 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study was to determine whether photodynamic therapy (PDT) alone or combined with cisplatin (DDP), can deactivate cisplatin-resistant cancer cells. Human cancer cell lines A549 and SKOV3, and chemoresistant sublines A549/DDP and SKOV3/DDP, were subjected to PDT, DDP, or PDT combined with DDP. Cell viability and apoptosis were analyzed, and then intracellular reactive oxygen species (ROS) and proteins related to apoptosis were determined. PDT caused cell death, and PDT combined with DDP led to the highest percentage of dead cells in 4 cell lines; similar results were detected in ROS; a quantification evaluation manifested that the combined effect was addition. DDP increased the percentage of apoptotic cells, and the ROS level in A549 and SKOV3 cells, which was not observed in A549/DDP and SKOV3/DDP cells. Western blot revealed an increase of caspase 3 and Bax, and a decrease of Bcl-2, demonstrating the occurrence of apoptosis. The data suggest that PDT can efficiently deactivate resistant cells and enhance the action of DDP against resistant cancer cells.
| Abbreviations | ||
| DDP | = | Cisplatin |
| MPPa | = | pyropheophorbide-α methyl ester |
| PDT | = | photodynamic therapy |
Introduction
Cisplatin (DDP) is the first-line chemotherapeutic agents for cancers of lung and ovary.Citation1 However, the gradual increase of chemoresistance decreases the therapeutic responses, and ultimately leads to a failure of treatments.Citation2,Citation3 Therefore, strategies that can efficiently deactivate refractory cancer cells should be developed to improve the prognosis.
Photodynamic therapy (PDT) has been clinically used to treat cancers, where a photosensitizer is activated under light exposure producing reactive oxygen species (ROS). ROS causes cytotoxicity, thereby deactivating cancer cells.Citation4,Citation5 Limited by the poor penetration of light in tissues, PDT is only employed for managements of superficial lesions in the present regimen.Citation6 Utilizing a longer wavelength and tissue-specifically photosensitizers are considered the potential solution, and combining other therapeutic modalities (e.g., chemotherapy) may be an alternative.Citation7 However, the effects of PDT on DDP-resistant cancers cells (particularly when combining an anticancer drug) remain unclear.
Here pyropheophorbide-α methyl ester (MPPa) was used as the photosensitizer, and then responses of DDP-resistant human lung/ovary cancer cells to PDT alone, or combined with DDP, were explored. Data suggest that PDT can efficiently deactivate resistant cells, and enhance the action of DDP against resistant cells.
Results
Cytotoxicity of DDP, light, or MPPa
Light exposure (0-10 J/cm2) or MPPa (0‒16 μmol/L) alone did not decrease viability of A549, A549/DDP, SKOV3 or SKOV3/DDP cells (light: p = 0.294, p = 0.217, p = 0.055, p = 0.828, MPPa: p = 0.212, p = 0.066, p = 0.076, p = 0.197) (). DDP led to a higher percentage of dead cells in A549 cells in comparison with A549/DDP cells (p < 0.001) and similar result was observed in SKOV3 and SKOV3/DDP cells (p < 0.001), confirming the resistance phenotype in A549/DDP and SKOV3/DDP sublines; IC50 was 18.0, 35.3, 19.7 and 35.3 μM, in A549, A549/DDP, SKOV3, and SKOV3/DDP cells, respectively (). Therefore, 14 μM DDP was chosen for following trials.
Figure 1. Cytotoxicity of light (A, D), MPPa (B, E) or DDP (C, F) on A549 and A549/DDP (A‒C), and SKOV3 and SKOV3/DDP (D‒F) cells. Light or MPPa alone caused no cytotoxicity; DDP led to a less percentage of survival cells in A549/DDP or SKOV3/DDP, compared with A549 or SKOV3 cells. Data were mean ± standard deviation for 3 independent experiments.
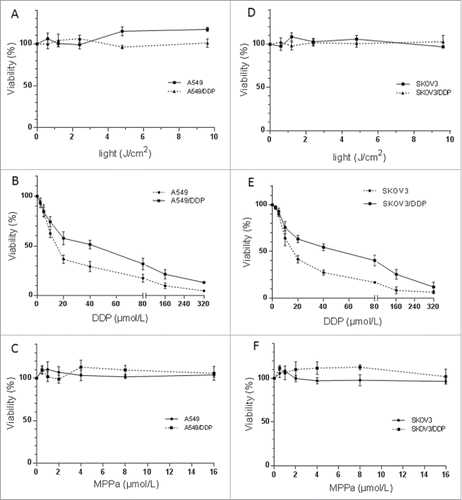
Figure 2. Cytotoxicity of the combination of MPPa and light (i.e., PDT) on A549 (A), A549/DDP (B), SKOV3 (C), and SKOV3/DDP (D) cells. Based on the cell-survival curve, 2.4 J/cm2 light and 2.0 μM MPPa were employed to perform PDT. Data were mean ± standard deviation for 3 independent experiments.
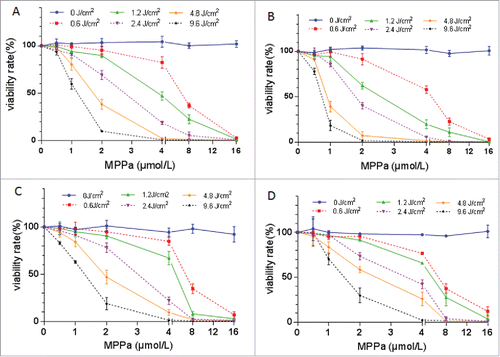
The percentage of survival cells decreased with increasing dose of light and drug, when combining light and MPPa (). At 2.4 J/cm2 of light exposure, IC50 of MPPa was about 2 μM, and therefore these parameters were employed for PDT.
Combined effect of PDT and DDP was addition
PDT or DDT decreased the percentage of survival cells in 4 cell lines, and the highest percentage of dead cells was observed in group PDT + DDP (p = 0.001, p = 0.005, p < 0.001, p < 0.001) (). The combination index (CI) values were 1.11, 1.10, 1.07 and 1.14 in A549, A549/DDP, SKOV3 and SKOV3/DDP cells, respectively. These data indicated that the combined effect of PDT and DDP was addition.
Figure 3. Percentages of dead cells in A549 and A549/DDP (A), and SKOV3 and SKOV3/DDP (B) cells. PDT or DDP deactivated cells, and the highest cell-death fraction was detected in group PDT + DDP in 4 cell lines. Data were mean ± standard deviation for 3 independent experiments. *: vs. group Ctrl, p < 0.05; &: vs. group DDP, p < 0.05; #: vs. group PDT, p < 0.05.
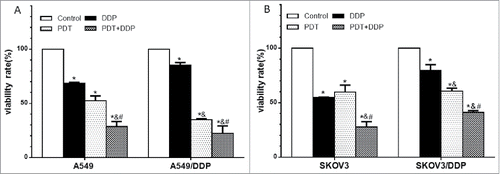
Figure 4. Apoptosis in A549 and A549/DDP (A), and SKOV3 and SKOV3/DDP (B) cells. PDT induced apoptosis in 4 cell lines, DDP exposure increased the percentage of apoptotic cells in A549 and SKOV3 cells, and the highest apoptotic rate was noted in group PDT + DDP in 4 cell lines. Data were mean ± standard deviation for 3 independent experiments. *: vs. group Ctrl, p < 0.05; &: vs. group DDP, p < 0.05; #: vs. group PDT, p < 0.05.
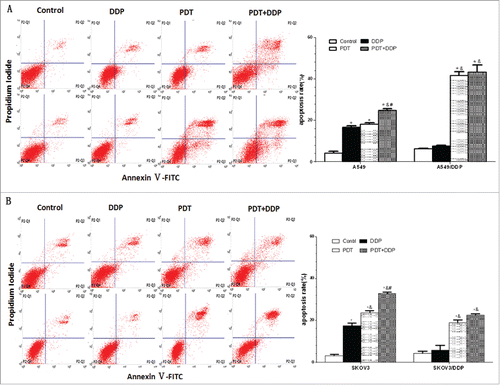
Figure 5. Intracellular level of ROS in A549 and A549/DDP (A), and SKOV3 and SKOV3/DDP (B) cells. PDT increased the level in 4 cell lines, but DDP increased the level in only A549 and SKOV3 cells; the highest ROS level occurred in group PDT + DDP in 4 cell lines. Data were mean ± standard deviation for 3 independent experiments. *: vs. group Ctrl, p < 0.05; &: vs. group DDP, p < 0.05; #: vs. group PDT, p < 0.05.
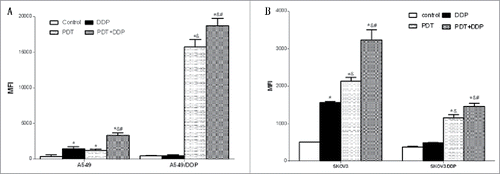
Combination of PDT and DDP induced the highest apoptotic percentage
The percentage of apoptotic cells in group PDT did not differ from that in group DDP, in A549 cells (p = 0.251). The apoptotic percentage in group DDP was not higher than that in group Ctrl in A549/DDP and SKOV3/DDP cells (p = 0.216, p = 0.448), indicting a failure of apoptosis induction. The highest apoptotic percentage was detected in group PDT + DDP in A549 and SKVO3 cell lines (p < 0.001, p < 0.001) (). CI was 0.78 in A549 cells, indicating slight antagonism; values were 0.94, .089 and 0.96 in A549/DDP, SKOV3 and SKOV3/DDP cells, respectively, demonstrating addition. These data indicated that the combination of PDT and DDP caused addition in A549/DDP, SKOV3 and SKOV3/DDP cells.
Combination of PDT and DDP induced the highest level of ROS
PDT alone or combined with DDP induced the generation of ROS in 4 cell lines, with the highest level in group PDT + DDP. Interestingly, DDP caused the production of ROS in A549 and SKOV3 cells (p = 0.001, p = 0.007), but which was not detected in A549/DDP and SKOV3/DDP cells (p = 0.970; p = 0.068). When comparing sensitive and resistant cells, the combination of PDT and DDP led to a higher ROS level in resistant lung cancer cells A549/DDP (p = 0.022), or sensitive ovarian cancer cells SKOV3 (p = 0.045) (). These findings suggest that the production of ROS varied between cell types.
PDT combined with DDP induced apoptosis via the mitochondrial pathway
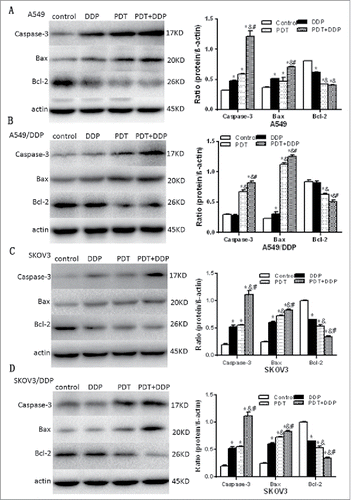
Immunofluorescence microscopy demonstrated that MPPa localized in mitochondria (). Proteins related to mitochondrial apoptosis were analyzed. Western blot demonstrated an increase of caspase-3 and Bax proteins and a decrease of Bcl-2 protein after PDT and/or DDP exposure, in 4 cell lines (). These manifested that PDT and/or DDP induced apoptosis via the mitochondrial pathway.
Figure 6. Intracellular localization of MPPa. Red fluorescence indicated MPPa and green fluorescence showed mitochondria; orange appeared in the merged image, demonstrating that MPPa located in mitochondria (×200).
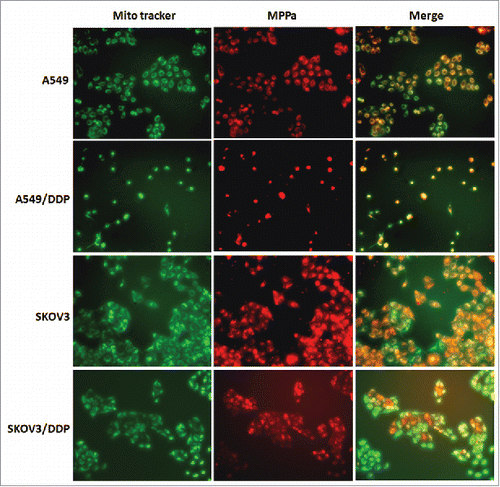
Figure 7. Cleaved caspase 3, Bax and Bcl-2 proteins validated by western blot, in A549 and A549/DDP (A, B), and SKOV3 and SKOV3/DDP (C, D) cells. In 4 cell lines, caspase 3 and Bax were increased and Bcl-2 decreased, in groups PDT and PDT + DDP, demonstrating the occurrence of apoptosis. However, Bcl-2/Bax/caspase 3 was not altered in group DDP, in A549/DDP and SKOV3/DDP cells, indicating the malfunction of apoptosis. Data were mean ± standard deviation for 3 independent experiments. *: vs. group Ctrl, p < 0.05; &: vs. group DDP, p < 0.05; #: vs. group PDT, p < 0.05.
Discussion
Multiple mechanisms resulted in DDP resistance, and varied between tissue types; further, the response to a physical factor depended on cell type.Citation8, Citation9 These have been hindered the development of therapeutic strategies.Citation10 Therefore, lung and ovary cancer cells were employed as the model in this study. MPPa was used as the photosensitizer for a better safety in humans.Citation11,Citation12
The present data manifested that PDT can efficiently deactivate chemoresistant cells. A higher percentage of dead cells in A549/DDP indicated that resistant cells were more sensitive to PDT but this effect was not detected in SKOV3/DDP cells, indicating the response to PDT varied between cell types (i.e., the therapeutic regimen should be specifically set for a specific cancer type). The difference may be related to variation of intracellular behavior of MPPa, and of the cells’ response to ROS. Interestingly, PDT combined DDP can decrease cells viability more potently compared with only PDT, however cells apoptosis were not increased effectively in A549/DDP and SKOV3/DDP cells. This suggested that other cell-death mechanisms may be involved in cells besides apoptosis.
PDT was limited by the poorer penetration of light, and therefore chemotherapy was used to improve the therapeutic efficacy. A combination of PDT and DDP led to an additive effect, suggesting the combined modality can be used to treat refractory cancers. This was due to an increase of apoptotic cells and ROS yield. Since MPPa located in mitochondria, ROS produced under light exposure directly damaged mitochondria to trigger apoptosis. Cytotoxicity of DDP was mediated frequently via inducing apoptosis. Thus, the combined modality deactivated more cells via the mitochondria apoptosis pathway. These were confirmed with the activation of caspase 3, up-regulation of Bax, and down-regulation of Bcl-2 (i.e., decreasing the ratio of Bcl-2 to Bax). Bcl-2 was not decreased in group DDP in both A549/DDP and SKOV3/DDP cells, which indicated that the apoptosis deficiency played an important part in chemoresistance since Bcl-2 was an apoptosis inhibitor. PDT decreased the level of Bcl-2 to enhance DDP-induced apoptosis, thus realizing chemosensitization. Interestingly, CI utilizing apoptotic percentages demonstrated antagonism between PDT and DDP in A549 cells, but the value utilizing cell-death fractions indicated the combined effect was addition; this suggested that other cell-death mechanisms may be involved in sensitization. This inconsistency was not observed in other cell lines, demonstrating the response to PDT combined with DDP depended on cell-type, and therefore the therapeutic regimen should be specifically set.
Cytotoxicity of DDP was mediated by both nuclear (i.e., DNA damages) and cytoplasmic factors.Citation13 DDP induced the formation of ROS, causing insults to organelles; damaging mitochondria can arouse apoptosis. The present data confirmed that either PDT or DDP led to intracellular ROS and that the highest level occurred in group PDT + DDP. This was the mechanism of chemosensitization due to PDT: targeting at the cytoplasmic sites. Most present chemomodulators sensitize DDP via inhibiting DNA repair.Citation14 Therefore, PDT should be considered a bypass. This effect was not detected in A549 cells, suggesting that this alternative may not exist in non-resistant cells. The response difference should be considered when formulating a therapeutic regimen.
In conclusion, the present findings indicated that PDT can deactivate chemoresistant cells, and the combination of PDT and DDP produced addition. The combined modality improved the efficacy via enhancing the mitochondria-dependent apoptosis. This strategy can be developed to treat refractory cancers of lung and ovary.
Materials and methods
Cell culture
Human cancer cell lines A549, A549/DDP, SKOV3 and SKOV3/DDP were cultured in RPMI-1640 (Gibco, Beijing, China) medium supplemented with 10% fetal bovine serum (FBS) (Gibco), at 37 °C and 5% CO2. Tissue type of A549 was lung and that of SKOV3 was ovary; A549/DDP and SKOV3/DDP were the DDP-resistant sublines; 2 or 0.75 μg/ml DDP was added into A549/DDP or SKOV3/DDP medium to maintain the property of chemoresistance. Cells were transferred into drug-free medium for 7 d before experiments, thereby avoiding interferences due to residual DDP.Citation15 All experiments were performed in dark to avoid the activation of MPPa.
Cytotoxicity of MPPa, light or DDP
Cells were seeded in a 96-well plate (5 × 103/well), and exposed to light (0‒9.6 J/cm2 with a wavelength of 630 nm; Wuhan Hi-tech Hengda Photoelectric Co., Ltd., Wuhan, China), MPPa (0‒16 μM; Sigma-Aldrich, St. Louis, MO) or DDP (0‒320 μM; Qilu Pharm. Co. Ltd., Jinan, China). Cells viability was determined with a CCK-8 assay (Dojindo Lab., Kumamoto, Japan).
For determining the parameter for PDT, cells were incubated with MPPa for 24 h, drugs washed away, and then exposed to light. Cell viability was assayed after 24 h. Based on the cell-survival curve, 2 μM of MPPa and 2.4 J/cm2 of light were employed for PDT.
When determining cytotoxicity of DDP, cells were exposed to DDP for 4 h, and then drugs washed away; cell viability was assayed after 24. A level of 14 μM was used in following trials, based on the percentage of survival cells. This manner indicated that both the drug peak level and “concentration × time” were with the range of human pharmacokinetics, thereby having clinical relevancy.Citation16,Citation17
Combination of PDT and DDP
Experiments were performed in 4 groups. Cells were exposed to DDP in group DDP, to PDT in group PDT, and to PDT combined with DDP in group PDT + DDP; control cells received sham exposure (i.e., with 0 μM drug and 0 J/cm2 light). In groups DDP and PDT+DDP, DDP was added for 4 h before light exposure. Drugs were washed away, and then cells were subjected to light. Cell viability was determined after 24 h, and then the percentage of survival cells was calculated.
Detection of apoptotic cells
Apoptotic cells were detected using the Annexin V/PI (propidium iodide) assay (Keygen Biotech., Nanjing, China).
Quantifying the combined effect of PDT and DDP
Percentages of dead (1 – percentage of survival cells) or apoptotic cells were used to (CI), thereby evaluating the combined effect.Citation16EA+B was the effect of PDT combined DDP, and EA/EB was the effect of PDT/DDP. A CI of >1.15, 0.85‒1.15, or <0.85 indicated synergy, addition, or antagonism, respectively.
Measurement of intracellular ROS
Intracellular ROS was detected with the DCFH-DA assay (2',7'-dichlorodihydrofluorescein diacetate; Keygen Biotech.).Citation12 Serum-free medium containing 10 μM DCFH-DA was added, and cells were kept in dark at 37°C for 30 min. Cells were washed with PBS, and then received flow cytometry; the fluorescence intensity reflected the level of ROS.
Subcellular localization of MPPa
Cells were seeded in a 24-well plate (5 × 104 cells/well). 2 μM MPPa was added after attachment, and cells were incubated at 37 °C for 24 h. Medium was removed, and the MitoTracker Green FM dye (Invitrogen, Eugene, OR) was added. Cells were washed with serum-free media for three times, and observed under a fluorescence microscope (Ti-E; Nikon, Tokyo, Japan).
Western blot
Proteins was extracted using RIPA kit (Beyotime Biotechnol., Shanghai, China). Total protein (30 μg/well) were separated by 15% SDS-PAGE and transferred onto a PVDF membrane. Rabbit monoclonal antibodies against cleaved caspase-3, Bax and Bcl-2 (Cell Signaling Technol., Danvers, MA) were used; β-actin served as the reference, with a rabbit polyclonal antibody (Cell Signaling Technol.). Proteins were visualized using an enhanced chemiluminescence kit (Pierce Biotechnol., Rockford, IL).
Statistics
The statistical analysis was performed with the software SPSS 17.0 (Chicago, IL). Data were expressed as means ± standard deviation (SD), and multiple analysis of variance (ANOVA) was used. the critical value was set p < 0.05.
Conflict of interests
We declare that we have no conflict of interest.
Ackownowledgment
This work was supported with grants from the Natural Science Foundation of China (31470822).
References
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. European journal of pharmacology. 2014;740:364–378.
- Chen J, Solomides C, Parekh H, Simpkins F, Simpkins H. Cisplatin resistance in human cervical, ovarian and lung cancer cells. Cancer chemotherapy and pharmacology. 2015;75(6):1217–1227.
- Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A, Castedo M, Kroemer G. Systems biology of cisplatin resistance: past, present and future. Cell death & disease. 2014;5:e1257.
- Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. Journal of photochemistry and photobiology B. 2009;96(1):1–8.
- Oniszczuk A, Wojtunik-Kulesza KA, Oniszczuk T, Kasprzak K. The potential of photodynamic therapy (PDT)—Experimental investigations and clinical use. Biomedicine & Pharmacotherapy. 2016;83:912–929.
- Park YK, Park CH. Clinical efficacy of photodynamic therapy. Obstet Gynecol Sci. 2016;59(6):479–488.
- Szymanski W, Reeßing F. Beyond Photodynamic Therapy: Light-Activated Cancer Chemotherapy. Curr Med Chem. 2016;23:1–46. doi:10.2174/0929867323666160906103223.
- Hu W, Jin P, Liu W. Periostin Contributes to Cisplatin Resistance in Human Non-Small Cell Lung Cancer A549 Cells via Activation of Stat3 and Akt and Upregulation of Survivin. Cell Physiol Biochem. 2016;38(3):1199–1208.
- Han X, Zhen S, Ye Z, Lu J, Wang L, Li P, Li J, Zheng X, Li H, Chen W, et al. A Feedback Loop Between miR-30a/c-5p and DNMT1 Mediates Cisplatin Resistance in Ovarian Cancer Cells. Cell Physiol Biochem. 2017;41(3):973–986.
- Zhang Y, Li J, Yu T. Pharmacokinetic profiles of cancer sonochemotherapy. Expert Opin Drug Deliv. 2017;14(6):745–753.
- Huang Q, Ou YS, Tao Y, Yin H, Tu PH. Apoptosis and autophagy induced by pyropheophorbide-alpha methyl ester-mediated photodynamic therapy in human osteosarcoma MG-63 cells. Apoptosis. 2016;21(6):749–760.
- Tu PH, Huang WJ, Wu ZL, Peng QZ, Xie ZB, Bao J, Zhong MH. Induction of cell death by pyropheophorbide-alpha methyl ester-mediated photodynamic therapy in lung cancer A549 cells. Cancer medicine. 2017;6(3):631–639.
- Brozovic A, Ambriovic-Ristov A, Osmak M. The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Critical reviews in toxicology. 2010;40(4):347–359.
- Li P, Yang X, Cheng Y, Zhang X, Yang C, Deng X, Li P, Tao J, Yang H, Wei J, et al. MicroRNA-218 Increases the Sensitivity of Bladder Cancer to Cisplatin by Targeting Glut1. Cell Physiol Biochem. 2017;41(3):921–932.
- Yu T, Yang Y, Zhang J, He H, Ren X. Circumvention of cisplatin resistance in ovarian cancer by combination of cyclosporin A and low-intensity ultrasound. Eur J Pharm Biopharm. 2015;91:103–110. doi: 10.1016/j.ejpb.2015.02.003.
- He H, Yu T, Zhang Y. The interaction between a drug and ultrasound in sonochemotherapy against ovarian cancers. Ultraschall in Med. 2012;33:275–282.
- Yu T, Luo L, Wang L. Ultrasound as a cancer chemotherapy sensitizer: the gap between laboratory and bedside. Expert Opinion on Drug Delivery. 2015;13(1):37–47.

