brain metastasis. However, its treatment sequence is not related with prognosis relevance, In terms of the recent and long-term curative effects of chemotherapy drugs applied for brain metastasis, further studies will be made to determine which chemotherapy drugs combined with radiotherapy can effectively treat SCLC with brain metastasis, and further improve the survival period of patients, as well as to determine whether target drugs such as bevacizumab can increase these curative effects and provide potential treatment effects for supportive treatment in patients with SCLC and brain metastasis, who have favorable prognosis. The fact that patients with SCLC, but without brain metastasis, and initially did not receive PCI, may be the main factor for brain metastasis. Hence, patients with SCLC and brain metastasis should be encouraged to receive PCI. Further studies will be made on the correlation between the start time of PCI and prognosis, as well as the potential benefits of PCI for patients who did not reach PR or CR after first-line chemotherapy. Staging, whether complicated with brain metastasis at the beginning of treatment, with or without brain metastasis symptoms and baseline NSE are good prognosis factors for patients with SCLC and brain metastasis. It is necessary to investigate the best treatment method for patients with SCLC and brain metastasis, who have a bad prognosis after combined therapies. Indeed, this preference also impacts the results of this study. Therefore, a prospective large-cohort study will be further conducted to promote the development of these treatment strategies for patients with SCLC and brain metastasis.
References
- Howlader N, Noone AM, Krapcho ME. SEER Cancer Statistics Review, 1975-2011, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. Bethesda (MD): National Cancer Institute; 2014.
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. SEER Cancer Statistics Review, 1975-2011, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. Bethesda (MD): National Cancer Institute; 2014.
- Wenwen Z, Wenjing J, Linlin L. Prophylactic cranial irradiation for patients with small-cell lung cancer: A systematic review of the literature with meta-analysis,BMC Cancer. BMC Cancer. 2014 Oct 31;14:793. doi:10.1186/1471-2407-14-793
- Waqar SN, Morgensztern D, Govindan R. Systemic treatment of brain metastases. Hematol Oncol Clin North Am. 2017;31(1):157-76. doi:10.1016/j.hoc.2016.08.007. PMID:27912831
- Califano R1, Abidin AZ, Peck R, Faivre-Finn C, Lorigan P. Management of small cell lung cancer: Recent developments for optimal care. Drugs. 2012;72(4):471-90. doi:10.2165/11597640-000000000-00000. PMID:22356287
- NCCN Guidelines Version 1. 2016 Small Cell Lung Cancer. Available online: http://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. doi:10.2165/11597640-000000000-00000. PMID:22356287
- Altan M, Chiang AC. Management of small cell lung cancer: Progress and updates. Cancer J. 2015;21(5):425-33. doi:10.1097/PPO.0000000000000148. PMID:26389768
- Chan BA, Coward JI. Chemotherapy advances in small-cell lung cancer. J Thorac Dis. 2013;5 Suppl 5:S565-78.
- Kalemkerian GP. Advances in pharmacotherapy of small cell lung cancer. Expert Opin Pharmacother. 2014;15:2385-96. PMID:25255939
- Quan AL, Videtic GM, Suh JH. Brain metastases in small cell lung cancer. Oncology (Williston Park). 2004;18(8):961-72. doi:10.1517/14656566.2014.957180. PMID:15328892
- Preusser M, Berghoff AS, Schadendorf D, Lin NU, Stupp R. Brain metastasis: Opportunity for drug development? Curr Opin Neurol. 2012;25(6):786-94. doi:10.1097/WCO.0b013e328359320d. PMID:23108247
- Postmus PE, Haaxma-Reiche H, Smit EF, Groen HJ, Karnicka H, Lewinski T, van Meerbeeck J, Clerico M, Gregor A, Curran D, et al. Treatment of brain metastases of small-cell lung cancer: Comparing teniposide and teniposide with whole-brain radiotherapy-a phase III study of the european organization for the research and treatment of cancer lung cancer cooperative group. J Clin Oncol. 2000;18(19):3400-8. doi:10.1200/JCO.2000.18.19.3400. PMID:11013281
- Wong J, Hird A, Zhang L, Tsao M, Sinclair E, Barnes E, Danjoux C, Chow E. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1125-31. doi:10.1016/j.ijrobp.2008.12.013. PMID:19231099
- Sadikov E, Bezjak A, Yi QL, Wells W, Dawson L, Millar BA, Laperriere N. Value of whole brain re-irradiation for brain metastases–single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-8. doi:10.1016/j.clon.2007.06.001. PMID:17662582
- Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. Outcomes after whole brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-72. doi:10.1016/j.ijrobp.2011.03.020. PMID:21620583
- Reveiz L, Rueda JR, Cardona AF. Chemotherapy for brain metastases from small cell lung cancer. Cochrane Database Syst Rev. Cochrane Database Syst Rev. 2012;13(6):CD007464. doi:10.1002/14651858.CD007464.pub2. PMID:22696370.
- Kristjansen PE, Soelberg Sørensen P, Skov Hansen M, Hansen HH. Prospective evaluation of the effect on initial brain metastases from small cell lung cancer of platinum-etoposide based induction chemotherapy followed by an alternating multidrug regimen. Ann Oncol. 1993;4(7):579-83. doi:10.1093/oxfordjournals.annonc.a058592. PMID:8395873
- Seute T, Leffers P, Wilmink JT, ten Velde GP, Twijnstra A. Response of asymptomatic brain metastases from small-cell lung cancer to systemic first-line chemotherapy. J Clin Oncol. 2006;24(13):2079-83. doi:10.1200/JCO.2005.03.2946. PMID:16648509
- Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H, Wagner H, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N Engl J Med. 1999;341(7):476-84. doi:10.1056/NEJM199908123410703. PMID:10441603
- Arinc S, Gonlugur U, Devran O, Erdal N, Ece F, Ertugrul M, Derince D, Oruc O, Hazar A. Prognostic factors in patients with small cell lung carcinoma. Med Oncol. 2010;27(2):237-41. doi:10.1007/s12032-009-9198-8. PMID:19399653