ABSTRACT
SRC and its activated form, phospho-SRC (pSRC), are aberrantly activated in pancreatic cancer and SRC represents a potential target for pancreatic cancer therapy. In this paper, we examined the inhibitory effect of dasatinib, a potent SRC inhibitor in combination with paclitaxel or gemcitabine on human and murine pancreatic cancer cell lines. The results showed that p-SRC can be highly expressed in most human and mouse pancreatic cancer cell lines compared with normal human cell lines and can be induced by paclitaxel or gemcitabine in HPAC cells. Dasatinib can enhance the efficacy of paclitaxel or gemcitabine by reducing the cell viability and inhibiting the cell proliferation. Dasatinib with paclitaxel combination exhibits statistically greater inhibition of the cell migration ability than single agent alone, paclitaxel with gemcitabine or FOLFIRINOX (combination of fluorouracil, leucovorin, irinotecan, and oxaliplatin) in HAPC, PANC-1, and BXPC-3 human pancreatic cancer cell lines as well as 8–285 APR and 8–365 APR mouse pancreatic cancer cell lines. In addition, dasatinib with gemcitabine combination also showed statistically greater inhibition of cell migration than single agent alone, paclitaxel with gemcitabine, or FOLFIRINOX in HAPC, PANC-1 and 8–285 APR cells. The combination of dasatinib with paclitaxel or gemcitabine also showed greater inhibition of the colony formation ability of pancreatic cancer cells compared with single-agent monotherapy or FOLFIRINOX. Dasatinib with paclitaxel or gemcitabine combination also inhibits p-SRC, p-STAT3, p-AKT, and/or p-ERK in these pancreatic cancer cells. Therefore, our results support that combined dasatinib and paclitaxel or gemcitabine therapy may be a viable therapeutic approach for human pancreatic cancer.
Introduction
Pancreatic cancer is the third leading cause of cancer death in the United States .Citation1 While curative resection and chemotherapy remains the standard of care in pancreatic cancers, the 5-y overall survival for pancreatic cancer is still less than 10% .Citation2,Citation3 High resistance to traditional chemo- and radiation therapy, including both intrinsic (de novo) and acquired (therapy induced) is regarded as a major reason for the limited benefit of most pancreatic cancer therapies .Citation4
Single-agent gemcitabine, a pyrimidine antimetabolite and analog of deoxycytidine, the most commonly used chemotherapeutic drug for pancreatic cancer, has modest clinical benefit and may not improve overall survival (OS) to a clinically meaningful degree. The lack of significant clinical response to monotherapy of gemcitabine is likely due to the inherent or acquired chemoresistance.Citation5,Citation6 Although recent study shows that newer combination chemotherapy regimen, FOLFIRINOX (combination of fluorouracil, leucovorin, irinotecan, and oxaliplatin) is a superior option for patients with advanced pancreatic cancer, the disease still rapidly develops resistance to the drug and no apparent improvement in OS rate was achieved.Citation7 Another regimen, consisting of nab-paclitaxel plus gemcitabine, was introduced to increase the available options for the refractory advanced pancreatic adenocarcinoma, the OS or progression-free survival (PFS) was still comparatively low.Citation8 Taken together, the failure of conventional chemotherapeutic regimens highlights a desperate need for novel treatment strategy.
Src-family kinases are non-receptor tyrosine kinases with a critical role in cellular proliferation. Src, one of the nine members of the Src-family kinases, was reported to be over-expressed with the progression of pancreatic neoplasia and correlated with poor prognosis.Citation9,Citation10 Hyper-activation of Src has been known to confer therapeutic resistance to a few current anticancer therapies and indicated poor prognosis in pancreatic cancer patients. It involves in a few of signaling pathways. Src is also known to be crucial during tumor metastasis, mainly as a result of its role in regulating the cytoskeleton, cell migration, adhesion, and invasion in a variety of human cancers including pancreatic cancer.Citation11 Given the essential role of Src in tumor development and extensive pre-clinical evidence of metastasis suppression by Src inhibition, inhibition of Src therefore represents a promising oncologic therapeutic target for pancreatic cancer. Dasatinib is a potent oral Src inhibitor which was approved in the treatment of chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia.Citation12 It not only shows promising results in hematological malignancy with well tolerance and limited toxicity but can also go across the blood–brain barrier.Citation13 Previously, several papers found that dasatinib can inhibit pancreatic tumor growth in xenograft mouse model but not in clinical practice.Citation10,Citation14 Its monotherapy shows no clinical benefit for small-cell-lung cancer and for metastatic colorectal cancer and should be combined with other drugs.Citation15,Citation16 Src activation appears to be a facilitator but may not necessarily a driving force for tumor progression as shown in some clinical models.Citation11 Preclinical data have demonstrated that dasatinib has additive or synergistic activity in combination with several cytotoxic and biologic agents, providing rationale for combination therapy.Citation17 Therefore, we hypothesize that Src inhibitors may provide better outcome if it combines with conventional therapeutic drugs and its combination may provide additional clinical benefit for pancreatic patients.
Paclitaxel is an anticancer agent used in pancreatic cancers as well as many other types of solid cancer. It may exert its role by stabilizing polymerized microtubules and enhances microtubule assembly, which arrests the cell cycle in G0/G1 and G2/M phases, and leads to cell death.Citation18 The combination efficacy of dasatinib and paclitaxel in pancreatic cancer has not been reported before. Gemcitabine is another single agent commonly used in pancreatic cancer for many years. Although several reports have confirmed the inhibitory effects of gemcitabine with dasatinib in pancreatic cancer cells,Citation19,Citation20 the exact role in overcoming the resistance of pancreatic cancers has not been investigated. Here, we investigated the combination efficacy of dasatinib with paclitaxel or gemcitabine on human and mice pancreatic cancer cells to find a better treatment strategy for pancreatic cancer treatment.
Materials and methods
Cell lines and reagents
Human pancreatic cancer cell lines HPAC, PANC-1, Capan-1, AsPC-1, and BxPC-3 (KRAS wild-type); human chorionic mesenchymal stromal cells (HCMSC); and normal human lung fibroblasts (NHLF) were purchased from the American Type Culture Collection (Manassas, VA, USA). Short-term cultured primary murine pancreatic cancer cell lines (8–365 APR, 8–285 APR and 22–614 APR) from a clinically relevant p16lox/lox; LSL-KrasG12D; Pdx1-Cre genetically engineered mouse model were provided by Dr Gloria H. Su at Columbia University Medical Center. All cell lines were routinely propagated in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum (FBS) as well as 1% penicillin/streptomycin and then grown in a humidified 37°C incubator with 5% CO2. Routinely, they were assessed microscopically for the expected morphologies.
All reagents in the study were purchased as follows: dimethyl sulfoxide (DMSO) (Sigma, USA), dasatinib (LC Laboratories, Woburn, MA, USA), paclitaxel (LC Laboratories), gemcitabine (LC Laboratories), oxaliplatin (LC Laboratories), Irinotecan (LC Laboratories), leucovorin calcium (Sigma), and 5-fluorouracil (Sigma). All the drugs were stored at −20°C with 20 mM stock solution.
Cell viability and cell proliferation assay
Human pancreatic cancer cell lines (HPAC, PANC-1, Capan-1, and ASPC-1) were seeded in 96-well plates at a density of 3,000 cells per well and cultured in the presence of 10% FBS at 37°C. The next day, the culture medium in each well was not only maintained at 100 µL as the final volume, but also respectively supplemented with either various concentrations of dasatinib (0.25 or 0.5 μM), paclitaxel (0.1 or 0.5 μM), and gemcitabine (0.25 or 0.5 μM) or in combination under the same cultivation condition. After 72 h, 25 μl of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) was added to each well. After incubation for 4 h, 150 μl of N,N-dimethylformamide (Sigma) solubilization solution were added to each well. The absorbance was read at a wavelength of 595 nm. Combination index (CI) was determined using data obtained from the MTT assay with CompuSyn software.Citation21 CI values indicate a synergistic effect when <1, an antagonistic effect when >1, and an additive effect when equal to 1.
To evaluate the combination effects on cell growth, HAPC, PANC-1, and Capan-1 PDAC cells were incubated in the six-well plates (3 × 105 cells per hole) in triplicate. The next day, the cells were treated with 0.25 µM dasatinib, 0.1 µM paclitaxel, 0.1 µM gemcitabine, 0.25 µM dasatinib with 0.1 µM paclitaxel, or 0.25 µM dasatinib with 0.1 µM gemcitabine. The culture of cell proliferation was measured, cell in 2 ml medium, counted manually after 2, 4, 6, and 8 d using a hemacytometer (Hawksley, West Sussex, UK) and a bright field microscope. It combined with Trypan blue staining method to determine the growth state of dispersed cells.
Wound healing assay assessed cell migratory ability
Human pancreatic cancer cells (HAPC, PANC-1, and BXPC-3) and mouse pancreatic cancer cells (8–285 APR and 8–365 APR) were seeded in six-well plates. When the cells reached 100% confluence, the monolayer was scratched in a uniform width using a pipette tip. After washing, cells were treated with DMSO, dasatinib alone, paclitaxel alone, gemcitabine alone, dasatinib with paclitaxel, dasatinib with gemcitabine, gemcitabine with paclitaxel, and FOLFIRINOX. When the DMSO group was completely healed after 24 h to 6 d after scratching, images were captured by inverted microscope (Nikon, Eclipse TS100, Japan). The percentage of wound healing was measured by software ImageJ (National Institutes of Health, Bethesda, Maryland, USA) and calculated by the equation: percent wound healing = average of (gap area before treatment – gap area after treatment)/gap area before treatment.
Colony formation assay
Human pancreatic cancer cells (HPAC, PANC-1, CAPAN-1, ASPC-1, and BXPC-3 cells) were seeded in six-well plates at a density of 1,000 cells per well. On the next day, cells were incubated with vehicle DMSO, or treated with dasatinib alone (0.1–1 μM), paclitaxel alone (0.005–0.01 μM), gemcitabine alone (0.01 μM), dasatinib with paclitaxel, dasatinib with gemcitabine, and FOLIFOX. After treatment for 72 h, cells were replenished with fresh growth medium comprising the same drug ingredient. One two weeks later when the assay ended, cells were washed twice with PBS and fixed with cold methanol for 30 min at 4°C followed by a staining with 1% crystal violet dye (dissolved in 25% methanol) at room temperature for 1 h. After staining, the plates were washed with distilled water, dried, and then quantified by colorimetric scanning.
Caspase-3/7 activity
Cells were cultured in the respective media and treated with 0.1 µM dasatinib, 0.1 µM paclitaxel, 0.1 µM gemcitabine, 0.1 µM dasatinib with 0.1 µM paclitaxel or 0.1 µM dasatinib with 0.1 µM gemcitabine. Caspase-3/7 activity was measured using the Caspase-3/7 Fluorescence Assay kit (Cayman, Ann Arbor, MI, USA) according to the manufacturer’s instruction. The fluorescence intensity of each well was read using excitation at 485 nm and emission at 535 nm.
Western blotting analysis
Five human pancreatic cancer cell lines (HPAC, PANC-1, CAPAN-1, ASPC-1, and BXPC-3), two normal human cell lines (HCMSC and NHLF), and three pancreatic mouse cancer cell lines (8–365 APR, 8–285 APR, and 22–614 APR) were harvested when cells were cultured to reach 80–90%. In addition, human pancreatic cancer cell lines (HPAC and PANC-1) and mouse pancreatic cancer cell line (8–285 APR) cultured to reach 80–90% confluence were subjected to treatments either with vehicle DMSO or with dasatinib alone (0.1 μM), paclitaxel alone (0.1 μM), gemcitabine alone (0.1 μM), dasatinib with paclitaxel (0.1 μM respectively), and dasatinib with gemcitabine (0.1 μM respectively). Twenty-four hours later, treated cells were harvested and lysed in the ice-cold RIPA buffer (Cell Signaling Technology) comprising cocktail inhibitors to block protease and phosphatase activities. The lysates were subjected to an electrophoresis on a 10% SDS-PAGE gel and the resolved proteins were transferred to a polyvinylidene difluoride membrane (GE Healthcare). Membranes were further incubated with 1,000-fold diluted primary antibody of interest for an overnight followed by with 10,000-fold diluted horse radish peroxidase-conjugated secondary antibody for 1.5 h. Primary antibodies employed in this study, respectively, recognized phosphorylated Src (Tyr416), p-SRC (Tyr527), phospho-specific extracellular signal-regulated kinase (p-ERK) 1/2 (Threonine 202/Tyrosine 204), phosphorylated STAT3 (Tyr705, p-STAT3Y705), pan STAT3, phosphorylated AKT (Ser473), SRC, ERK GAPDH, and secondary antibody (all from Cell Signaling Technology, USA). Membranes were analyzed using enhanced chemiluminescence plus reagents and scanned with the Storm Scanner (Amersham Pharmacia Biotech Inc., USA)
Statistical analysis
Significance of correlations was assessed using GraphPad Prism software 7.0 (GraphPad Software, Inc., USA). Unpaired t-tests were used for analyses, assuming Gaussian populations with a 95% confidence interval. Data are presented as mean ± standard deviation. Differences were analyzed with the Student's t-test, and significance was set at p < 0.05. *, **, and *** indicates p < 0.05, p < 0.01, and p < 0.001, respectively.
Results
p-SRC was highly expressed in pancreatic cancer cells and can be activated by paclitaxel or gemcitabine
To determine the expression of SRC activation in pancreatic cancer cells, we have performed Western blotting analysis in five human pancreatic cancer cell lines (HPAC, BXPC-3, ASPC-1, PANC-1, and CAPAN-1) and three mouse pancreatic cancer cell lines (22–614 APR, 8–285 APR, and 8–365 APR). The results showed that all these pancreatic cancer cell lines express p-SRC (Y416 and Y527, ) and the relative ratio level of p-SRC (Y416 to Y527) was higher than the NHLF and HCSMC normal tissue cell lines ().
Figure 1. p-SRC was highly expressed in pancreatic cancer cell lines and can be induced by paclitaxel or gemcitabine.
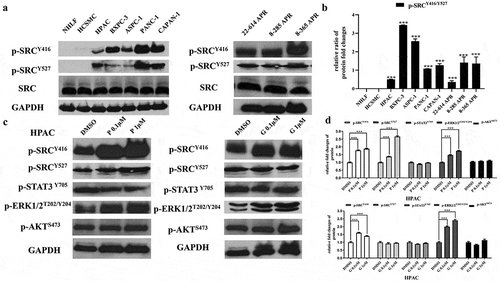
Since paclitaxel and gemcitabine are first-line chemotherapeutic drugs currently used in pancreatic chemotherapy, we then tested if these two drugs can reduce the SRC/STAT3 pathway. The results, however, showed that paclitaxel can induce the activation of p-SRC (Y416 and Y527) and p-ERK1/2T202/Y204 in HPAC cells. It did not reduce the expression of p-STAT3Y705 and p-AKTS473. Gemcitabine can also slightly induce the expression of p-SRC (Y416) and p-ERK1/2T202/Y20. It did not reduce the expression of p-AKT S473 and p-STAT3 Y705 (–). p-AKTT308 was not detectable in HPAC cells.
Dasatinib can enhance the efficacy of paclitaxel or gemcitabine by reducing the cell viability and inhibiting the cell proliferation
Since Src protein was highly expressed and can be induced by paclitaxel or gemcitabine, we then tested whether dasatinib can enhance the efficacy of paclitaxel or gemcitabine. The MTT array showed that dasatinib with paclitaxel can reduce the cell viabilities than single drug alone in all four human pancreatic cancer cells (HPAC, PANC-1, CAPAN-1, and ASPC-1, , CI<1). Similarly, dasatinib with gemcitabine can reduce the cell viabilities than single drug alone in all four human pancreatic cancer cells (HPAC, PANC-1, CAPAN-1, and ASPC-1, , CI<1).
Figure 2. Dasatinib shows synergistic effects when combined with paclitaxel or gemcitabine.
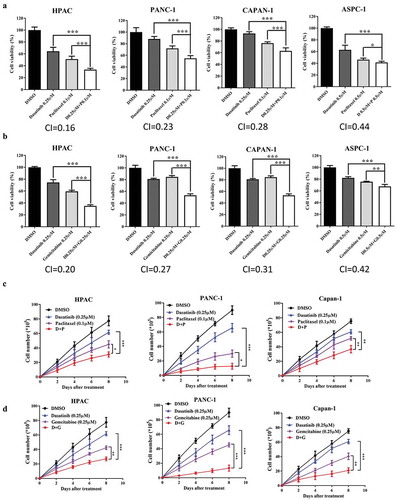
To assess whether dasatinib with paclitaxel or dasatinib with gemcitabine plays a suppressive role in pancreatic cancer cells development, we then evaluated the effect of these drugs on cell proliferation using the Trypan blue staining method in HAPC, PANC-1, and Capan-1 cells. The results showed that dasatinib with paclitaxel exhibited significantly lower growth rates compared with dasatinib or paclitaxel alone in HAPC, PANC-1, and Capan-1 cells (). Dasatinib with gemcitabine also exhibited significantly lower growth rates compared with dasatinib or gemcitabine alone in HAPC, PANC-1, and Capan-1 cells ().
Dasatinib inhibited the cell migration of pancreatic cancer cells when combined with paclitaxel or gemcitabine
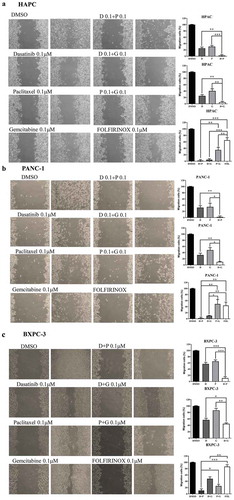
Cell migration ability is an important process for cancer cell metastasis. Wound healing assay was also performed to see the additive effect of dasatinib with paclitaxel or gemcitabine. We chose three pancreatic cancer cell lines (HPAC, PANC-1, and BXPC-3) and two mouse pancreatic cancer cell lines (8-285 APR and 8-365 APR) to test. The results showed that in HPAC and PANC-1 cells (–), dasatinib with paclitaxel or gemcitabine inhibited cell migration ability better than single agent alone, paclitaxel with gemcitabine and FOLFIRINOX under same concentration. In BXPC-3 cells (), dasatinib with paclitaxel was better than paclitaxel with gemcitabine or FOLFIRINOX at the same concentration.
Figure 3. Combination of dasatinib with paclitaxel or gemcitabine can greatly inhibit the cell migration of human pancreatic cancer cells.
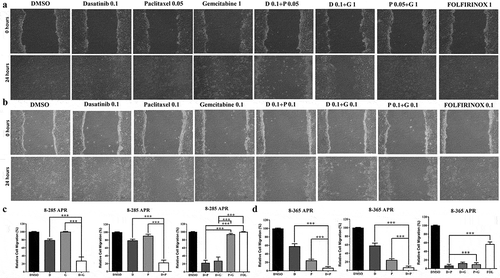
In 8–285 APR pancreatic cancer cell lines ( and ), dasatinib with paclitaxel or gemcitabine can inhibit cell migration ability better than single agent alone as well as paclitaxel and gemcitabine or FOLFIRINOX at the same concentration. In 8–365 APR pancreatic cancer cell lines ( and ), dasatinib with paclitaxel can inhibit cell migration ability better than single agent alone as well as paclitaxel and gemcitabine or FOLFIRINOX at the same concentration.
Figure 4. Combination of dasatinib with paclitaxel or gemcitabine can greatly inhibit the cell migration of mouse pancreatic cancer cells.
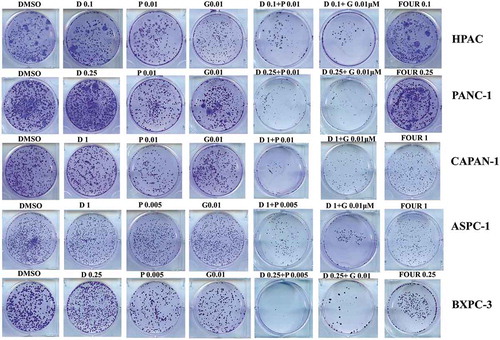
Dasatinib inhibited colony formation of pancreatic cancer cells when combined with paclitaxel or gemcitabine
We next sought to investigate whether these combinations might inhibit the colony formation capability than single agent alone. Since FOLFIRINOX proved to be the current most effective treatment strategy in the pancreatic chemotherapy, the same concentration of these combinations was used to compare the dasatinib with paclitaxel or gemcitabine. As shown in , the cells showed a decreased ability to recover and form colonies following treatment with dasatinib and paclitaxel or gemcitabine. The less colonies were formed compared with FOLFIRINOX at the same concentration.
Figure 5. Dasatinib with paclitaxel or with gemcitabine can greatly reduce the cell colony formation ability of human pancreatic cancer cells.
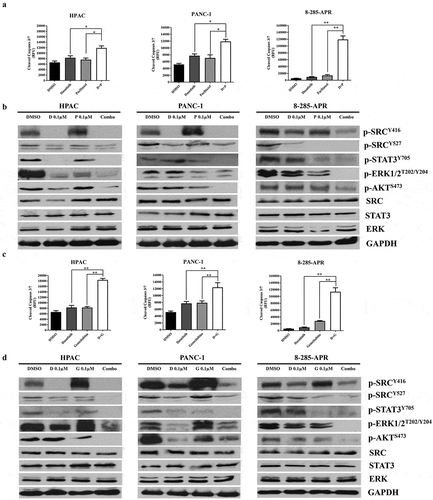
Combination of dasatinib with paclitaxel or gemcitabine can induce apoptosis and inhibit the activation of SRC/STAT3 pathway
We then tested whether the combination of dasatinib with paclitaxel or gemcitabine can induce pancreatic cancer cell apoptosis. The results showed that the level of cleaved caspase-3/7 was elevated with drug combination (dasatinib plus paclitaxel) compared with levels for either drug alone in HPAC, PANC-1, and 8–285-APR cells (). Since p-SRC was highly expressed in all pancreatic cancer cell lines and activation of SRC or ERK could occur through paclitaxel, we then assessed the phosphorylation of STAT3, ERK1/2, AKT, and SRC after treatment of pancreatic cancer cells with dasatinib and paclitaxel. The results showed that p-SRC (Y416 and Y527) and p-ERK1/2T202/Y204 induced by paclitaxel in HPAC pancreatic cancer cell lines were demolished with the combination use of dasatinib with paclitaxel in HAPC, PANC-1, and 8–285 APR cells. Dasatinib with paclitaxel can also reduce the level of p-STAT3Y705 and p-AKTS473 in HAPC, PANC-1, and 8–285 APR cells (). Similarly, the level of cleaved caspase-3/7 was elevated with dasatinib and gemcitabine compared with levels for either drug alone in HPAC, PANC-1, and 8–285-APR cells (). p-SRC (Y416) induced in HAPC, PANC-1, and 8–285 APR cells was demolished with the combination use of dasatinib with gemcitabine. This combination can also reduce the level of p-SRC (Y527), p-STAT3Y705 and p-AKTS473, and p-ERK1/2T202/Y204 in HAPC, PANC-1, and 8–285 APR cells, respectively ().
Figure 6. Combination of dasatinib with paclitaxel or gemcitabine can induce apoptosis and inhibit the activation of SRC/STAT3 pathway.
Discussion
Due to resistance to cytotoxic chemotherapy, pancreatic cancer is still an incurable disease with poor outcomes.Citation20 Overexpression of Src is known to be crucial during tumor metastasis by promoting dissociation of cell–cell adherents junction and facilitating cell mobility,Citation11 and this can cause insensitive to most conventional chemotherapeutic drugs. Hence, there is considerable interest in exploiting Src kinase as a novel therapeutic target in malignant disease and in inhibiting the development of metastatic disease.Citation22 The Src’s role in the migration and invasion steps of the metastatic cascade might imply the potential for Src inhibitors in the early adjuvant setting to reduce the risk of metastatic recurrence during or after definitive therapies.Citation11 As our results indicated, overexpression of Src can be observed in many pancreatic cancer cell lines. Although exact mechanisms of Src overexpression remain complex and elusive, it was likely associated with their deregulated upstream regulators and signaling like EGFR, HER2, and/or c-MET.Citation23–Citation25
Interestingly, our results found that the overexpressed p-Src can be further induced by paclitaxel and gemcitabine in HPAC and PANC-1 cells and this suggests us Src may be a potential target with combination of these two drugs in the treatment of pancreatic cancer. The major downstream signaling upon Src activation includes activation of Akt, enhancement of cell proliferation, STAT3 activation, and transcriptional upregulation of secretary factors involved in metastasis and angiogenesis.Citation11 Inhibition of Src signaling has been shown to restore the sensitivity to gemcitabine and 5-flourouracil in human pancreatic cancer cells.Citation10,Citation26 This implicates that Src inhibition in restoring the chemosensitivity and suggests a novel approach using dasatinib in combination with gemcitabine for the treatment of pancreatic cancer.
Gemcitabine, which was confirmed as the standard drug for advanced pancreatic cancer in 1997, showed little effects on improving survival outcomes.Citation27,Citation28 Many gemcitabine-based combinations demonstrated no more efficacy than gemcitabine alone, aside from when administered in patients with good performance status. Previously, one data showed that gemcitabine resistance correlates with increased Src activity, and Src inhibition can overcome this resistance.Citation29 There is one report indicating that dasatinib synergized with gemcitabine in MIA-PaCa-2 by decreasing the levels of ALDH1A1.Citation19 In consistent of these findings, our results showed that dasatinib can enhance the effects of gemcitabine in five pancreatic cancer cell lines and three mouse pancreatic cancer cell lines. STAT3 is a key oncogenic factor in many human cancers and is required for oncogenesis in the mouse model of cancers. Hyperactivation of STAT3 in Tyr705 has been reported in many pancreatic cancer cell lines. Its activation form can regulate oncogenic signaling and increase the tumor cell survival, proliferation and tumor growth.Citation30 The combination uses of dasatinib and gemcitabine not only reduce the level of Src but also target multiple tumor-associated pathways including STAT3, ERK, and p-AKT and may overcome resistance to monotherapy by multiple mechanisms.
As a microtubule-stabilizing agent, paclitaxel possesses both mitosis-dependent and mitosis-independent activities against cancer cells.Citation31 Resistance of paclitaxel limits its use in pancreatic cancer treatment. Some has tried dasatinib with paclitaxel in advanced breast cancer, uterine cervical adenocarcinoma cells, ovarian cancer, and lung adenocarcinoma.Citation32–Citation35 In these studies, the mechanism underlying the interaction of Src inhibitor enhanced paclitaxel sensitivity include enhancing apoptosis, inducing autophagic death, enhancing microtubule stability, and/or targeting tumor neovasculature. Our investigation is the first report to combine dasatinib with paclitaxel in pancreatic cancer cell lines. Our primitive results showed great efficacy when combining these two drugs in vitro. Similar to dasatinib with gemcitabine, it may enhance the efficacy of paclitaxel through inhibition of induced SRC expression.
Our results showed elevated cleaved caspase-3/7 was observed with dasatinib and gemcitabine or dasatinib and paclitaxel. Several mechanisms of apoptosis and autophagy induced by dasatinib were investigated in a certain type of solid tumors or CML. It induces autophagic cell death in ovarian cancer that partially depends on Beclin-1, AKT, and Bcl-2.Citation36 In gastric cancers, it promoted tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis via upregulation of CCAAT/enhancer-binding protein homologous protein (CHOP)-dependent death receptor 5 (DR5) expression.Citation37 In CML model (K562R cells), dasatinib promoted apoptosis through downregulation of AKT/mTOR activities, while preventing exosomal release and inhibiting autophagy by reducing expression of beclin-1 and Vps34.Citation38 MEK/ERK and p38 MAPK were found to control dasatinib/valproic acid-induced apoptosis as upstream regulators.Citation39
There are two clinical trials investigating the efficacy of dasatinib and gemcitabine, but the results are quite different. One phase I results showed an encouraging clinical activity in advanced and metastatic pancreatic cancer.Citation40 Another clinical trial however showed that dasatinib failed to show increased OS or PFS in patients with locally advanced PDCA .Citation41 Specific biomarkers have not yet been identified that predict the ability of Scr inhibitors to potentiate gemcitabine or paclitaxel treatment. There are no any clinical trials for dasatinib and paclitaxel.
Taken together, our results clearly show that dasatinib can greatly enhance the efficacy of paclitaxel and gemcitabine activity in human pancreatic cancer cells through inhibition of paclitaxel- and gemcitabine-induced activation of Src and ERK. More clinical trials are needed to further confirm these combination effects.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported by the University of Maryland School of Medicine and Comprehensive Cancer Center start-up fund. We are grateful to Dr Richard Eckert for his kind assistance of using a microscope for wound healing assays.
Additional information
Funding
References
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi:10.1158/0008-5472.CAN-14-0155.
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi:10.1056/NEJMra0901557.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387.
- Long J, Zhang Y, Yu X, Yang J, LeBrun DG, Chen C, Yao Q, Li M. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets. 2010;15(7):817–828. doi:10.1517/14728222.2011.566216.
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi:10.1056/NEJMoa1011923.
- Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O’Reilly DA, Cunningham D, Wadsley J, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–1024. doi:10.1016/S0140-6736(16)32409-6.
- Thibodeau S, Voutsadakis IA. FOLFIRINOX chemotherapy in metastatic pancreatic cancer: A systematic review and meta-analysis of retrospective and phase II studies. J Clin Med. 2018;7(1). pii: E7. doi:10.3390/jcm7010007.
- Palacio S, Hosein PJ, Reis I, Akunyili II, Ernani V, Pollack T, Macintyre J, Restrepo MH, Merchan JR, Rocha Lima CM. The nab-paclitaxel/gemcitabine regimen for patients with refractory advanced pancreatic adenocarcinoma. J Gastrointest Oncol. 2018;9(1):135–139. doi:10.21037/jgo.2017.10.12.
- Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94(2):344–351. doi:10.1002/cncr.10221.
- Nagaraj NS, Smith JJ, Revetta F, Washington MK, Merchant NB. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Mol Cancer Ther. 2010;9(8):2322–2332. doi:10.1158/1535-7163.MCT-09-1212.
- Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012;33(3):122–128. doi:10.1016/j.tips.2011.11.002.
- Foa R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, Elia L, Paoloni F, Fazi P, Cimino G, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521–6528. doi:10.1182/blood-2011-05-351403.
- Porkka K, Koskenvesa P, Lundan T, Rimpilainen J, Mustjoki S, Smykla R, Wild R, Luo R, Arnan M, Brethon B, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112(4):1005–1012. doi:10.1182/blood-2008-02-140665.
- Bartscht T, Rosien B, Rades D, Kaufmann R, Biersack H, Lehnert H, Gieseler F, Ungefroren H. Dasatinib blocks transcriptional and promigratory responses to transforming growth factor-beta in pancreatic adenocarcinoma cells through inhibition of Smad signalling: implications for in vivo mode of action. Mol Cancer. 2015;14:199. doi:10.1186/s12943-014-0278-9.
- Parseghian CM, Parikh NU, Wu JY, Jiang ZQ, Henderson L, Tian F, Pastor B, Ychou M, Raghav K, Dasari A, et al. Dual inhibition of EGFR and c-Src by cetuximab and dasatinib combined with FOLFOX chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23(15):4146–4154. doi:10.1158/1078-0432.CCR-16-3138.
- Sesumi Y, Suda K, Mizuuchi H, Kobayashi Y, Sato K, Chiba M, Shimoji M, Tomizawa K, Takemoto T, Mitsudomi T. Effect of dasatinib on EMT-mediated-mechanism of resistance against EGFR inhibitors in lung cancer cells. Lung Cancer. 2017;104:85–90. doi:10.1016/j.lungcan.2016.12.012.
- Araujo J, Logothetis C. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36(6):492–500. doi:10.1016/j.ctrv.2010.02.015.
- Verma S, Kumar N, Verma V. Role of paclitaxel on critical nucleation concentration of tubulin and its effects thereof. Biochem Biophys Res Commun. 2016;478(3):1350–1354. doi:10.1016/j.bbrc.2016.08.127.
- Duong HQ, Yi YW, Kang HJ, Bae I, Jang YJ, Kwak SJ, Seong YS. Combination of dasatinib and gemcitabine reduces the ALDH1A1 expression and the proliferation of gemcitabine-resistant pancreatic cancer MIA PaCa-2 cells. Int J Oncol. 2014;44(6):2132–2138. doi:10.3892/ijo.2014.2357.
- Nagaraj NS, Washington MK, Merchant NB. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res. 2011;17(3):483–493. doi:10.1158/1078-0432.CCR-10-1670.
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi:10.1158/0008-5472.CAN-09-1947.
- Brunton VG, Frame MC. Src and focal adhesion kinase as therapeutic targets in cancer. Curr Opin Pharmacol. 2008;8(4):427–432. doi:10.1016/j.coph.2008.06.012.
- Ahluwalia MS, de Groot J, Liu WM, Gladson CL. Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett. 2010;298(2):139–149. doi:10.1016/j.canlet.2010.08.014.
- Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14(7):667–678. doi:10.1634/theoncologist.2009-0009.
- Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6(3):209–214. doi:10.1016/j.ccr.2004.09.001.
- Ischenko I, Camaj P, Seeliger H, Kleespies A, Guba M, De Toni EN, Schwarz B, Graeb C, Eichhorn ME, Jauch KW, et al. Inhibition of Src tyrosine kinase reverts chemoresistance toward 5-fluorouracil in human pancreatic carcinoma cells: an involvement of epidermal growth factor receptor signaling. Oncogene. 2008;27(57):7212–7222. doi:10.1038/onc.2008.326.
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi:10.1200/JCO.1997.15.6.2403.
- Arshad A, Al-Leswas D, Al-Taan O, Stephenson J, Metcalfe M, Steward WP, Dennison AR. Pooled survival and response data from phase III randomized controlled trials for gemcitabine-based regimes in the treatment of advanced pancreatic cancer. Am J Clin Oncol. 2013;36(4):411–414. doi:10.1097/COC.0b013e3182124216.
- Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res. 2004;10(7):2307–2318.
- Byers LA, Sen B, Saigal B, Diao L, Wang J, Nanjundan M, Cascone T, Mills GB, Heymach JV, Johnson FM. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clin Cancer Res. 2009;15(22):6852–6861. doi:10.1158/1078-0432.CCR-09-0767.
- Le XF, Bast RC Jr. Src family kinases and paclitaxel sensitivity. Cancer Biol Ther. 2011;12(4):260–269.
- Xiao J, Xu M, Hou T, Huang Y, Yang C, Li J. Dasatinib enhances antitumor activity of paclitaxel in ovarian cancer through Src signaling. Mol Med Rep. 2015;12(3):3249–3256. doi:10.3892/mmr.2015.3784.
- Diao Y, Ma X, Min W, Lin S, Kang H, Dai Z, Wang X, Zhao Y. Dasatinib promotes paclitaxel-induced necroptosis in lung adenocarcinoma with phosphorylated caspase-8 by c-Src. Cancer Lett. 2016;379(1):12–23. doi:10.1016/j.canlet.2016.05.003.
- Takiguchi E, Nishimura M, Mineda A, Kawakita T, Abe A, Irahara M. Growth inhibitory effect of the Src inhibitor dasatinib in combination with anticancer agents on uterine cervical adenocarcinoma cells. Exp Ther Med. 2017;14(5):4293–4299. doi:10.3892/etm.2017.5061.
- Morris PG, Rota S, Cadoo K, Zamora S, Patil S, D’Andrea G, Gilewski T, Bromberg J, Dang C, Dickler M, et al. Phase II study of paclitaxel and dasatinib in metastatic breast cancer. Clin Breast Cancer. 2018. doi:10.1016/j.clbc.2018.03.010.
- Le XF, Mao W, Lu Z, Carter BZ, Bast RC Jr. Dasatinib induces autophagic cell death in human ovarian cancer. Cancer. 2010;116(21):4980–4990. doi:10.1002/cncr.25426.
- Wang X, Xue Q, Wu L, Wang B, Liang H. Dasatinib promotes TRAIL-mediated apoptosis by upregulating CHOP-dependent death receptor 5 in gastric cancer. FEBS Open Bio. 2018;8(5):732–742. doi:10.1002/2211-5463.12404.
- Liu J, Zhang Y, Liu A, Wang J, Li L, Chen X, Gao X, Xue Y, Zhang X, Liu Y. Distinct dasatinib-induced mechanisms of apoptotic response and exosome release in imatinib-resistant human chronic myeloid leukemia cells. Int J Mol Sci. 2016;17(4):531. doi:10.3390/ijms17040531.
- Heo SK, Noh EK, Yoon DJ, Jo JC, Park JH, Kim H. Dasatinib accelerates valproic acid-induced acute myeloid leukemia cell death by regulation of differentiation capacity. PLoS One. 2014;9(2):e98859. doi:10.1371/journal.pone.0098859.
- Cardin DB, Goff LW, Chan E, Whisenant JG, Dan Ayers G, Takebe N, Arlinghaus LR, Yankeelov TE, Berlin J, Merchant N. Dual Src and EGFR inhibition in combination with gemcitabine in advanced pancreatic cancer: phase I results: A phase I clinical trial. Invest New Drugs. 2018 Jun;36(3):442–450.
- Evans TRJ, Van Cutsem E, Moore MJ, Bazin IS, Rosemurgy A, Bodoky G, Deplanque G, Harrison M, Melichar B, Pezet D, et al. Phase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancer. Ann Oncol. 2017;28(2):354–361. doi:10.1093/annonc/mdw607.

