Abstract
Disease management programs improve outcomes in subjects with chronic obstructive pulmonary disease (COPD), but their effect in subjects with alpha-1 antitrypsin deficiency (AATD) has not been evaluated. To assess the impact of a disease management program, applicable to subjects with AATD-associated COPD throughout the United States, on exacerbations, healthcare resource utilization and health-related quality of life (HRQoL). The Alpha-1 Disease Management and Prevention Program (ADMAPP) consisted of comprehensive written educational patient-directed material for self-study and treatment plans. Program reinforcement was performed through monthly phone calls by specialized coordinators. Outcomes were collected prospectively for 12 months before, and 12 months after enrollment into the program. Exacerbations and healthcare resource utilization were recorded monthly. HRQoL was measured with the St George's Respiratory Questionnaire (SGRQ) every 6 months and the Short Form-36 (SF-36) every 12 months. A total of 878 subjects completed the 2-year study. During the intervention year, there was a significant increase in the use of long-acting bronchodilators, better compliance with oxygen therapy, and more use of steroid courses during exacerbations. Total exacerbation rates, unscheduled physician visits and emergency room visits significantly decreased. There was also a statistically significant slowing in the deterioration of the SGRQ's activity domain, while total SGRQ scores remained stable during the study. Significant improvements were observed in some of the SF-36 domains, particularly in the general health domain. The ADMAPP improved health outcomes in subjects with AATD-associated COPD.
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is associated with significant morbidity and mortality and imposes a profound burden on the health care system. Despite the development of effective pharmacological therapies to relieve airflow obstruction and reduce exacerbations, COPD subjects continue to experience a slow deterioration of lung function with progressive disability and significant impairment in health-related quality of life (HRQoL). The impact of COPD may be even more pronounced in subjects with alpha-1 antitrypsin deficiency (AATD), a genetic condition associated with early-onset COPD, as affected subjects usually become symptomatic during their most productive years. The burden of disease on subjects with AATD is further aggravated by the stigma of carrying a genetic condition and the additional commitment to regular intravenous infusions of expensive augmentation therapy. Therefore, more effective therapeutic approaches are needed for the treatment of subjects with COPD, and benefits from such approaches may be magnified in individuals with AATD.
Pulmonary rehabilitation, combining exercise programs with patient education, is one of the non-pharmacologic therapeutic modalities for COPD that has been associated with the most favorable outcomes in term of improvement in exercise capacity, dyspnea and HRQoL (Citation[1]). Unfortunately, rehabilitation programs are not widely utilized because of reimbursement inconsistencies (Citation[2]). Another approach to improve patient-oriented COPD outcomes has been the development of disease management programs aimed at patient education with self-management principles.
Unfortunately, the results of available studies regarding the effect of disease management programs in improving HRQoL (Citation[3], Citation[4], Citation[5], Citation[6]) or in reducing exacerbation rates (Citation[3], Citation[6]), hospitalizations (Citation[5], Citation[7]) and physician visits (Citation[6], Citation[8], Citation[9]) have been variable, largely due to the wide heterogeneity in their designs and the patient populations evaluated. In a recent meta-analysis, it was concluded that self-management education is associated with a reduction in hospital admissions with no indication of detrimental effects in other outcome parameters, but current data is insufficient to establish clear recommendations regarding the form or content of self-management programs in COPD (Citation[10]). The impact of self-management programs in specific COPD populations, such as those with AATD, has not previously been evaluated.
In the present report, we present the results of a 12-month disease management program applied to a large cohort of individuals with COPD due to AATD in the United States (Alpha-1 Disease Management and Prevention Program or ADMAPP).
METHODS
Study participants
Study participants were all members of AlphaNet, a not-for-profit, patient-run health management company that coordinates services for subjects with AATD. These subjects were distributed geographically throughout the United States and were followed on a regular basis by one of 23 regionally based disease management coordinators, all of whom are individuals with AATD. As members of AlphaNet, all study participants were diagnosed with AATD by their own physicians and were placed on augmentation therapy because of symptomatic obstructive lung disease, primarily with Prolastin® (alpha-1 proteinase inhibitor, Talecris Biotherapeutics, Research Triangle Park, NC) although about 3% received Zemaira® (alpha-1 proteinase inhibitor, CSL Behring, King of Prussia, PA).
All members of AlphaNet were invited to participate in the study. Subjects who had lung transplantation were excluded from the analysis. For the study, all subjects verbally confirmed their diagnosis of AATD. In an attempt to further confirm this, 68% responded to the investigators' request to send a copy of their AAT genotypes/phenotypes and AAT levels at the time of diagnosis. From this group, 94% were genotype ZZ with AAT levels below 11 μ M. The protocol and enrollment process has been previously reported (Citation[11]) and was approved by the University of Miami's Institutional Review Board. All subjects signed a written informed consent and HIPAA authorization.
Study design
The protocol was designed to evaluate the impact of the ADMAPP in subjects with AATD. The main outcome was effect on HRQoL scores and secondary outcomes were effect on exacerbation rates, medication utilization, healthcare resource utilization (physician visits, emergency room visits and hospitalizations), and patient satisfaction.
The study is a non-randomized and non-concurrent trial comparing outcomes in the same subjects a 2-year period: an initial 1-year observation period, or pre-ADMAPP year, and a post-intervention or ADMAPP year. Throughout the study, telephone surveys were conducted on a monthly basis by the AlphaNet coordinators to reinforce the components of the program and to collect data on disease exacerbations, healthcare resource utilization, medication use, and the evaluation of exercise. HRQoL data were collected by experienced surveyors (Qessential Medical Market Research LLC, Exeter, NH) using the St. George's Respiratory Questionnaire (SGRQ) (Citation[12], Citation[13]) administered at 0,6,12,18 and 24 months, and with the Short Form-36 (SF-36) administered at 0, 12 and 24 months (Citation[14]). All SF-36 scores were normalized to the general U.S. population (1998) on a scale of 0 (worst) to 100 (best) with 50 being the general U.S. population mean and each 10-point variation representing one standard deviation (SD).
Exacerbations were defined according to a consensus definition by an expert panel (Citation[15]). Participants were asked to provide the results of a pulmonary function test performed during the initial study year for classification according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Citation[16]). All survey data were entered directly at the time of collection into a secure database via an encrypted Web-based system. The study was conducted between February 2003 and July 2005.
Alpha-1 disease management and prevention program (ADMAPP)
The ADMAPP was developed as part of AlphaNet's commitment to improve the care of individuals with AATD. It is an integrated system of directed patient self-education, organized supervision, healthcare provider education, and outcome measurement. The program was begun to empower individuals with AATD to take an active role in understanding and managing their own health with the overall goal to improve their HRQoL and optimize clinical and economic outcomes. The program included a comprehensive reference guide with detailed scientific and lay explanations about COPD and AATD, specifically directed towards a well-educated, informed patient. It was not intended to be a substitute for physician diagnosis and treatment.
The guide, entitled the “Big Fat Reference Guide to Alpha-1 (BFRG)”, is a written document authored by over 30 recognized experts in AATD and/or specific aspects of the management program and is divided into easily understood sections. Included are sections covering general information regarding AATD, prevention of disease in AATD, and severity-based descriptions of AATD-associated lung disease. Management recommendations focused on chronic care, highlighting the risks and benefits of the different classes of medications that can be used in COPD, including the advantages of long-acting bronchodilators. The BFRG also provided guidance regarding healthcare provider visits, follow-up care, and intercurrent illness management. In addition, there are specific supplements related to management of exacerbations, augmentation therapy, oxygen therapy, preoperative evaluation, sleep problems, inpatient management, optimization of nutrition, exercise, sexuality, travel considerations and psychosocial issues. The document provides a set of guidelines, checklists, and help sheets for the patients and their physicians. The entire document is available online by registering at http://www.alphanetbfrg.org.
Each participant in the program was provided a hard copy of the program (the 1000-page BFRG), and also received an additional copy for their healthcare provider. Disease management coordinators, all individuals with AATD, were trained and tested according to a predefined curriculum. The coordinators then contacted study subjects by telephone a minimum of once monthly throughout the 2-year study. After distribution of the BFRG at study month 12, coordinators were in charge of enforcing the reading, discussion and understanding of each of the components of the ADMAPP. During the study period, a checklist assessment was performed to determine the subject's comprehension of each of the main components of the program. Every subject was required to work through a long-term treatment plan document, which involved visiting every section of the BFRG.
Statistical analysis
All data were analyzed using the SAS version 9.13 software (SAS institute Inc., Cary, North Carolina, USA). Descriptive statistics are displayed as the mean and standard deviation, or percentage. Categorical data were analyzed using the Chi-square test and continue variables were compared using the Student t test and analysis of variance (ANOV). Variables that did not have a normal distribution were compared with the Mann–Whitney U-test. Subanalysis of outcomes was also done by age groups (< 49, 50–59 and > 59 years) and by GOLD stage. Age groupings were selected to roughly equalize the number of enrolled subjects in each group. The repeat measurement mixed models method was employed to compare the changes of HRQoL within and between-age-group differences. Statistical significance was accepted at the 5% level.
RESULTS
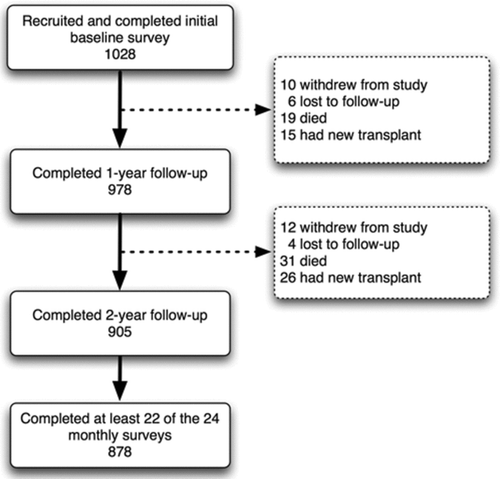
A total of 1028 subjects without a history of lung transplantation consented to participate, completed the baseline survey and were enrolled in the study. A total of 905 individuals completed the two study years (88%). The reasons for non-completion are outlined in . During the intervention period, 87% of all subjects successfully completed the long-term treatment plan that required at least minimal understanding of every section of the written educational material. The final analysis of the effect of the disease management program included 878 subjects who completed at least 22 of the 24 monthly surveys and all the HRQoL questionnaires (85% of the initial group).
Figure 1 Study flow diagram. The final analysis included 878 subjects who completed at least 22 monthly surveys and all health-related quality of life surveys.
Characteristics of the study population
The sociodemographic characteristics of the 878 evaluable participants are summarized in . The average age was 54 years and in general, subjects were mostly overweight, had relatively severe lung disease (79% GOLD stage III or IV), and had significant impairment in baseline HRQoL scores in both the disease-specific (SGRQ) and more generic (SF-36) surveys (). Most expressed moderate impairment in their daily activities due to AATD. Overall, the 15% of subjects who did not complete the 2-year follow-up did not have any significant differences compared with the group as a whole. However, the 50 subjects (4.8%) who died during follow-up had significantly worse SGRQ total scores and worse SF-36 composite scores at baseline.
Table 1 Baseline sociodemographic characteristics of the study cohort
Table 2 Baseline clinical characteristics of the study cohort
Impact of ADMAPP on chronic COPD care
After institution of the ADMAPP, there were significant changes towards a more optimal use of COPD medications, in particular an increase in the use of long-acting bronchodilators and significant reductions in the use of theophylline and chronic systemic steroids (). This was observed across all age groups and among all GOLD stages in the subgroup of subjects who had spirometry results available. After ADMAPP, there was significantly better compliance with oxygen use. No significant changes in the participant's exercise practices were observed.
Table 3 Impact of ADMAP program on chronic COPD care.
Impact of ADMAPP on acute COPD exacerbations
There was a significant reduction in the mean number of exacerbations during the intervention year compared with the first year (), mostly due to a reduction in the frequency of moderate exacerbations. This was observed across all age groups and in subjects with GOLD stages III and IV (not shown). Significant reductions in the total time spent with exacerbation symptoms during the year were observed, mainly due to both a reduction in the number of exacerbations and a reduction in the duration of moderate exacerbations. The frequency and duration of severe exacerbations did not change.
Table 4 Impact of ADMAP program on acute COPD care
After ADMAPP, there was an increased use of short-term systemic steroids to manage acute COPD exacerbations. No changes were noted in antibiotic use or self-treatment. There was a reduction in calls to primary care physicians and more subjects visited a physician office for evaluation and treatment of acute exacerbations ().
Table 5 Frequency, severity and duration of exacerbations in the 12 months prior and after introduction of the ADMAP program (N = 878)
Impact of ADMAPP on healthcare resource utilization
Significant reductions in overall healthcare resource utilization were reported during the 12 months post-ADMAPP introduction compared with the 12 months prior for a number of items including primary care physicians office visits, unscheduled physician visits, and emergency room visits (). Hospitalization rates did not differ.
Table 6 Impact of ADMAP program on healthcare utilization
Impact of ADMAPP on HRQoL
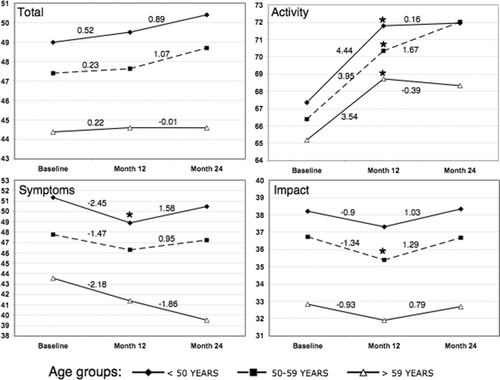
The overall SGRQ total scores at the end of the study period (48.0 ± 16.3) were not clinically different (> 4 points) from the scores recorded at baseline (47.7 ± 17.5). The activity domain was the domain with the greatest impairment at baseline and the domain with the greatest deterioration during the 12 months prior to ADMAPP (from 66.3 ± 21.7 to 70.3 ± 22.4). However, by slope analysis, the activity domain deterioration significantly stabilized by the end of year 2 (+4 vs. +0.56, respectively, p < 0.001). This slowing in deterioration of the activity domain scores and stabilization of SGRQ total scores at the end of the intervention year were observed across all age groups, including the subgroup with the least HRQoL impairment at baseline (the group > 59 years) (). Interestingly, this older group had the least deterioration in all SGRQ scores during the intervention year. This same pattern in SGRQ changes was observed across all GOLD stages (not shown).
Figure 2 Saint George Respiratory Questionnaire variation during the study period according to age groups. The slopes of SGRQ changes for each of the study years are shown. The slopes were calculated by mixed model analysis using the baseline, 6th and 12th month measurement for year 1 and 12th, 18th and 24th month measurements for year 2 (intervention year). *: p < 0.05.
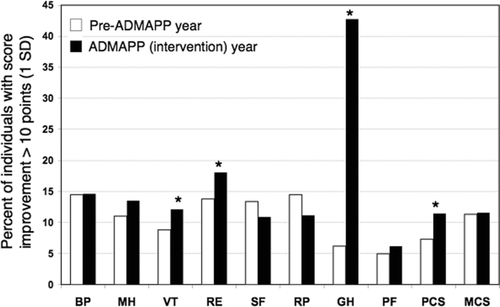
At study entry, participants had significant impairment in the physical domain scores (PF, RP, GH, PCS) of the SF-36 compared with the general U.S. population (). Since no consensus is available for interpreting SF-36 changes, in order to evaluate the effect of ADMAPP on general HRQoL we compared the proportion of individuals improving > 1 SD between the first and second year. There was a significant improvement in the proportion of subjects that report improvements in VT, RE, PCS and particularly GH (). There were reciprocal changes of less worsening in these domains (not shown).
Figure 3 Proportion of subjects that improved SF-36 scores more than 10 points (1 standard deviation for the 1998 United States population) during the pre-ADMAPP and ADMAPP (intervention) year. *: p < 0.05. BP: Bodily Pain; MH: Mental Health; VT: Vitality; RE: Role impairment due to Emotional problems; SF: Social Function; RP: Role impairment due to Physical problems; GH: General Health; PF: Physical Functioning; PCS: Physical Component Summary; MCS: Mental Component Summary.
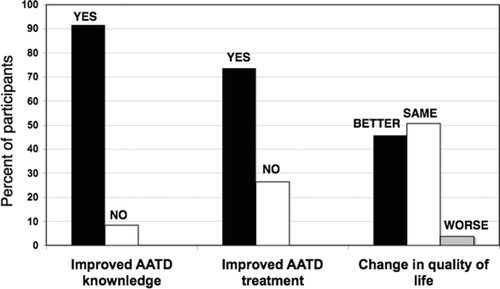
Patient satisfaction
At the end of the intervention year, subjects were asked to evaluate the effect the program had on them. Most stated that it improved their knowledge about AATD and improved their current treatment. Subjectively, the majority felt that their quality of life either stabilized or improved ().
DISCUSSION
This study showed that ADMAPP, a program designed to provide patient education using an original educational document enforced by regular telephone contacts, improves the patients' knowledge of AATD and its treatment. This translates into more optimal long-term and short-term medication use, and into positive changes in several patient-centered COPD outcomes such as HRQoL, exacerbation rates and duration, unscheduled physician and ER visits, and overall patient satisfaction.
We studied a large group of individuals diagnosed with AATD that were receiving augmentation therapy due to pulmonary compromise. As a group, HRQoL at study entry was severely impaired, with scores in SGRQ and SF-36 comparable with those reported for older subjects included in other large non-AATD COPD trials (Citation[17], Citation[18]). Although our study did not have a control group without intervention, we believe that the overall effect of ADMAPP on our subject's HRQoL was a positive one. It has been shown that SGRQ total scores in COPD deteriorate by a clinically significant degree (> 4 points) every 15 months (Citation[18]).
In contrast, our subjects had stable SGRQ scores throughout the 24-month study. We observed that most of this reduction in the rate of deterioration was due to a halt in decline in the activity domain during the intervention year. We speculate that this effect is directly related to improved medication use, in particular the use of long acting bronchodilators, which have important effects in stabilizing HRQoL (Citation[19], Citation[20]) and reducing exacerbations rates (Citation[21], Citation[22]). This may also explain the observed improvement in SF-36, particularly in the General Health domain, despite the lack of increase in exercise habits. It is important to emphasize that the benefits of the program were observed across all age groups, with different levels of baseline HRQoL impairment.
The ADMAPP improved subject's awareness about the significance and consequences of acute exacerbations and the importance of rapid optimal therapy. During the intervention year, participants were more likely to use a course of short-term systemic steroids to treat exacerbations and sought medical evaluation by a health professional more often. This appears to be attributable to patients' increased awareness of the worsening of their symptoms and seeking of help, rather than to an adverse effect of the disease management program. In contrast, the total number of ER visits and unscheduled physician visits were significantly less during the intervention year. Although this could be due to a reduction in the total number and duration of exacerbations, an improved understanding of their disease may have provided patients with increased confidence in evaluating their own symptoms, resulting in fewer visits or consultations with their physicians.
Disease management programs have been previously evaluated in more traditional COPD cohorts but to the best of our knowledge, this is the first trial to evaluate its effectiveness in subjects with COPD associated with AATD. In COPD, disease management programs have beneficial effects on different outcomes. For example, two studies evaluating disease-specific education programs supported by weekly patient contact by trained health professionals and an action plan, showed lower hospital admissions for acute exacerbations, as well as decreased ER and unscheduled physician visits (Citation[3], Citation[23]). In a systematic review, interventions that included at least two chronic care model components (also provided by the ADMAPP) led to lower rates of ER and unscheduled visits as observed in our study (Citation[24]).
Improved medication use, such as increased use of systemic steroid during exacerbations, resulted from a program that combined self-education with short group sessions (Citation[25]). Regarding HRQoL, some studies have shown improvements with disease management programs (Citation[26]) while others have shown no differences between intervention and control groups (Citation[5], Citation[6], Citation[8]). In a recent review of randomized trials, it was concluded that disease management plans overall have a positive effect in the HRQoL of subjects with COPD (Citation[27]). None of these programs have been shown to affect mortality in patients with COPD. The higher number of deaths observed during the second year of our study suggests that despite the ADMAPP, subjects continued to suffer from ongoing disease progression. Over the nine years that AlphaNet has followed individuals with AATD, the annual mortality rate has shown remarkable variability.
Most of the studied disease management programs in COPD combine different strategies such as educational brochures (Citation[25], Citation[26], Citation[28]), audiotapes (Citation[28]), small group or individual education sessions (Citation[3], Citation[4], Citation[6], Citation[25], Citation[28], Citation[29], Citation[30]), home visits (Citation[3], Citation[5]), exercise programs (Citation[4]) and action plans (Citation[3], Citation[5], Citation[6], Citation[7], Citation[25], Citation[26], Citation[30]). Our program had the particularity of being based on self-education reinforced by frequent personalized phone calls by trained disease management coordinators. These two features of the ADMAPP allow reaching a large number of subjects with a wide geographic distribution, as is the case with AATD-affected individuals. This is particularly important for a population in which affected individuals are scattered among the vast population of patients with respiratory diseases with very few under the care of physicians with specific expertise in AATD. It also allows its application to patients undergoing care by different health care professionals and health plans. In addition, the flexibility of telephone call timing and the convenience of not leaving home favors patient compliance. One limitation of our program is that it lacks direct “face to face” interaction, and therefore may not allow effective exercise counseling and training in breathing techniques or proper use of inhaled medications, although these topics were covered with text and illustrations in the BFRG.
The lack of a parallel control group to better estimate the true effect of the program may be viewed as a limitation of our study. Given the nature of our cohort, randomization of treatment and controls would not have been possible. Since each of the disease management coordinators supervised more than 100 subjects at a time, it was not practical to have them perform different types of calls to subjects who were part of the ADMAPP or not. In addition, subjects with AATD in the United States are frequently involved in support groups, receive newsletters, and are in frequent contact with each other, which would have jeopardized randomization. As an alternative, we used the same cohort as its own control, observing baseline outcomes during the year prior to the intervention. The wide geographical location of the participants in our study did not allow us to gather them for objective measures of exercise capacity and lung function. For the same reason, we chose to administer the surveys via telephone calls, including the HRQoL instruments as described in other studies (Citation[31], Citation[32]). The HRQoL data recorded, with scores and impairment patterns similar to those reported in other COPD studies (Citation[17], Citation[18]), suggests that our measurements were reliable. Despite these efforts, the nature of the cohort and the study design still does not eliminate the possibility of other potential biases, which can only be controlled in randomized clinical trials in this population.
The subjects with AATD studied in our cohort exhibited marked impairment in several aspects of their life, despite being a group with access to healthcare resources such as augmentation therapy. This highlights the importance of instituting efforts such as the ADMAPP. To the best of our knowledge, this is the first report that demonstrates that a self-management program, that empowers AATD individuals to take a more active role in the management of their disease, leads to beneficial effects in affected individuals with severe lung disease. The positive effects observed are particularly encouraging given that our subjects were already self-motivated, as evidenced by their high rate of participation and low dropout rates. It is possible that a longer follow-up may have allowed us to document further improvements in outcomes, given that not all subjects comprehend the educational material at the same pace and many need a longer time to incorporate the lifestyle changes recommended by the program. The longer-term effects of the ADMAPP warrant ongoing evaluation.
ACKNOWLEDGMENTS
To John Walsh from the Alpha-1 Foundation and to Bonnie Boyd and Rosemarie Carillo from AlphaNet. We are extremely grateful for the superb work of all the AlphaNet Coordinators: Terry Young, Gayle Allison, Roger Bray, Dick Bueker, Patti Brown, Victoria Cameron, Robert Campbell, Shirley Dennis, Joyce Finch, Phil Freeman, Kathy Haduck, Randy Harwell, Pat MacInnes, Darrel Nall, Diana Patterson, Mary Pierce, Judy Rose, Sandy Singleton, Kathleen Sivesind, Marta Strock, Kay Kinsel Swift, Doug Turley, Liz Veronda and Fred Walsh.
Funding for this article provided by AlphaNet, Inc.
Presented as a poster in the 2007 American Thoracic Society International Meeting.
REFERENCES
- Ries A L, Bauldoff G S, Carlin B W, Casaburi R, Emery C F, Mahler D A, Make B, Rochester C L, Zuwallack R, Herrerias C. Pulmonary rehabilitation: Joint accp/aacvpr evidence-based clinical practice guidelines. Chest 2007; 131: 4S–42S
- Hill N S. Pulmonary rehabilitation. Proc Am Thorac Soc 2006; 3: 66–74
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, Renzi P, Nault D, Borycki E, Schwartzman K, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: A disease-specific self-management intervention. Arch Intern Med 2003; 163: 585–591
- Boxall A M, Barclay L, Sayers A, Caplan G A. Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardiopulm Rehabil 2005; 25: 378–385
- Coultas D, Frederick J, Barnett B, Singh G, Wludyka P. A randomized trial of two types of nurse-assisted home care for patients with copd. Chest 2005; 128: 2017–2024
- Monninkhof E, van der Valk P, van der Palen J, van Herwaarden C, Zielhuis G. Effects of a comprehensive self-management programme in patients with chronic obstructive pulmonary disease. Eur Respir J 2003; 22: 815–820
- Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J 2004; 34: 608–614
- Gallefoss F, Bakke P S, Rsgaard P K. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 812–817
- Gourley G A, Portner T S, Gourley D R, Rigolosi E L, Holt J M, Solomon D K, Bass G E, Wicke W R, Braden R L. Humanistic outcomes in the hypertension and copd arms of a multicenter outcomes study. J Am Pharm Assoc (Wash) 1998; 38: 586–597
- Effing T, Monninkhof E M, van der Valk P D, van der Palen J, van Herwaarden C L, Partidge M R, Walters E H, Zielhuis G A. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007, CD002990
- Campos M A, Wanner A, Zhang G, Sandhaus R A. Trends in the diagnosis of symptomatic patients with alpha1-antitrypsin deficiency between 1968 and 2003. Chest 2005; 128: 1179–1186
- Jones P W, Quirk F H, Baveystock C M, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The st. George's respiratory questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327
- Jones P W. St. George's respiratory questionnaire: Mcid. COPD 2005; 2: 75–79
- Ware J E, Snow K K, Kosinski M. Sf-36 health survey: Manual and interpretation guide. QualityMetric Incorporated, Lincoln, RI 2002
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000; 117: 398S–401S
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease (gold), Available at www.Goldcopd.Org Accessed 7/17/07, 2006
- Calverley P M, Anderson J A, Celli B, Ferguson G T, Jenkins C, Jones P W, Yates J C, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356: 775–789
- Spencer S, Calverley P M, Sherwood Burge P, Jones P W. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 122–128
- Casaburi R, Mahler D A, Jones P W, Wanner A, San P G, ZuWallack R L, Menjoge S S, Serby C W, Witek T, Jr. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19: 217–224
- Donohue J F, van Noord J A, Bateman E D, Langley S J, Lee A, Witek T J, Jr., Kesten S, Towse L. A 6-month, placebo-controlled study comparing lung function and health status changes in copd patients treated with tiotropium or salmeterol. Chest 2002; 122: 47–55
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with copd. Thorax 2003; 58: 399–404
- Niewoehner D E, Rice K, Cote C, Paulson D, Cooper J A, Jr., Korducki L, Cassino C, Kesten S. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: A randomized trial. Ann Intern Med 2005; 143: 317–326
- Gadoury M A, Schwartzman K, Rouleau M, Maltais F, Julien M, Beaupre A, Renzi P, Begin R, Nault D, Bourbeau J. Self-management reduces both short- and long-term hospitalisation in copd. Eur Respir J 2005; 26: 853–857
- Adams S G, Smith P K, Allan P F, Anzueto A, Pugh J A, Cornell J E. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med 2007; 167: 551–561
- Gallefoss F, Bakke P S. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication?. Am J Respir Crit Care Med 1999; 160: 2000–2005
- Watson P B, Town G I, Holbrook N, Dwan C, Toop L J, Drennan C J. Evaluation of a self-management plan for chronic obstructive pulmonary disease. Eur Respir J 1997; 10: 1267–1271
- Niesink A, Trappenburg J C, de Weert-van Oene G H, Lammers J W, Verheij T J, Schrijvers A J. Systematic review of the effects of chronic disease management on quality-of-life in people with chronic obstructive pulmonary disease. Respir Med 2007; 101: 2233–2239
- Blake R L, Jr., Vandiver T A, Braun S, Bertuso D D, Straub V. A randomized controlled evaluation of a psychosocial intervention in adults with chronic lung disease. Fam Med 1990; 22: 365–370
- Howland J, Nelson E C, Barlow P B, McHugo G, Meier F A, Brent P, Laser-Wolston N, Parker H W. Chronic obstructive airway disease. Impact of health education. Chest 1986; 90: 233–238
- Martin I R, McNamara D, Sutherland F R, Tilyard M W, Taylor D R. Care plans for acutely deteriorating copd: A randomized controlled trial. Chron Respir Dis 2004; 1: 191–195
- Desikan R, Mason H L, Rupp M T, Skehan M. Health-related quality of life and healthcare resource utilization by copd patients: A comparison of three instruments. Qual Life Res 2002; 11: 739–751
- Anie K A, Jones P W, Hilton S R, Anderson H R. A computer-assisted telephone interview technique for assessment of asthma morbidity and drug use in adult asthma. J Clin Epidemiol 1996; 49: 653–656