ABSTRACT
Sepsis is a systemic inflammatory condition caused by an unbalanced immunological response to infection, which affects numerous organs, including the intestines. Lipopolysaccharide (LPS; also known as endotoxin), a substance found in Gram-negative bacteria, plays a major role in sepsis and is mostly responsible for the disease’s morbidity and mortality. Berberine is an isoquinoline alkaloid found in a variety of plant species that has anti-inflammatory properties. For many years, berberine has been used to treat intestinal inflammation and infection. Berberine has been reported to reduce LPS-induced intestinal damage. The potential pathways through which berberine protects against LPS-induced intestinal damage by inhibiting NF-κB, suppressing MAPK, modulating ApoM/S1P pathway, inhibiting COX-2, modulating Wnt/Beta-Catenin signaling pathway, and/or increasing ZIP14 expression are reviewed.
Abbreviations: LPS, lipopolysaccharide; TLR, Toll-like receptor; MD-2, myeloid differentiation factor 2; CD14, cluster of differentiation 14; LBP, lipopolysaccharide-binding protein; MYD88, myeloid differentiation primary response 88; NF-κB, nuclear factor kappa light-chain enhancer of activated B cells; MAPK, mitogen-activated protein kinase; IL, interleukin; TNFα, tumor necrosis factor-alpha; Caco-2, cyanocobalamin uptake by human colon adenocarcinoma cell line; MLCK, myosin light-chain kinase; TJ, tight junction; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; IBS, irritable bowel syndrome; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase (JNK; GVB, gut-vascular barrier; ApoM, apolipoprotein M; S1P, sphingosine-1-phosphate; VE-cadherin, vascular endothelial cadherin; AJ, adherens junction; PV1, plasmalemma vesicle-associated protein-1; HDL, high-density lipoprotein; Wnt, wingless-related integration site; Fzd, 7-span transmembrane protein Frizzled; LRP, low-density lipoprotein receptor-related protein; TEER, transendothelial/transepithelial electrical resistance; COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor-binding protein; ZIP, Zrt-Irt-like protein; PPAR, peroxisome proliferator-activated receptors; p-PPAR, phosphorylated-peroxisome proliferator-activated receptors; ATF, activating transcription factors; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; SARA, subacute ruminal acidosis; IPEC-J2, porcine intestinal epithelial cells; ALI, acute lung injury; ARDS, acute respiratory distress syndrome
1. Introduction
Sepsis is a condition that develops when the body’s immune system reacts to an infection destructively [Citation1], often causing a cascade of events that damage multiple organ systems potentially causing organ failure and even death. Sepsis presents with a range of signs and symptoms [Citation2]. Fever, hypotension with insufficient tissue perfusion, and disseminated intravascular coagulation are all signs of Gram-negative infection and septicemia that can lead to mortality [Citation3]. Multiple organ dysfunctions, such as acute lung injury (ALI), acute respiratory distress syndrome (ARDS), significant myocardial depression microvascular dysfunction, acute liver function, brain microabscesses, alterations in brain neurotransmitters, and reduced cerebral blood flow, make sepsis a severe condition with considerable morbidity and mortality [Citation4–7]. Regarding the failure of conventional treatments and subsequently significant morbidity and mortality, various attempts have been made to enhance the understanding of the host response dysregulation in sepsis [Citation1].
Endotoxins, DNA, and peptidoglycan, which are found in gram-negative bacteria, may have a role in causing deadly septic shock [Citation8]. The most effective microbial mediators in the pathophysiology of sepsis are the lipopolysaccharides (LPS). The Gram-negative cell wall is composed of a thin, inner layer of peptidoglycan and an outer membrane consisting of molecules of phospholipids, lipopolysaccharides (LPS), lipoproteins, and surface proteins [Citation9]. LPS comprises three covalently connected regions: lipid A, an oligosaccharide core, and an O side chain. The toxicity of LPS resides in the innermost layer, the lipid A, which is composed of fatty acyl chains connected to two N-acetyl glucosamine residues [Citation10]. Small levels of LPS generated by infecting pathogens trigger the potent innate immune response, preparing the immune system to defend against infection. However, if the LPS-related reaction is not appropriately managed, septic shock syndrome, which could be lethal, may develop [Citation11].
One of the most significant organs affected by sepsis is the gastrointestinal tract [Citation12]. Although a large number of bacteria live in the intestine, they generally do not enter the bloodstream. Intestinal mucosal integrity helps to maintain this physiological homeostasis [Citation13]. Damaging factors such as severe burns or sepsis can launch a series of reactions leading to the breakdown of the intestinal mucosa, allowing the entrance of bacteria and toxicants into the bloodstream. Burn damage causes a severe dysbiosis of the gut microbiome in both humans and mice, allowing for similar Gram-negative aerobic bacteria overgrowths. Burn damage also causes mesenteric vasoconstriction, resulting in a hypoxic environment for the intestine. As a result, the intestine is thought to be the “motor” of sepsis [Citation14,Citation15]. Accordingly, one critical goal in the prevention and treatment of sepsis is to protect the lining of the gastrointestinal tract [Citation16,Citation17].
Berberine () is a plant alkaloid found in the roots and bark of a variety of plants, including Coptis chinensis (goldenthread), Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry), Hydrastis canadensis (goldenseal), and Berberis aristata (tree turmeric) [Citation18–20]. Berberine has a diverse range of pharmacological actions, including anticancer [Citation21–23], antidiabetic [Citation24–26], antiobesity, anti-hyperlipidemic [Citation25,Citation27], cardioprotective [Citation25,Citation28], and spatial memory improvement [Citation25,Citation29], immunosuppressive [Citation30] properties, as well as antioxidant and anti-inflammatory activity [Citation31,Citation32]. Berberine has been used to treat intestinal inflammation and infection [Citation33,Citation34]. In the experiments, both oral and injectable delivery methods were employed. Berberine could be absorbed from the GI tract in animals, and its pharmacokinetic characteristics in humans are similar to those shown in animal research. However, the oral bioavailability of berberine appears to be very poor, but there are ways to improve it, such as P-gp inhibitors, enhancers, and lipid microparticle delivery systems [Citation35].
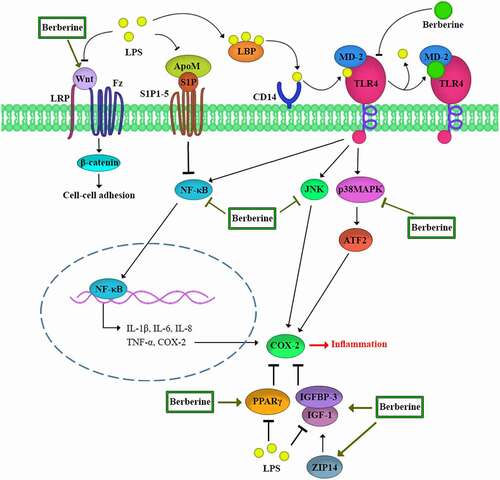
The aim of this review was to describe the potential protective role of berberine on LPS-induced intestine injury and to summarize the different pathways such as those summarized in .
2. LPS induces TLR4 signaling
Toll-like receptors (TLRs) have a critical function in innate immunity [Citation36]. TLRs are classified into one of two types: cell surface TLRs and intracellular TLRs. TLR4/MD-2, TLR1/TLR2, and TLR6/TLR2 are cell surface TLR dimers that identify microbial membrane lipids, while TLR3, TLR7, TLR8, and TLR9 are intracellular TLR dimers that identify microbial nucleic acids. The lipopolysaccharide or lipopeptides in the microbial membrane are detected by cell-surface TLRs [Citation10]. TLR4, one of the most studied TLRs, is an LPS receptor [Citation1]. LPS must be delivered to the TLR4 and the MD-2 receptor complex to be activated [Citation10]. LPS stimulates mammalian cells by interacting with several proteins, including the LPS binding protein (LBP), CD14, MD-2, and TLR4. LBP is a soluble shuttle protein that binds to LPS and promotes the interaction of LPS with CD14 [Citation36]. CD14 is a glycosylphosphatidylinositol-anchored protein that transports LPS to the TLR4 and the MD-2 receptor complexes [Citation37]. On the cell surface, MD-2 is non-covalently linked with TLR4 and supports the LPS/TLR4 complex-related cellular response in its response to LPS. As a result, MD-2 serves as a connection between TLR4 and LPS signaling [Citation38].
LPS/TLR4 signaling can be classified as a MyD88-dependent or MyD88-independent pathway. Both can activate the inflammatory pathways regulated by NFκ-B and MAPK [Citation36]. The NFκ-B and MAPK pathways are essential in the production of inflammatory cytokines [Citation39]. TLR4 and MyD88 are upstream mediators of NFκ-B and MAPK [Citation40].
Berberine has been shown to inhibit LPS-induced TLR4 and MyD88 expression, suggesting that berberine may interfere with the LPS-induced TLR4 pathway. Berberine binds to the TLR4/MD-2 receptor with a greater affinity than LPS [Citation41,Citation42]. It has been proposed that berberine may inhibit the generation of pro-inflammatory cytokines by decreasing LPS-induced activation of the TLR4-mediated NFκ-B and MAPK pathways [Citation39,Citation43]. In sepsis, berberine has an important function in organs such as the lung and heart. Guanghui Xu and colleagues reported that berberine given by injection or by inhalation in mice reduced lung injury and enhanced pulmonary morphology by inhibiting LPS-induced TLR4 [Citation44]. Berberine was also found to be effective in reversing myocardial damage in male rats by suppressing the activation of the TLR4 signaling pathway [Citation45].
2.1. Berberine and NF-κB inhibition
NF-κB regulates the transcription of genes involved in the inflammatory response, resulting in increased IL-1β, IL-6, IL-8, and TNF-α [Citation46–49]. IL-1 β can stimulate cell proliferation, differentiation, and death as well as activate the inflammatory response [Citation50]. IL-6 has a range of biological functions in the cytokine network, including the acute-phase response, immune modulation, and inducing lymphocyte differentiation [Citation50,Citation51]. IL-8 causes neutrophil migration and degranulation [Citation52]. TNF-α can affect the infection site by increasing vascular endothelial cell production, stimulating interleukin (IL) and chemokine production, and inducing a large number of leucocytes [Citation53]. Berberine inhibits NF-κB gene expression, which in turn reduces the synthesis of these inflammatory mediators [Citation47]. The inhibitory effect of berberine on TNF-α was shown to be related to its ability to modulate the gut microbiota and reduce endotoxemia in rats [Citation54]. Another in vivo study found that pretreatment with neutral sulfate berberine significantly reduced mortality and tissue damage in the lungs and small intestine of mice exposed to LPS by decreasing plasma TNF-α levels [Citation55].
Under endotoxemic conditions, the NF-κB p65 subunits in the gastrointestinal tract are activated [Citation56]. The activated NF-κB p65 then interacts with the myosin light-chain kinase (MLCK) promoter region, stimulating the production of MLCK mRNA [Citation57,Citation58]. The intestinal tight junctions are regulated by MLCK. MLC phosphorylation by MLCK induces actin-myosin filament contraction, which can lead to changes in the tight junction [Citation59] protein distribution and expression, as well as the functional opening of the TJ barrier. As a result, chemicals that inhibit the NFκB and MLCK, such as berberine, may restore the intestinal TJ barrier in cases of endotoxemia [Citation60,Citation61].
NF-κB inhibitors like IκBa typically sequester NF-κB in the cytoplasm of non-stimulated cells [Citation62]. IκBa is phosphorylated and then is degraded to activate NF-κB [Citation63–65]. In the case of endotoxemia, IκBa, p-IκBa, and p-65 are increased. The expression of IκBa, p-IκBa, and p-65 decreased following exposure to berberine [Citation47]. Zhao and colleagues have suggested that berberine treatment prevents the overactivation of the NF-κB signaling pathway caused by LPS and reduces phosphorylation of IκB and p65 in rumen epithelial cells [Citation39]. These results suggest that berberine inhibits the generation of pro-inflammatory cytokines by inhibiting the activity of the NF-κB pathway [Citation47]. In addition, berberine improved IBS signs by suppressing the TNF-α-NF-κB-MLCK pathway overexpression in rats [Citation66]. According to outcomes of a research in cats, berberine can restore gut microbiota homeostasis and modulate the TLR4/NF-κb pathway, hence reducing inflammatory responses. These results provide insight into the mechanisms of berberine treatment in cats with IBD [Citation67].
2.2. Berberine and MAPK inhibition
Mitogen-activated protein (MAP) kinase is a signaling module found in eukaryotic cells that allow cells to convert extracellular signals such as hormones, cytokines, and growth factors into intracellular responses and thereby control cell growth, differentiation, migration, and inflammation [Citation68]. In some pathologic conditions, the MAPK signaling pathway is disrupted. Activation of MAPK has been confirmed to increase pro-inflammatory cytokine production [Citation69].
ERK1/2, JNK, and p38 MAPK are three well-known MAPK subfamilies [Citation69]. ERK1/2 regulates cell survival, differentiation, and proliferation [Citation70]. JNK and p38MAPK play essential roles in inflammation by inhibiting cell cycle progression and promoting apoptosis. JNK and p38MAPK may perform a crucial function as pro-apoptotic cellular signals in damaged intestinal epithelial cells [Citation71].
Berberine reduced JNK and p38MAPK phosphorylation but not ERK phosphorylation in rumen epithelial cells of Holstein calves indicating that berberine may inhibit JNK and p38MAPK over-activation produced by LPS and prevent the development of downstream inflammatory factors. As a result, berberine’s anti-inflammatory activity on LPS-induced inflammation in rumen epithelial cells might be related to its ability to suppress TLR4-mediated NF-κB and MAPK signaling pathways, and it could be a prospective therapeutic agent for rumen inflammation in subacute ruminal acidosis (SARA) of cows [Citation39]. However, in piglets, berberine decreased both ERK and JNK protein expression, modulated oxidative stress, and preserve intestinal health [Citation72]. According to the study of Zhu and colleagues, Berberine pretreatment may significantly inhibit LPS-induced NF-κB/MAPK signaling pathway in porcine intestinal epithelial cells (IPEC-J2), and inhibit the generation of downstream inflammatory factors. Consequently, berberine is effective in preventing and treating diarrhea caused by Escherichia coli in weaned pigs by decreasing the interaction between the NF-κB/MAPK signaling pathway and associated inflammatory mediators. These findings suggested that berberine might be an effective substance for reducing LPS-induced intestinal inflammation [Citation47].
3. Berberine modulates ApoM/S1P pathway
S1P is a sphingomyelin metabolite that stimulates five G-coupled receptors, including S1P1-5 [Citation73]. S1P has a broad range of biological functions including cell proliferation, apoptosis, immune, and coagulation system control [Citation74]. S1P is also required for vascular integrity and gut-vascular barrier maintenance. The primary carrier of plasma S1P is ApoM, which is found in about 5% of HDL particles [Citation75].
The gut-vascular barrier (GVB) prevents microorganisms from entering the bloodstream and regulates antigen translocation [Citation76]. Increased capillary permeability in the intestinal mucosa contributes to the spread of germs throughout the body [Citation77]. As a result, lowering gut capillary hyperpermeability is important for avoiding gut-derived sepsis [Citation78].
VE (vascular endothelial)-cadherin and beta-catenin are the major adherent junction (AJ) molecules in the gastrointestinal vascular endothelium, while occludin and claudin-12 are the major tight junction [Citation59] molecules [Citation79]. TJ and AJ proteins are both involved in the function of the vascular endothelial barrier. In LPS-stimulated cells, the level of PV1, an endothelial permeability marker, increased, and occludin and β-catenin decreased [Citation78,Citation80]. Thus, endothelial barrier function is disrupted in sepsis, and the vascular wall becomes leaky, resulting in low blood pressure and the potential development of septic shock [Citation81,Citation82].
S1P activates the S1P1 receptor, which raises the trans-monolayer electric resistance in endothelial cells [Citation81,Citation82]. S1P also decreases LPS-induced PV1 expression while increasing occludin and β-catenin levels that are suppressed by LPS [Citation78,Citation80].
LPS, TNF-α, and IL-1 have been shown to suppress ApoM production in the liver [Citation83,Citation84]. Berberine enhanced the hepatic ApoM mRNA and plasma ApoM level during sepsis by inhibiting gluconeogenesis, insulin resistance, and proinflammatory molecule secretion. Berberine has been suggested to protect against GVB damage through modulating the ApoM/S1P pathway [Citation78].
4. Berberine modulates Wnt/beta-catenin signaling pathway
The Wnt genes encode a large family of secreted protein growth factors, cysteine-rich glycoproteins that signal in either a paracrine or autocrine fashion by passing signals into a cell through cell surface receptors. Wnts interact with the 7-span transmembrane protein Frizzled (Fzd) and the single-span low-density lipoprotein receptor-related protein (LRP) and contribute to a variety of biological responses [Citation85]. Wnts activate the release of β-catenin in combination with Fz/LRP [Citation86]. β-Catenin enhances cell–cell adhesion by aggregating at cell–cell contact areas known as adherent junctions [Citation87].
Transepithelial/transendothelial electrical resistance (TEER) is a well-recognized quantitative technique used to evaluate the integrity of the endothelial barrier [Citation88]. In cell culture models of endothelial monolayers, TEER was significantly reduced by LPS. When LPS and berberine were combined, the detrimental effects of LPS were eliminated. He et al. reported that when Wnt/beta-catenin inhibitors were combined with berberine, upregulation of TJ/AJ production was reduced, indicating that berberine stimulation of the Wnt/beta-catenin signaling pathway was involved in the preservation of GVB function. In sepsis, berberine has been proposed to preserve GVB function through modulating the Wnt/beta-catenin signaling pathway [Citation89].
In addition, an in vivo study showed that by physically binding to NF-κb and inhibiting its activation, beta-catenin inhibits NF-κb activity in the same mechanism as IκBα does, indicating that beta-catenin is a negative regulator of NF-κb. As a result, berberine might be beneficial through Wnt/beta-catenin signaling pathway regulation [Citation90].
5. Berberine and COX-2 inhibition
Cyclooxygenase-2 (COX-2) is a pro-inflammatory enzyme that plays a role in the development of intestinal inflammation [Citation33]. In the intestine, basal levels of COX-2 are required for the maintenance of epithelial integrity, proliferation, and homeostasis [Citation91–93]. However, excessively high levels of this enzyme are harmful to the intestinal barrier [Citation91,Citation93,Citation94].
LPS can significantly increase different pro-inflammatory molecules, including iNOS and COX-2, that induce gut mucosal sloughing, while iNOS and COX-2 inhibitors may ameliorate such gut mucosal damage [Citation95,Citation96]. This function of berberine may be beneficial in a variety of pathologic conditions. According to Hsu et al., berberine reduced COX-2 production and induced apoptosis in SW620 human colon cancer cells [Citation97].
5.1. MAPKs and NF-κB induce COX-2 transcription
One of the most important signaling pathways involved in COX-2 gene expression is the mitogen-activated protein kinase (MAPK) pathway [Citation98]. In the enterocyte, COX-2 gene transcription is initiated by p38, inducing and activating ATFs (activating transcription factors) [Citation98]. ATF2 and ATF3 have an important function in the induction of COX-2 gene expression [Citation93,Citation99,Citation100]. LPS injection activated mucosal p38 and ATF2 via enhancing phosphorylation and upregulating ATF2 and ATF3 protein levels in the mucosa. LPS-induced phosphorylation and overproduction of p38 and ATFs were reduced by berberine administration [Citation38]. Berberine may have the potential to reduce LPS-induced COX-2 through suppression of the p38 pathway [Citation101].
NF-kB is an inducible transcription factor that controls the expression of a variety of genes (including cox-2) involved in inflammation [Citation102]. The cox-2 promoter contains two NF-kB-binding sites and NF-kB increases cox-2 expression in response to numerous cytokines [Citation98]. Furthermore, exposure to LPS activates NF-kB, leading in increased COX-2 expression [Citation103]. As a result, berberine might block COX-2 transcription by blocking NF-kB [Citation33].
5.2. IGF-1/IGFBPs signaling and COX-2 overexpression
Overexpression of iNOS and COX-2 has been linked to the inactivation of IGF-1/IGFBPs signaling [Citation104,Citation105]. Insulin-like growth factor 1 (IGF-1) is a polypeptide that regulates immune and inflammation processes [Citation106,Citation107]. IGF-1 enhances enterocyte count, crypt depth, villus height, and surface in the gut, as well as maintaining intestinal barrier performance and inhibiting luminal bacteria and toxicants transfer [Citation108–110]. IGF-1 has a role in the production of claudin-1 and occludin in the gut, and subsequently, the severity of endotoxemia could be reduced by improving the gut tight junctions [Citation111,Citation112]. Ninety-nine percent of IGFs in the plasma is bound to proteins such as IGFBP-3 that control the availability of free IGF-1 [Citation113]. IGFBP-3 is the most common IGFBP in plasma and has the highest affinity to IGF-1. The proper ratio of IGF-1/ IGFBP-3 is necessary for maintaining intestinal homeostasis [Citation114].
LPS significantly inhibits IGF-1 and IGFBP-3 mRNA and their protein expression, leading to disruption of the IGF-1/IGFBP-3 axis. Pretreatment of rats suffering from acute endotoxemia with berberine improved IGF-1/IGFBP3 expression, which was likely due to its inhibition of pro-inflammatory mediators like COX-2 and iNOS [Citation115]. According to Fukuda et al., berberine suppressed COX-2 gene transcription in colon cancer cells at levels greater than 0.3 μM, which might describe the mechanism of the anti-inflammatory and antitumor effect of berberine [Citation116].
5.3. Berberine ameliorates COX-2 expression through the PPARγ pathway
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors that are activated by peroxisome proliferators [Citation117]. Three PPAR isotypes have been identified, α, δ, and γ, which play a role in regulating the expression of genes involved in lipid storage and mobilization, glucose metabolism, morphogenesis, and the inflammatory response [Citation118]. PPARα is highly expressed in cells with considerable fatty acid catabolism. PPARδ has the most variable expression pattern, and the amount of expression in different tissues is determined by the level of cell proliferation and differentiation. This isotype has an important role in the skin, intestines, placenta, skeletal muscle, adipose tissue, and brain [Citation119]. PPARγ has been suggested to reduce pro-inflammatory reactions through interaction with PPARγ ligands [Citation120,Citation121]. PPARγ is a negative regulator of inflammation [Citation33]. Intestinal epithelium, macrophages, and vascular endothelial cells all express PPARγ in the gut, which helps preserve gut homeostasis in cases of sepsis [Citation122–125]. The anti-inflammatory mechanism involves some transcription factors, such as NFκB, signal transducer and activator of transcription (STAT), and activator protein 1 (AP-1) or intracellular signaling proteins, including mitogen-activated protein (MAP) kinases [Citation126]. As a result, the elevation of PPARγ expression in the small intestinal mucosa during systemic inflammation is a self-regulating protective mechanism [Citation33]. TLR4 is a powerful PPAR inducing mediator. LPS stimulates the TLR4 signal pathway, which might explain the rise in PPARγ expression in small intestine mucosa [Citation125].
p-PPARγ generation, which is mediated by ERK1/2, is normally low in rats without LPS stimulation; LPS treatment increased the amount of p-PPARγ in endotoxemic rats. LPS not only enhanced PPARγ expression but also stimulated PPARγ phosphorylation [Citation33,Citation126,Citation127]. It has been shown that PPARγ phosphorylation reduces its bioactivity. As a result, LPS-induced phosphorylation of PPARγ may impair the PPARγ pathway [Citation33,Citation128,Citation129].
Feng et al. showed that berberine inhibited LPS-induced phosphorylation of PPARγ, demonstrating a potential protective effect of berberine in PPARγ activity. Berberine acts like a PPARγ pathway modulator and reduces intestinal mucosal inflammation [Citation101]. Berberine reduced the inducible COX-2 overproduction via activating the PPARγ pathway but did not affect the constitutive expression of COX-2 [Citation33]. It has been suggested that berberine could have a protective effect on other organs such as the lungs and liver through the PPARγ pathway [Citation130,Citation131].
6. Berberine and ZIP14 expression
Berberine increases the Zrt-Irt-like protein-14 (ZIP14) expression and influences zinc redistribution to help maintain the intestinal barrier in sepsis through the activation of IGF-1 signaling [Citation132]. The ZIP family is involved in regulating the absorption and transfer of zinc into the cytoplasm. ZIP14 plays a key role in gut zinc homeostasis. ZIP14 is found in intestinal epithelial cells [Citation133,Citation134]. One of the most important effects of zinc is to modulate both inflammatory and immunological responses. In sepsis, redistribution causes zinc dyshomeostasis, impaired immunity, and gut barrier dysfunction [Citation83–85]. Berberine has been shown to increase IGF-1 expression in enterocytes [Citation115]. According to an in vitro study in Caco-2 cells treated with LPS, IGF-I increased intracellular zinc concentration. He et al. have hypothesized that berberine might impact zinc redistribution, thus preserving the intestinal mucosal barrier in sepsis by activating IGF-1 signaling, while pretreatment with AG1024, an inhibitor of IGF-1, blocked the effect of berberine. Berberine may also help alleviate hypozincemia by inducing ZIP14 expression, which subsequently influences zinc translocation into the intestinal mucosa, therefore protecting the gut barrier through the activation of the IGF-1 pathway [Citation132].
7. Conclusion and future perspectives
Despite remarkable developments in our understanding of sepsis immunopathology, therapeutic progress has been less impressive. Due to the high mortality rate of sepsis, numerous attempts have been made to develop better therapeutic approaches to treating sepsis. The therapeutic use of natural products is one of several scientific and clinical approaches currently being investigated for the treatment of sepsis.
Berberine has been used in Chinese traditional medicine for centuries. The pharmacological properties of berberine suggest that it could be used to treat various diseases and pathologies, including diabetes, cancers, cardiovascular disease, hyperlipidemia, hypertension, diarrhea, inflammation, and bacterial and viral infections. In addition to the effects of berberine on the GI tract summarized in , berberine has also been reported to inhibit oxidative stress and to modulate the gut microbiota [Citation54,Citation135]. Despite some clinical trial studies regarding the effects of berberine on diabetes [Citation136], chronic heart failure [Citation137], hyperlipidemia [Citation138], cancer [Citation139], oral diseases [Citation140], and diarrhea [Citation141], berberine needs to be investigated as a possible therapy for GI illnesses in well-designed clinical trials to further define the potential therapeutic function of berberine in the gut and for treating sepsis.
Table 1. Summary of the protective role of berberine in the gut.
Acknowledgments
The authors thank Mashhad University of Medical Sciences for financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891.
- Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. Bmj. 2007;335(7625):879–883.
- Mayeux PR. Pathobiology of lipopolysaccharide. J Toxicol Environ Health. 1997 August 01;51(5):415–435.
- Bowton D. CNS effects of sepsis. Crit Care Clin. 1989;5(4):785–792.
- Woźnica EA, Inglot M, Woźnica RK, et al. Liver dysfunction in sepsis. Adv Clin Exp Med. 2018;27(4):547–551.
- Gill SE, Rohan M, Mehta S. Role of pulmonary microvascular endothelial cell apoptosis in murine sepsis-induced lung injury in vivo. Respir Res. 2015;16(1):1–13.
- Merx M, Weber C. Sepsis and the heart. Circulation. 2007;116(7):793–802.
- Horn DL, Morrison DC, Opal SM, et al. What are the microbial components implicated in the pathogenesis of sepsis? Report on a symposium. Clin Infect Dis. 2000;31(4):851–858.
- Pålsson‐mcdermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll‐like receptor‐4. Immunology. 2004;113(2):153–162.
- Miyake K, editor Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Seminars in immunology. Elsevier. 2007;19: 3–10.
- Park BS, Lee J-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45(12):e66–e66.
- Appiah MG, Park EJ, Darkwah S, et al. Intestinal epithelium-derived luminally released extracellular vesicles in sepsis exhibit the ability to suppress TNF-α and IL-17A expression in mucosal inflammation. Int J Mol Sci. 2020;21(22):8445.
- Okumura R, Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm Regen. 2018;38(1):1–8.
- Meng M, Klingensmith NJ, Coopersmith CM. New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care. 2017;23(2):143.
- Earley ZM, Akhtar S, Green SJ, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PloS one. 2015;10(7):e0129996.
- Yao P, Cui M, Li Y, et al. Effects of rhubarb on intestinal flora and toll-like receptors of intestinal mucosa in rats with severe acute pancreatitis. Pancreas. 2015;44(5):799–804.
- Chen F-Q, Xu W-Z, Gao H-Y, et al. Clinical effect of changweishu on gastrointestinal dysfunction in patients with sepsis. J Int Med Res. 2020;48(8):0300060520919579.
- Gu L, Li N, Li Q, et al. The effect of berberine in vitro on tight junctions in human Caco-2 intestinal epithelial cells. Fitoterapia. 2009;80(4):241–248.
- Li G-X, Wang X-M, Jiang T, et al. Berberine prevents intestinal mucosal barrier damage during early phase of sepsis in rat through the toll-like receptors signaling pathway. Korean J Physiol Pharmacol. 2015;19(1):1–7.
- Gu L, Li N, Gong J, et al. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J Infect Dis. 2011;203(11):1602–1612.
- Gong C, Hu X, Xu Y, et al. Berberine inhibits proliferation and migration of colorectal cancer cells by downregulation of GRP78. Anticancer Drugs. 2020;31(2):141–149.
- Jin F, Xie T, Huang X, et al. Berberine inhibits angiogenesis in glioblastoma xenografts by targeting the VEGFR2/ERK pathway. Pharm Biol. 2018;56(1):665–671.
- Wang Y, Zhang S. Berberine suppresses growth and metastasis of endometrial cancer cells via miR-101/COX-2. Biomed Pharmacother. 2018;103:1287–1293.
- Yu Y, Liu L, Wang X, et al. Modulation of glucagon-like peptide-1 release by berberine: in vivo and in vitro studies. Biochem Pharmacol. 2010;79(7):1000–1006.
- Zhang Y, Li X, Zou D, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93(7):2559–2565.
- Zhang H, Wei J, Xue R, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59(2):285–292.
- Kim WS, Lee YS, Cha SH, et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296(4):E812–E819.
- Derosa G, Maffioli P, Cicero AF. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther. 2012;12(8):1113–1124.
- Wang K, Chen Q, Wu N, et al. Berberine ameliorates spatial learning memory impairment and modulates cholinergic anti-inflammatory pathway in diabetic rats. Front Pharmacol. 2019;10:1003.
- Yarmohammadi F, Karbasforooshan H, Hayes AW, et al. Inflammation suppression in doxorubicin-induced cardiotoxicity: natural compounds as therapeutic options. Naunyn-Schmiedeberg’s Arch Pharmacol. 2021 October 01;394(10):2003–2011.
- Habtemariam S. Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol Res. 2020;155:104722.
- Wu S-J, Don T-M, Lin C-W, et al. Delivery of berberine using chitosan/fucoidan-taurine conjugate nanoparticles for treatment of defective intestinal epithelial tight junction barrier. Mar Drugs. 2014;12(11):5677–5697.
- Feng A-W, Gao W, Zhou G-R, et al. Berberine ameliorates COX-2 expression in rat small intestinal mucosa partially through PPARγ pathway during acute endotoxemia. Int Immunopharmacol. 2012;12(1):182–188.
- Shan C, Yang J, Kong Y, et al. Alteration of the intestinal barrier and GLP2 secretion in berberine-treated type 2 diabetic rats. J Endocrinol. 2013;218(3):255–262.
- Liu C-S, Zheng Y-R, Zhang Y-F, et al. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia. 2016;109:274–282.
- Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151.
- Frey E, Miller D, Jahr TG, et al. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176(6):1665–1671.
- Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189(11):1777–1782.
- Zhao C, Wang Y, Yuan X, et al. Berberine inhibits lipopolysaccharide-induced expression of inflammatory cytokines by suppressing TLR4-mediated NF-ĸB and MAPK signaling pathways in rumen epithelial cells of Holstein calves. J Dairy Res. 2019;86(2):171–176.
- Nyati KK, Masuda K, Zaman MM, et al. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017 Mar 17;45(5):2687–2703. PubMed PMID: 28168301; PubMed Central PMCID: PMCPMC5389518. eng
- Chu M, Ding R, Chu Z-Y, et al. Role of berberine in anti-bacterial as a high-affinity LPS antagonist binding to TLR4/MD-2 receptor. BMC Complement Altern Med. 2014;14(1):1–9.
- Li H-M, Wang Y-Y, Wang H-D, et al. Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisms. Acta Pharmacol Sin. 2011;32(11):1364–1372.
- Xu X, Zhang L, Zhao Y, et al. Anti‑inflammatory mechanism of berberine on lipopolysaccharide‑induced IEC‑18 models based on comparative transcriptomics. Mol Med Rep. 2020;22(6):5163–5180.
- Xu G, Wan H, Yi L, et al. Berberine administrated with different routes attenuates inhaled LPS-induced acute respiratory distress syndrome through TLR4/NF-κB and JAK2/STAT3 inhibition. Eur J Pharmacol. 2021;908:174349.
- Chen H, Liu Q, Liu X, et al. Berberine attenuates septic cardiomyopathy by inhibiting TLR4/NF-κB signalling in rats. Pharm Biol. 2021;59(1):121–128.
- Hu Y, Chen X, Duan H, et al. Chinese herbal medicinal ingredients inhibit secretion of IL-6, IL-8, E-selectin and TXB2 in LPS-induced rat intestinal microvascular endothelial cells. Immunopharmacol Immunotoxicol. 2009;31(4):550–555.
- Zhu Z, Xueying L, Chunlin L, et al. Effect of berberine on LPS-induced expression of NF-κ B/MAPK signalling pathway and related inflammatory cytokines in porcine intestinal epithelial cells. Innate Immun. 2020;26(7):627–634.
- Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18(49):6853–6866.
- Detmer K, Wang Z, Warejcka D, et al. Endotoxin stimulated cytokine production in rat vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;281(2):H661–H668.
- Vardam TD, Zhou L, Appenheimer MM, et al. Regulation of a lymphocyte–endothelial–IL-6 trans-signaling axis by fever-range thermal stress: hot spot of immune surveillance. Cytokine. 2007;39(1):84–96.
- Duan H, Zhang Y, Xu J, et al. Effect of anemonin on NO, ET-1 and ICAM-1 production in rat intestinal microvascular endothelial cells. J Ethnopharmacol. 2006;104(3):362–366.
- Kucharzik T, Hudson JT, Lügering A, et al. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54(11):1565–1572.
- Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003;14(3–4):185–191.
- Liu D, Zhang Y, Liu Y, et al. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp Clin Endocrinol Diabetes. 2018;126(8):513–520.
- Li F, WANG H, LU D, et al. Neutral sulfate berberine modulates cytokine secretion and increases survival in endotoxemic mice 1. Acta Pharmacol Sin. 2006;27(9):1199–1205.
- Schlegel N, Leweke R, Meir M, et al. Role of NF-κB activation in LPS-induced endothelial barrier breakdown. Histochem Cell Biol. 2012;138(4):627–641.
- Ma TY, Boivin MA, Ye D, et al. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288(3):G422–G430.
- Al-Sadi R, Ye D, Dokladny K, et al. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180(8):5653–5661.
- Gatica-Andrades M, Vagenas D, Kling J, et al. WNT ligands contribute to the immune response during septic shock and amplify endotoxemia-driven inflammation in mice. Blood Adv. 2017;1(16):1274–1286.
- Ranaivo HR, Carusio N, Wangensteen R, et al. Protection against endotoxic shock as a consequence of reduced nitrosative stress in MLCK210-null mice. Am J Pathol. 2007;170(2):439–446.
- Shen L, Black ED, Witkowski ED, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119(10):2095–2106.
- Li C, Yu J, Ai K, et al. IκBα phosphorylation and associated NF-κB activation are essential events in lymphocyte activation, proliferation, and anti-bacterial adaptive immune response of Nile tilapia. Dev Comp Immunol. 2020;103:103526.
- Cui L, Wang H, Lin J, et al. Progesterone inhibits inflammatory response in E. coli-or LPS-Stimulated bovine endometrial epithelial cells by NF-κB and MAPK pathways. Dev Comp Immunol. 2020;105:103568.
- Mahmoudi M, Zamani Taghizadeh Rabe S, Balali-Mood M, et al. Immunotoxicity induced in mice by subacute exposure to berberine. J Immunotoxicol. 2016;13(2):255–262.
- Yarmohammadi F, Hayes AW, Karimi G. Possible protective effect of resolvin D1 on inflammation in atrial fibrillation: involvement of ER stress mediated the NLRP3 inflammasome pathway. Naunyn-Schmiedeberg’s Arch Pharmacol. 2021 August 01;394(8):1613–1619.
- Hou Q, Zhu S, Zhang C, et al. Berberine improves intestinal epithelial tight junctions by upregulating A20 expression in IBS-D mice. Biomed Pharmacother. 2019;118:109206.
- Guo M, Cao J, Chen M, et al. Berberine maintains intestinal homeostasis through modulating TLR4/NF-κB/MTORC pathway and autophagy in cats. 2022.
- Coulombe P, Meloche S. Atypical mitogen-activated protein kinases: structure, regulation and functions. Biochim Biophys Acta-Mol Cell Res. 2007;1773(8):1376–1387.
- Yang S-H, Sharrocks AD, Whitmarsh AJ. MAP kinase signalling cascades and transcriptional regulation. Gene. 2013;513(1):1–13.
- Roskoski Jr R. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66(2):105–143.
- He S, Hou X, Xu X, et al. Quantitative proteomic analysis reveals heat stress-induced injury in rat small intestine via activation of the MAPK and NF-κB signaling pathways. Mol Biosyst. 2015;11(3):826–834.
- Tang M, Yuan D, Liao P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ Pollut. 2021;289:117865.
- Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Semin Immunopathol. 2012;34(1):73–91.
- Ding R, Han J, Tian Y, et al. Sphingosine-1-phosphate attenuates lung injury induced by intestinal ischemia/reperfusion in mice: role of inducible nitric-oxide synthase. Inflammation. 2012;35(1):158–166.
- Frej C, Linder A, Happonen KE, et al. Sphingosine 1‐phosphate and its carrier apolipoprotein M in human sepsis and in Escherichia coli sepsis in baboons. J Cell Mol Med. 2016;20(6):1170–1181.
- Sorribas M, Jakob MO, Yilmaz B, et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 2019;71(6):1126–1140.
- Vermette D, Hu P, Canarie MF, et al. Tight junction structure, function, and assessment in the critically ill: a systematic review. Intensive Care Med Exp. 2018;6(1):1–18.
- Li Y, Zhou J, Qiu J, et al. Berberine reduces gut-vascular barrier permeability via modulation of ApoM/S1P pathway in a model of polymicrobial sepsis. Life Sci. 2020;261:118460.
- Spadoni I, Pietrelli A, Pesole G, et al. Gene expression profile of endothelial cells during perturbation of the gut vascular barrier. Gut Microbes. 2016;7(6):540–548.
- Spadoni I, Zagato E, Bertocchi A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350(6262):830–834.
- Wilkerson BA, Grass GD, Wing SB, et al. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J Biol Chem. 2012;287(53):44645–44653.
- Garcia JG, Liu F, Verin AD, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701.
- Zhang X, Jiang B, Luo G, et al. Hyperglycemia down-regulates apolipoprotein M expression in vivo and in vitro. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids. 2007;1771(7):879–882.
- Jiang B, Zhang X, Di D, et al. Hyperglycemia-induced downregulation of apolipoprotein M expression is not via the hexosamine pathway. Lipids Health Dis. 2015;14(1):1–6.
- Silva-García O, Valdez-Alarcón JJ, Baizabal-Aguirre VM. Wnt/β-catenin signaling as a molecular target by pathogenic bacteria. Front Immunol. 2019;10:2135.
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20(1):781–810.
- Pai SG, Carneiro BA, Mota JM, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):1–12.
- Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20(2):107–126.
- He Y, Yuan X, Zuo H, et al. Berberine exerts a protective effect on gut-vascular barrier via the modulation of the Wnt/beta-catenin signaling pathway during sepsis. Cell Physiol Biochem. 2018;49(4):1342–1351.
- Duan Y, Liao AP, Kuppireddi S, et al. β-Catenin activity negatively regulates bacteria-induced inflammation. Lab Invest. 2007;87(6):613–624.
- Grishin A, Wang J, Hackam D, et al. p38 MAP kinase mediates endotoxin-induced expression of cyclooxygenase-2 in enterocytes. Surgery. 2004;136(2):329–335.
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–144.
- Grishin AV, Wang J, Potoka DA, et al. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J Immunol. 2006;176(1):580–588.
- Singer II, Kawka DW, Schloemann S, et al. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115(2):297–306.
- Unno N, Wang H, Menconi MJ, et al. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113(4):1246–1257.
- Short SS, Wang J, Castle SL, et al. Low doses of celecoxib attenuate gut barrier failure during experimental peritonitis. Lab Invest. 2013;93(12):1265–1275.
- Hsu W-H, Hsieh Y-S, Kuo H-C, et al. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch Toxicol. 2007;81(10):719–728.
- Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68:95–114.
- Bottone Jr FG, Martinez JM, Alston-Mills B, et al. Gene modulation by Cox-1 and Cox-2 specific inhibitors in human colorectal carcinoma cancer cells. Carcinogenesis. 2004;25(3):349–357.
- Guo Y-S, Hellmich MR, Wen XD, et al. Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. J Biol Chem. 2001;276(25):22941–22947.
- Feng A-W, Yu C, Mao Q, et al. Berberine hydrochloride attenuates cyclooxygenase-2 expression in rat small intestinal mucosa during acute endotoxemia. Fitoterapia. 2011;82(7):976–982.
- Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87(1):13–20.
- Kojima M, Morisaki T, Izuhara K, et al. Lipopolysaccharide increases cyclo-oxygenase-2 expression in a colon carcinoma cell line through nuclear factor-κB activation. Oncogene. 2000;19(9):1225–1231.
- Granado M, Martín AI, Villanúa MÁ, et al. Experimental arthritis inhibits the insulin-like growth factor-I axis and induces muscle wasting through cyclooxygenase-2 activation. Am J Physiol Endocrinol Metab. 2007;292(6):E1656–E1665.
- Priego T, Ibáñez de Cáceres I, Martín AI, et al. NO plays a role in LPS-induced decreases in circulating IGF-I and IGFBP-3 and their gene expression in the liver. Am J Physiol Endocrinol Metab. 2004;286(1):E50–E56.
- Onnureddy K, Onteru SK, Singh D. IGF-1 attenuates LPS induced pro-inflammatory cytokines expression in buffalo (Bubalus bubalis) granulosa cells. Mol Immunol. 2015;64(1):136–143.
- Haydon AM, MacInnis RJ, English DR, et al. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55(5):689–694.
- Zhang W, Frankel WL, Adamson WT, et al. Insulin-like growth factor-I improves mucosal structure and function in transplanted rat small intestine. Transplantation. 1995;59(5):755–761.
- Scopa CD, Koureleas S, Tsamandas AC, et al. Beneficial effects of growth hormone and insulin-like growth factor I on intestinal bacterial translocation, endotoxemia, and apoptosis in experimentally jaundiced rats. J Am Coll Surg. 2000;190(4):423–431.
- Lorenzo-Zuñiga V, Rodriguez-Ortigosa CM, Bartoli R, et al. Insulin-like growth factor I improves intestinal barrier function in cirrhotic rats. Gut. 2006;55(9):1306–1312.
- Zhao T-Y, Su L-P, Ma C-Y, et al. IGF-1 decreases portal vein endotoxin via regulating intestinal tight junctions and plays a role in attenuating portal hypertension of cirrhotic rats. BMC Gastroenterol. 2015;15(1):1–10.
- Hatakeyama N, Kojima T, Iba K, et al. IGF-I regulates tight-junction protein claudin-1 during differentiation of osteoblast-like MC3T3-E1 cells via a MAP-kinase pathway. Cell Tissue Res. 2008;334(2):243–254.
- Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20(6):761–787.
- Chen K, Okuma T, Okamura K, et al. Insulin‐like growth factor‐I prevents gut atrophy and maintains intestinal integrity in septic rats. J Parenteral Enteral Nutr. 1995;19(2):119–124.
- He Y, Yuan X, Zhou G, et al. Activation of IGF-1/IGFBP-3 signaling by berberine improves intestinal mucosal barrier of rats with acute endotoxemia. Fitoterapia. 2018;124:200–205.
- Fukuda K, Hibiya Y, Mutoh M, et al. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol. 1999;66(2):227–233.
- Torra P, Gervois P, Staels B. Peroxisome proliferator-activated receptor alpha in metabolic disease, inflammation, atherosclerosis and aging. Curr Opin Lipidol. 1999;10(2):151–159.
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53(1):409–435.
- Michalik L, Auwerx J, Berger JP, et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58(4):726–741.
- Baregamian N, Mourot JM, Ballard AR, et al. PPAR-γ agonist protects against intestinal injury during necrotizing enterocolitis. Biochem Biophys Res Commun. 2009;379(2):423–427.
- Ricote M, Huang J, Fajas L, et al. Expression of the peroxisome proliferator-activated receptor γ (PPARγ) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Nat Acad Sci. 1998;95(13):7614–7619.
- Huin C, Corriveau L, Bianchi A, et al. Differential expression of peroxisome proliferator-activated receptors (PPARs) in the developing human fetal digestive tract. J Histochem Cytochem. 2000;48(5):603–611.
- Su CG, Wen X, Bailey ST, et al. A novel therapy for colitis utilizing PPAR-γ ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104(4):383–389.
- Chinetti G, Fruchart J-C, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflammation Res. 2000;49(10):497–505.
- Eun CS, Han DS, Lee SH, et al. Attenuation of colonic inflammation by PPARγ in intestinal epithelial cells: effect on toll-like receptor pathway. Dig Dis Sci. 2006;51(4):693–697.
- Carvalho M, Gonçalves-de-albuquerque CF, Silva AR. PPAR gamma: from definition to molecular targets and therapy of lung diseases. Int J Mol Sci. 2021;22(2):805.
- Feng A, Zhou G, Yuan X, et al. Inhibitory effect of baicalin on iNOS and NO expression in intestinal mucosa of rats with acute endotoxemia. PloS one. 2013;8(12):e80997.
- Rangwala SM, Rhoades B, Shapiro JS, et al. Genetic modulation of PPARγ phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5(4):657–663.
- Diradourian C, Girard J, Pégorier J-P. Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie. 2005;87(1):33–38.
- Guan C, Qiao S, Lv Q, et al. Orally administered berberine ameliorates bleomycin-induced pulmonary fibrosis in mice through promoting activation of PPAR-γ and subsequent expression of HGF in colons. Toxicol Appl Pharmacol. 2018;343:1–15.
- Mahmoud AM, Hozayen WG, Ramadan SM. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARγ, and suppressing oxidative stress and apoptosis in rats. Biomed Pharmacother. 2017 October 01;94:280–291.
- He Y, Yuan X, Zuo H, et al. Berberine induces ZIP14 expression and modulates zinc redistribution to protect intestinal mucosal barrier during polymicrobial sepsis. Life Sci. 2019;233:116697.
- Guthrie GJ, Aydemir TB, Troche C, et al. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am J Physiol Gastrointest Liver Physiol. 2015;308(3):G171–G178.
- Aydemir TB, Cousins RJ. The multiple faces of the metal transporter ZIP14 (SLC39A14). J Nutr. 2018;148(2):174–184.
- Zhang Q, Piao X-L, Piao X-S, et al. Preventive effect of Coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem Toxicol. 2011;49(1):61–69.
- Rao A. Efficacy of berberine hydrochloride on biochemical parameters in Indian type 2 diabetic patients. Endocr Pract. 2017;23(1):18A.
- Zeng X-H, Zeng X-J, Li -Y-Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;92(2):173–176.
- Wang L, Peng L, Wei G, et al. Therapeutic effects of berberine capsule on patients with mild hyperlipidemia. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi= Chinese j integrated trad Western med. 2016;36(6):681–684.
- Li G-H, Wang D-L, Hu Y-D, et al. Berberine inhibits acute radiation intestinal syndrome in human with abdomen radiotherapy. Med Oncol. 2010;27(3):919–925.
- Jiang X-W, Zhang Y, Zhu Y-L, et al. Effects of berberine gelatin on recurrent aphthous stomatitis: a randomized, placebo-controlled, double-blind trial in a Chinese cohort. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(2):212–217.
- Chen C, Tao C, Liu Z, et al. A randomized clinical trial of berberine hydrochloride in patients with diarrhea‐predominant irritable bowel syndrome. Phytother Res. 2015;29(11):1822–1827.