Abstract
Combined use of β2-agonists and anticholinergic bronchodilators may have complementary benefits in patients with chronic obstructive pulmonary disease (COPD). The objective of this study was to compare combination treatment with formoterol (FORM) plus tiotropium (TIO) versus treatment with TIO alone in patients with COPD. In this active-controlled, double-blind, multicenter trial, a total of 255 subjects with diagnosed COPD were randomized to 12 weeks of either a combination of FORM 12 μ g twice-daily plus TIO 18 μ g once-daily in the morning (QD AM) or monotherapy with TIO 18 μ g QD AM. The primary efficacy variable was the area under the curve for forced expiratory volume in 1 second measured 0 to 4 hours after AM dosing (FEV1 AUC0−4h). Significantly greater improvements in the FEV1 AUC0−4h were seen with FORM + TIO (n = 116) versus TIO (n = 124) at all time points. The increase in FEV1 5 minutes after the first dose was 180 mL with FORM + TIO versus 40 mL with TIO (p < 0.001). At endpoint, FEV1 AUC0−4h increased 340 mL with FORM + TIO versus 170 mL with TIO (p < 0.001). Improvements in trough FEV1 with FORM + TIO versus TIO were 180 mL and 100 mL, respectively (p < 0.01). Significantly greater reductions from baseline in symptom scores (p < 0.05) and daytime albuterol use (p < 0.04) were seen at endpoint with combination FORM + TIO versus TIO monotherapy. Both treatments were well tolerated. This study demonstrated that concurrent treatment with FORM + TIO results in greater therapeutic benefits than TIO alone.
Key words: :
INTRODUCTION
Bronchodilators (i.e., β2-agonists and anticholinergics) have long been the foundation of pharmacologic therapy in patients with chronic obstructive pulmonary disease (COPD). These agents not only improve lung function in patients with COPD, evident primarily as increases in forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), but also reduce COPD symptoms and exacerbations while improving exercise tolerance and health status (Citation[1], Citation[2]).
Short-acting bronchodilators (i.e., albuterol and ipratropium) have been available for many years to treat COPD, and their therapeutic benefits and limitations are well established. These agents have a rapid onset of action that makes them useful for the prompt relief of dyspnea, but their short duration of action (Citation[4], Citation[5], Citation[6], Citation[7], Citation[8] h) necessitates 4 times daily administration for maintenance therapy (Citation[1], Citation[2]). Formoterol (FORM) and tiotropium (TIO) are long-acting bronchodilators now available for the treatment of COPD. These agents have proven efficacy and safety in the treatment of patients with stable COPD (Citation[3], Citation[4], Citation[5], Citation[6], Citation[7], Citation[8], Citation[9]). They provide sustained clinical effects with once-or twice-daily administration (Citation[4], Citation[5], Citation[7], Citation[9]) and are more convenient than short-acting bronchodilators for daily maintenance therapy (Citation[2], Citation[10]).
Formoterol and TIO differ from each other not only in terms of mechanism of action (FORM is a long-acting β2-agonist [LABA], whereas TIO is an anticholinergic agent), but also in terms of onset and duration of action. Formoterol has been shown to have a faster onset of action and a different efficacy profile than TIO (Citation[11], Citation[12]), whereas TIO has a longer duration of bronchodilatation than FORM (24 h vs 12 h, respectively). Combined short-acting β2-agonists and anticholinergics are often used to treat COPD, achieving additive effects via the complementary mechanisms of the individual agents. The benefits of therapy with combinations of long-acting bronchodilators are now being actively explored (Citation[10], Citation[13]). Five studies comparing combined FORM + TIO with the individual agents have been published (Citation[12], Citation[14], Citation[15], Citation[16], Citation[17]). However, 4 of these studies involved fewer than 100 patients and evaluated spirometric effects for ≤ 6 weeks (Citation[12], Citation[14], Citation[15], Citation[16]), and the 5th study evaluated nebulized FORM instead of FORM delivered via a dry powder inhaler (Citation[17]). Therefore, a more comprehensive, longer study evaluating the effects of FORM delivered via a dry powder inhaler + TIO in a larger population of patients with COPD was necessary to more thoroughly examine the benefits of concurrent administration of these 2 long-acting bronchodilators. Thus, a randomized placebo-controlled trial comparing the efficacy and safety of FORM + TIO with TIO alone during 12 weeks of treatment in patients with COPD was performed.
MATERIALS AND METHODS
Design overview
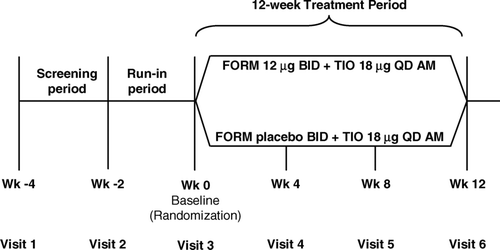
This was a 12-week, multicenter, double-blind, randomized, parallel-group study (). The study was approved by an appropriate Institutional Review Board or Independent Ethics Committee at each site, and all patients provided written informed consent.
Setting and participants
The trial was conducted at 35 study centers across the United States (mainly primary care centers). Male and nonpregnant female patients aged ≥ 40 years who had a clinical history of COPD were enrolled in this study. Each patient had a postbronchodilator FEV1 < 70% and > 30% predicted normal or > 0.75 L, whichever was less, at run-in, and a FEV1 to FVC ratio (FEV1/FVC) of < 0.70 at screening and run-in.
Daytime and/or nighttime symptoms of COPD, including dyspnea, must have been present on ≥ 4 of the 7 days before the baseline visit. Women of childbearing potential were required to use a medically accepted form of birth control during the study.
Study exclusion criteria included a current or previous history of asthma or other significant medical condition that may have interfered with study treatment as assessed by the investigator, smoking cessation within the previous 3 months, ventilator support for respiratory failure within the previous year, the use of oxygen (≥ 2 L/min or for > 2 h/d), initiation of pulmonary rehabilitation within the previous 3 months, the requirement for nasal continuous positive airway pressure or bilevel positive airway pressure, clinically significant lung disease other than COPD (i.e., bronchiectasis, sarcoidosis, pulmonary fibrosis, tuberculosis), sleep apnea, chronic narrow-angle glaucoma, symptomatic prostatic hyperplasia or bladder neck obstruction, and the need for chronic or prophylactic antibiotic therapy.
Randomization and interventions
Following screening, prohibited medications (i.e., β -agonists, β -blockers, cromolyn sodium, ipratropium bromide, leukotriene antagonists, cytotoxic agents, and theophylline) were withdrawn. Continued use of prior stable inhaled corticosteroid regimens and systemic corticosteroids for the treatment of exacerbations was permitted throughout the study. All patients were provided with albuterol inhalers for use as rescue medication.
Patients previously using TIO or FORM discontinued the drugs at least 4 weeks or 48 hours before screening, respectively. Patients completed a 2-week run-in period using placebo and as-needed rescue albuterol, after which eligible patients were randomized to 12 weeks of treatment with either FORM 12 μ g (Foradil Aerolizer®, Schering Corporation, Kenilworth, NJ) twice-daily and TIO 18 μ g (Spiriva Handihaler®, Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT/Pfizer Inc, New York, NY) once-daily in the morning delivered via 2 separate inhalers, or FORM-matched placebo twice-daily and TIO 18 μ g once-daily delivered via 2 separate inhalers. Training was given on the proper use of both inhalers. Patients were randomized sequentially as they qualified for the study according to a pregenerated computer code labelled on the medication kit.
Outcomes and follow-up
Patients visited the clinic at weeks 0 (time of randomization, baseline), 4, 8, and 12 of the treatment phase. During each treatment phase visit, spirometry was performed, diaries were collected and dispensed, and compliance with study medication and procedures was checked. In addition, the total symptom severity score, the baseline dyspnea index (BDI) score and/or transition dyspnea index (TDI) score (Citation[18]), the global therapeutic response, and the number and severity of exacerbations were assessed. At baseline and week-12 visits, patients completed the St. George's Respiratory Questionnaire (SGRQ) (Citation[19]).
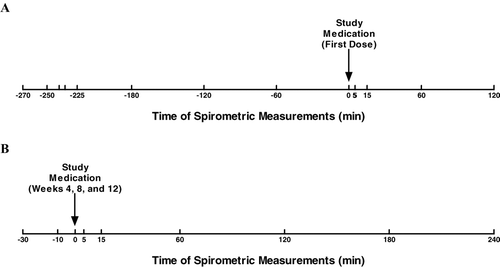
Spirometry testing was performed in the morning of each visit. At baseline, FEV1 and FVC were measured at 9 time points from 270 minutes predose to immediately before the morning dose of study treatment, and at 5, 15, 60, and 120 minutes postdose. At treatment weeks 4, 8, and 12, FEV1 and FVC were measured at 30 and 10 minutes predose; immediately before the morning dose; and at 5, 15, 60, 120, 180, and 240 minutes postdose ().
Figure 2 Timing of spirometry measurements. Spirometry was performed to measure FEV1 and FVC before and after administration of the first dose of study treatment (A) and before and after doses administered during visits at study weeks 4, 8, and 12 (B). FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
Patient diaries were used to record morning and evening peak expiratory flow (PEF), symptom severity scores, use of rescue albuterol, nocturnal awakenings requiring rescue albuterol, changes in study or concomitant medications, and adverse events. Symptoms were rated daily in the morning and evening using scales of increasing severity and included dyspnea (0 = none to 4 = severe), wheezing, cough, and chest tightness (0 = none to 3 = very uncomfortable).
To assess the impact of treatment on dyspnea, BDI and TDI scores were calculated, as originally described by Mahler et al. (Citation[18]). The global therapeutic response was assessed by the investigator and scored on a scale of 1 (much improved) to 5 (much worse).
Statistical analysis
Analyses of primary and secondary efficacy variables were performed on data derived from the intent-to-treat (ITT) population, which included all randomized patients who took at least 1 dose of study treatment. Superiority analyses were also performed on the primary efficacy variable for the efficacy evaluable population of patients who followed the protocol and met key criteria for eligibility and evaluability. The primary efficacy variable was the normalized area under the curve (AUC) for FEV1 measured 0 to 4 hours post-morning dose (FEV1 AUC0–4h) at the last visit (imputed using the last observation carried forward). Normalized FEV1 AUC0–4h values were obtained by dividing the computed FEV1 AUC0–4h by the time for which the subject was actually observed and were evaluated using analysis of covariance using the baseline value as the covariate. It was estimated that a sample size of approximately 250 patients per treatment group would provide 85% power to detect a between-group difference of 80 mL in the normalized FEV1 AUC0–4h (α = 0.05, 2-sided with a standard deviation of 300 mL).
Analysis of covariance models were used to analyze changes from baseline in trough (average of values obtained 10 and 30 min predose) FEV1 and FVC, weekly PEF, symptom severity scores, TDI, and SGRQ scores. Other secondary endpoints, including exacerbations, the global therapeutic response, discontinuations because of worsening COPD, and percentages of patients achieving targeted improvements in the SGRQ and TDI scores were analyzed using the chi-square test.
RESULTS
Patient disposition and demographics
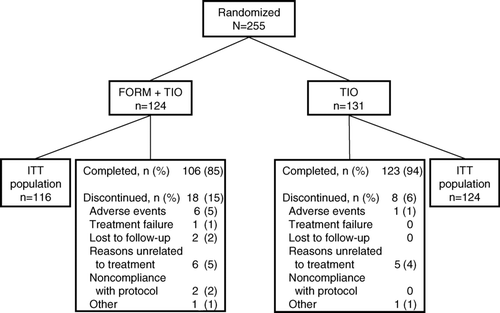
A total of 255 patients were randomized to treatment with FORM + TIO (n = 124) or TIO alone (n = 131). The majority of patients (85% [106/124] and 94% [123/131], respectively) completed 12 weeks of treatment (). Treatment groups were similar with regard to demographics and baseline characteristics, the majority being white males with no previous inhaled corticosteroid use ().
Table 1 Baseline demographic and disease characteristics (randomized patients)
Spirometric effects
For the primary efficacy variable, the mean increases from baseline in normalized FEV1AUC0–4h values at the last visit were significantly greater with FORM + TIO compared with TIO alone (340 mL and 170 mL, respectively, p < 0.001). Treatment differences were statistically significant at week 4 (150 mL; 95% CI, 90–220), week 8 (170 mL; 95% CI, 100–230), week 12 (180 mL; 95% CI, 120–240), and the last visit (170 mL; 95% CI, 120–230) (p < 0.001 for all) ().
Figure 4 Improvements by treatment visit in normalized forced expiratory volume in 1 second (FEV1) area under the response-time curve from 0–4 hours (AUC0–4h) (A) and normalized forced vital capacity (FVC) AUC0–4h(B). FORM = formoterol fumarate 12 μ g BID; TIO = tiotropium bromide 18 μ g QD. * p < 0.001 compared with TIO.
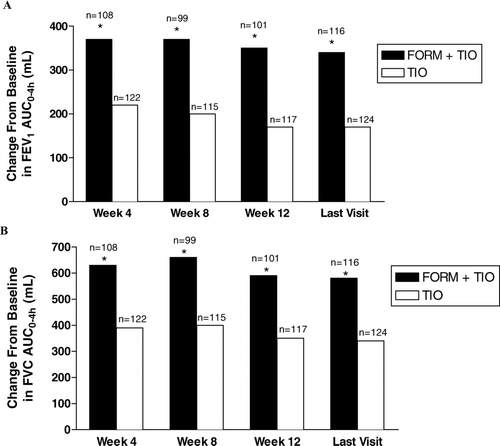
Significantly greater improvements from baseline in FVC AUC0–4h occurred following treatment with FORM + TIO compared with TIO at all study weeks and the final visit (), with between-treatment differences in favor of FORM + TIO ranging from 240 mL to 260 mL (p < 0.001). Significantly greater improvements in trough FVC values for FORM + TIO versus TIO were also observed at all study weeks, with between-treatment differences ranging from 120 mL to 180 mL (p < 0.05).
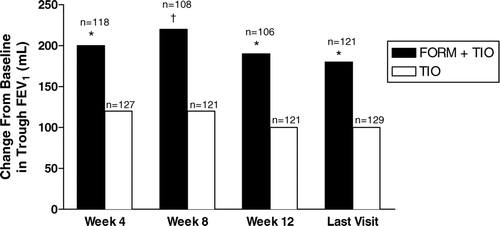
Greater improvements in trough FEV1values (not a predefined protocol-specified objective) were also seen with FORM + TIO compared with TIO alone at all study weeks and the last visit (). The between-treatment differences in trough FEV1 at weeks 4 (80 mL; 95% CI, 20–130), 8 (100 mL; 95% CI, 40–160), and 12 (90 mL; 95% CI, 30–140) and the last visit (80 mL; 95% CI, 30–140) were all statistically significant (p < 0.01).
Figure 5 Improvements by treatment visit in trough values for forced expiratory volume in 1 second (FEV1); shown are the averages of values obtained 30 and 10 minutes predose. FORM = formoterol fumarate 12 μ g BID; TIO = tiotropium bromide 18 μ g QD. *p < 0.01; †p < 0.001 compared with TIO.
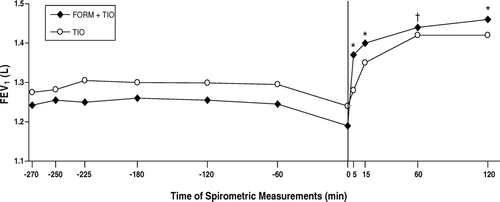
Five minutes after administration of the first dose of study treatment on day 1, the increase in FEV1 with FORM + TIO was significantly greater than the increase with TIO (180 mL vs 40 mL, respectively, p < 0.001) ().
Figure 6 Onset of improvements in postbronchodilator forced expiratory volume in 1 second (FEV1) following administration of the first dose of each study treatment; the FEV1 measurement at t = 0 was obtained immediately before administration of FORM + TIO (n = 118) and TIO (n = 125). FORM = formoterol fumarate 12 μ g BID; TIO = tiotropium bromide 18 μ g QD. *p < 0.001; † p = 0.005 compared with TIO.
AM and PM PEF
Improvements from baseline in AM PEF ranged from 16.1% to 18.9% in the FORM + TIO group and from 6.2% to 12.1% in the TIO group. Significant differences favoring FORM + TIO versus TIO were seen at the last visit (p = 0.015) and all treatment weeks except weeks 3, 4, and 11. Improvements in PM PEF ranged from 14.3% to 18.1% in the FORM + TIO group and from 9.5% to 17.6% in the TIO group, with the only significant differences seen at weeks 1 (p < 0.01) and 5 (p < 0.05).
Patient-centered efficacy variables
The improvement in dyspnea was greater in the FORM + TIO group than the TIO group at all visits, but the difference was significant only at week 8 (1.86 vs. 1.01, respectively; 95% CI, 0.18–1.51; p = 0.013). For both treatment groups, TDI exceeded the minimal clinically important difference (≥1 unit), but the difference in the TDI response between groups did not exceed this threshold. At the final visit, the TDI scores for the FORM + TIO and TIO groups were 1.60 and 1.53, respectively (95% CI, −0.69–0.82; P = 0.866) (). The SGRQ questionnaire was completed at baseline and week 12 by 242 patients.
Table 2 Changes from baseline to last visit in patient-centered outcomes
Mean baseline scores were similar for both treatment groups. Clinically meaningful decreases from baseline (≥4 points) were noted for total scores, as well as scores for impact and symptoms, in the FORM + TIO group and for scores in the activity domain in the TIO alone group. Greater statistically significant and clinically meaningful improvements in the SGRQ scores for the burden of symptoms was seen with FORM + TIO compared with TIO (p < 0.05) ().
Patients in each treatment group had similar total COPD symptom scores at baseline, and the scores were reduced by both treatments. The AM, PM, and average AM/PM scores were significantly lower at most study weeks in the FORM + TIO group compared with the TIO group and were reduced by a significantly greater extent at the last weekly interval post baseline (). Rescue medication use at baseline in each treatment group was between 2 to 3 daytime puffs and 1 to 2 nighttime puffs of albuterol. Although the use of rescue medication decreased in each treatment group at the last weekly interval (), reductions in daytime use (recorded as PM scores) were significantly greater with FORM + TIO than TIO alone at weeks 1 to 6, week 8, and the average of all time points post baseline (p < 0.04). The number of nocturnal awakenings decreased from baseline in each treatment group; the only significant difference between treatments was a greater reduction in nocturnal awakenings in the FORM + TIO group versus the TIO group at week 1 (p < 0.05).
COPD exacerbations occurred in 21 patients (17%) in the FORM + TIO group and 14 patients (11%) in the TIO group; the difference between treatments was not significant (p = 0.149). Most patients who needed treatment for their exacerbations received antibiotics alone or a course of antibiotics and systemic steroids. The mean duration of exacerbations was longer in the TIO group (19.4 d) than in the FORM + TIO group (16.2 d). Improvements in global therapeutic response were noted for a greater proportion of FORM + TIO patients than for TIO patients at all treatment weeks. Across all visits, 6% to 12% of FORM + TIO patients were much improved compared with 2% to 8% of TIO patients.
Safety and tolerability
Treatment with FORM + TIO was generally well tolerated (), and most adverse events (AEs) were of mild to moderate severity. Severe or life-threatening AEs were reported by 14 subjects overall, 7 in each treatment group. However, in the FORM + TIO group, there were no severe or life-threatening AEs that were considered to be treatment related. On the other hand, 4 patients in the TIO group experienced severe treatment-emergent AEs that were determined by the investigators to be treatment related. These severe treatment-emergent AEs were nausea, pneumonia, overdose, headache, and COPD. No clinically meaningful changes in vital signs, physical findings, or other observations related to safety were noted in either treatment group.
Table 3 Treatment-emergent adverse events reported by ≥ 3% of patients in either group
DISCUSSION
Our study was the first to evaluate the spirometric and clinical effects of 12 weeks of treatment with a combination of FORM + TIO in patients with COPD. Compared with TIO therapy alone, significant between-treatment differences favoring FORM + TIO were observed at each visit. The treatment differences in normalized values for FEV1 AUC0–4h were ≥150 mL in favor of FORM + TIO at all time points, a difference that exceeds the threshold of 100 mL representing a clinically significant treatment effect (Citation[20]). The findings from this study are important because they demonstrate the benefits of combined use of bronchodilators that act by different mechanisms. In addition to the greater improvements with FORM + TIO than TIO alone in the primary efficacy variable (normalized FEV1 AUC0–4h), significantly greater increases in trough FEV1 levels were seen with FORM + TIO versus TIO alone at all study visits. Furthermore, greater improvements in symptom scores and other patient-centered outcomes with FORM + TIO versus TIO were sustained over the 12-week treatment period. These results clearly show that FORM + TIO have additive effects when used in combination. This additivity is unlikely to be due simply to submaximal bronchodilator effects of each agent when used alone, but is more likely the result of different mechanisms of bronchodilator action of the two different drugs, since previous dose-response studies have shown that the individual doses of formoterol and tiotropium that were used in the present study produced maximal or near-maximal bronchodilatation (Citation[21], Citation[22]).
Both the SGRQ symptom scores and diary recorded symptom scores were significantly improved by FORM + TIO treatment compared with TIO alone. However, it is unclear why the TDI improvements did not differ between the two treatment groups. The TDI was measured by the method developed by Mahler and is graded by observers of the patient (Citation[18]). This version of TDI assessment is not as standardized as the automated version and may be affected by testing center and observer variability. This variability could contribute to the lack of statistically significant improvements. It is notable that although improvements in the SGRQ total score in response to FORM + TIO did not reach statistical significance compared with TIO, a clinically meaningful change of > 4 was observed. A longer time period of treatment may be necessary to achieve statistical significance.
Although a 50% increase in the rate of exacerbations was noted in the FORM + TIO group compared with TIO alone (Citation[21] vs 14 patients, respectively), the difference was not statistically significant. Moreover, no conclusions can be drawn from these observations because the 3-month study duration was too short to evaluate exacerbations adequately. We evaluated a more comprehensive range of treatment effects, including patient-centered outcomes, and over a longer period compared with 5 previously published studies of FORM + TIO (Citation[12], Citation[14], Citation[15], Citation[16], Citation[17]). This included one 6-week study that evaluated FORM administered via nebulizer (Citation[17]). In all 5 of the previously published studies, significantly greater improvements in lung function were found with FORM + TIO compared with at least one of the individual agents to which the combination was compared. Notably, one of these was a 3-day study that compared the acute bronchodilator effects of single doses of FORM + TIO and TIO alone in 20 outpatients with stable COPD. The study found that the increase in FEV1 10 minutes postdose was significantly greater with FORM + TIO than with TIO alone (p < 0.02) (Citation[14]). Indeed, the present study found that superior improvement in FEV1 with FORM + TIO vs TIO after the first dose was seen within 5 minutes postdose and sustained thereafter. Furthermore, spirometric improvements were accompanied by reductions in AM and PM symptoms that were significantly greater with FORM + TIO versus TIO at most study weeks, as well as improvements in the SGRQ symptom scores that were greater with FORM + TIO compared with TIO alone. Thus, patients not only exhibited improvements in key metrics of pulmonary function but, most importantly, patients on FORM + TIO experienced improvements in symptoms that were significantly greater than those reported by patients on TIO alone. Furthermore, use of AM and PM rescue medication was lower in patients using FORM + TIO than patients using TIO alone at all study weeks, with significantly greater reductions with FORM + TIO versus TIO alone at some study weeks. The reductions in rescue medication use with FORM + TIO were greater during the day than during the night, suggesting that FORM + TIO patients had less need for rescue medication when pursuing their daily activities than those treated with TIO alone.
The present study is limited by the lack of a FORM + placebo group, because TIO-matched placebo inhalation capsules were not available. Therefore, because all patients received TIO, it was not possible to evaluate the effects of FORM alone or determine if the improvements associated with FORM treatment were greater than those associated with TIO treatment. Placebo-controlled studies of FORM and TIO monotherapy lasting for up to 1 year have demonstrated that both agents significantly improve expiratory lung function, reduce COPD symptoms and rescue albuterol use, and improve health status as measured by the SGRQ (Citation[3], Citation[4], Citation[6], Citation[7]). Additional studies have demonstrated the faster onset of action with FORM vs TIO (Citation[11], Citation[14], Citation[15]).
Patients in a previous study that compared FORM + TIO with TIO alone completed a 2-week run-in on TIO before the study treatments were administered. This maneuver made it possible to establish a pharmacodynamic steady state of TIO and thereby discern an additional improvement of lung function achieved with FORM after a baseline improvement on TIO alone had been achieved (Citation[12]). In addition, 2 other previous studies evaluating combined treatment with FORM plus TIO or oxitropium showed that improvements were greater with FORM as the initial treatment compared with the anticholinergic agent as the initial treatment (Citation[11], Citation[23]). Our study expands on previous findings by demonstrating that administration of both agents together results in additive beneficial effects.
In conclusion, concurrent treatment with FORM + TIO demonstrated greater efficacy than TIO alone and was equally well tolerated. Significant differences between treatments were observed as early as 5 minutes postdose (the first scheduled measurement), and the improvements were sustained throughout the dosing interval. Furthermore, treatment with FORM + TIO preserved lung function and provided improvements in patient-centered health status over the entire study period.
ACKNOWLEDGMENTS
This work was supported by a grant from Schering Corporation, Kenilworth, NJ. The authors thank Weiyuan Chung, MSc, for statistical design and support, and Ken Kauffman, BSc, who provided editorial assistance. This assistance was funded by Schering-Plough. The authors would like to acknowledge specifically the contribution of Domenic Iezzoni, MD, to the development of the plan for this study, and to the preparation of this article. Dr. Iezzoni is to be respectfully remembered for the expertise, insight and enthusiasm he brought to this and many other scientific study projects at Schering-Plough.
Donald Tashkin, MD, has received industry sponsored grants, and advisory board and speaking engagement honoraria, from Schering-Plough. James Pearle, MD, has been an investigator in trials sponsored by Schering-Plough. Domenic Iezzoni, MD, and Santosh T. Varghese, MD, are both employees of Schering-Plough. This work was supported by a grant from Schering Corporation, Kenilworth, NJ. Editorial assistance was funded by Schering-Plough.
REFERENCES
- Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute. World Health Organization. 2006
- Casaburi R, Briggs D D, Jr., Donohue J F, Serby C W, Menjoge S S, Witek T J, Jr. The spirometric efficacy of once-daily dosing with tiotropium in stable COPD: a 13-week multicenter trial. The US Tiotropium Study Group. Chest 2000; 118: 1294–1302
- Casaburi R, Mahler D A, Jones P W, Wanner A, San P G, ZuWallack R L, Menjoge S S, Serby C W, Witek T, Jr. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19: 217–224
- Tashkin D, Kesten S. Long-term treatment benefits with tiotropium in COPD patients with and without short-term bronchodilator responses. Chest 2003; 123: 1441–1449
- Dahl R, Greefhorst L A, Nowak D, Nonikov V, Byrne A M, Thomson M H, Till D, Della Cioppa G. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 778–784
- Rossi A, Kristufek P, Levine B E, Thomson M H, Till D, Kottakis J, Della Cioppa G. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest 2002; 121: 1058–1069
- Rennard S I, Anderson W, ZuWallack R, Broughton J, Bailey W, Friedman M, Wisniewski M, Rickard K. Use of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 1087–1092
- Mahler D A, Donohue J F, Barbee R A, Goldman M D, Gross N J, Wisniewski M E, Yancey S W, Zakes B A, Rickard K A, Anderson W H. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115: 957–965
- Tashkin D P, Cooper C B. The role of long-acting bronchodilators in the management of stable COPD. Chest 2004; 125: 249–259
- Richter K, Stenglein S, Mucke M, Sieder C, Schmidtmann S, Harnest U, Weidinger G, Magnussen H. Onset and duration of action of formoterol and tiotropium in patients with moderate to severe COPD. Respiration 2006; 73: 414–419
- van Noord J A, Aumann J L, Janssens E, Verhaert J, Smeets J J, Mueller A, Cornelissen P J. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest 2006; 129: 509–517
- Rennard S I, Stoner J A. Challenges and opportunities for combination therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2: 391–393, discussion 394–395
- Cazzola M, Di Marco F, Santus P, Boveri B, Verga M, Matera M G, Centanni S. The pharmacodynamic effects of single inhaled doses of formoterol, tiotropium and their combination in patients with COPD. Pulm Pharmacol Ther 2004; 17: 35–39
- van Noord J A, Aumann J L, Janssens E, Smeets J J, Verhaert J, Disse B, Mueller A, Cornelissen P J. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J 2005; 26: 214–222
- Di Marco F, Verga M, Santus P, Morelli N, Cazzola M, Centanni S. Effect of formoterol, tiotropium, and their combination in patients with acute exacerbation of chronic obstructive pulmonary disease: a pilot study. Respir Med 2006; 100: 1925–1932
- Tashkin D P, Littner M, Andrews C P, Tomlinson L, Rinehart M, Denis-Mize K. Concomitant treatment with nebulized formoterol and tiotropium in subjects with COPD: A placebo-controlled trial. Respir Med 2008; 102: 479–487
- Mahler D A, Weinberg D H, Wells C K, Feinstein A R. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984; 85: 751–758
- Jones P W, Quirk F H, Baveystock C M. The St George's Respiratory Questionnaire. Respir Med 1991; 85(Suppl B)25–31, discussion 33–37
- Donohue J F. Minimal clinically important differences in COPD lung function. COPD 2005; 2: 111–124
- Littner M R, Ilowite J S, Tashkin D P, Friedman M, Serby C W, Menjoge S S, Witek T J, Jr. Long-acting bronchodilation with once-daily dosing of tiotropium (Spiriva) in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 1136–1142
- Cazzola M, Centanni S, Regorda C, di Marco F, di Perna F, Carlucci P, Boveri B, Santus P. Onset of action of single doses of formoterol administered via Turbuhaler in patients with stable COPD. Pulm Pharmacol Ther 2001; 14: 41–45
- Cazzola M, Di Perna F, Califano C, Vinciguerra A, D'Amato M. Incremental benefit of adding oxitropium bromide to formoterol in patients with stable COPD. Pulm Pharmacol Ther 1999; 12: 267–271