Abstract
Bronchiectasis is a heterogeneous disorder with a large number of etiologic factors. The main symptom is a chronic productive cough. The aim of this study was to describe the phenotypes of patients with bronchiectasis who had developed a chronic productive cough in childhood (before 16 years of age) compared with those who had developed a productive cough as adults. One hundred and eighty-two subjects with bronchiectasis diagnosed by computed tomography scanning were studied. Subjects all had a detailed clinical review and assessment of potential etiologic factors performed by the investigators. There were 107 (59%) subjects who developed a chronic productive cough in childhood and 75 (41%) subjects who developed a chronic productive cough in adulthood. There were significant differences in a number of parameters between the two groups including duration of cough, frequency of exacerbations, presence of rhinosinusitis, crackles on examination and lung function. The adult group could be further divided into those who had developed a cough whilst smoking and those who had no obvious relationship with smoking. In conclusion there were a number of significant differences between the child onset and adult onset group that may reflect different phenotypes of bronchiectasis.
Keywords:
INTRODUCTION
Bronchiectasis is characterized by chronic bronchial dilatation. Chronic inflammation that is thought to arise predominantly from the interaction between bacterial infection and the host immune response results in this airway change. The widespread availability of high resolution computed tomography (HRCT) scanning has made the diagnosis of bronchiectasis much easier and led to an increased awareness. At least 110,000 adults in the United States have bronchiectasis (Citation[1]). Two studies have described the incidence of bronchiectasis in chronic obstructive pulmonary disease (COPD) as being 29% (Citation[2]) and 50% (Citation[3]).
Bronchiectasis is generally classified by the likely cause and a large number of etiologic factors have been described (Citation[4], Citation[5]). Many of these factors are associative rather than definitive causes. However the identification of a specific etiology for bronchiectasis (such as cystic fibrosis, hypogammaglobulinaemia, allergic bronchopulmonary aspergillosis (ABPA) and foreign body aspiration) may have implications both for patient management and prognosis.
Recent studies have emphasized that patients with bronchiectasis have a gradual decline in lung function with progressive airway obstruction and develop clinical deterioration over a number of years (6–8). The dominant symptom in most subjects is a chronic productive cough (Citation[9], Citation[10]), which may have been present for many years before a diagnosis is made . When patients actually develop the radiologic features of bronchiectasis after the onset of symptoms is generally not known. The most common pattern described in the literature is a chronic productive cough arising in childhood (Citation[9], Citation[10]).
The authors have been studying bronchiectasis for a number of years. Recently we have been seeing an increasing proportion of subjects who developed symptoms, principally chronic sputum production as adults. The aim of this study was to assess whether adult subjects with bronchiectasis could be differentiated into 2 different phenotypic groups (childhood onset and adult onset) based on the time of onset of their productive cough (defined as productive cough on most days for at least 6 months).
We studied a cohort of 182 adult patients with radiologically confirmed bronchiectasis. Subjects were divided into childhood onset cough and adult onset cough groups. Subjects had a detailed assessment for clinical features and etiologic factors in which they had a clinical interview and examination performed by one of the investigators.
METHODS
Study subjects
A series of 195 adult patients with bronchiectasis was seen at Monash Medical Centre (MMC)/Southern Health between 1998 and 2007 by a respiratory physician (PK). These subjects had all been seen previously by a respiratory physician at MMC/Southern Health (PK, MF and PH) with a total of over 1200 clinical reviews being performed and were well known to the respiratory physicians. The diagnosis of bronchiectasis was confirmed by high resolution CT scanning using standardized criteria. Thirteen of these subjects did not have a history of chronic productive cough (defined as productive cough on most days for at least 6 months) and were excluded from the group. This left a cohort of 182 subjects who were assessed for this project. Ethical approval for this study was obtained from the Southern Health Ethics Committee, Monash Medical Centre.
Patients had been treated with a regime of narrow spectrum antibiotics for exacerbations, chest physiotherapy, bronchodilators and vaccinations. Sixty-two patients (34%) were taking inhaled corticosteroids at the time of assessment and four subjects were taking systemic corticosteroids. All subjects were living independently in the community and were seen as outpatients. There was a low incidence of co-morbid disease with 16 subjects having asthma, 14 having ischemic heart disease and 17 having hypertension. Other conditions included diabetes (n = 7), osteoporosis (n = 7) and prostate cancer (n = 3). Co-morbid disease in this cohort was well controlled and did not appear to affect the course of bronchiectasis. Subjects did not have current major drug or alcohol issues and as far as could be ascertained social disadvantage had not been a major issue in childhood.
Methods
All subjects had a detailed clinical assessment (by PK) performed at Monash Medical Centre with a standard interview to record findings, screening for underlying causation, lung function and sputum analysis. Subjects were assessed when clinically stable (i.e., they were living independently when at their usual baseline state and had not had an exacerbation for at least one month).
Patients were asked about the presence, frequency of productive cough and age of onset of productive cough. Details were also obtained about sputum volume (sputum volume was estimated by comparison with a teaspoon (5 ml), tablespoon (15 ml), eggcup (50 ml) and teacup (150 ml), presence of rhinosinusitis, haemoptysis in the past 5 years, frequency of exacerbations (defined by a sustained worsening in their clinical state with at least one of: increase in sputum volume, dyspnoea or fever) and symptoms of dyspnoea using the Medical Research Council (MRC) dyspnoea grade. Subjects were asked about the initial onset of productive cough and progression of their symptoms from this time. Childhood respiratory disease, general health (including questioning about symptoms of autoimmune disease and arthritis) and tobacco consumption (age of commencement/cessation and pack-years) was noted. Findings on physical examination; crepitations, wheeze and clubbing were also recorded.
To assess for predisposing factors for bronchiectasis clinical details on family history, unexplained infertility, and asthma and examination findings were recorded. Subjects had blood taken for full blood examination, immunoglobulin levels (including subclasses), alpha1-antitrypsin levels, allergic bronchopulmonary aspergillosis (aspergillus precipitins, antibody and skin test reactions), neutrophil function (phagocytosis and oxidative burst), cystic fibrosis mutation analysis and lymphocyte subsets. Further investigations where clinically indicated included sweat test analysis (widespread severe bronchiectasis), ciliary function (done for unexplained infertility/family history), and testing for human immune deficiency virus (CD4 lymphocyte count below the normal range).
Subjects had spirometry performed for forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) at Monash Medical Centre using a Jaeger pneumotach system that was calibrated daily with a 2-litre syringe. At least 2 measurements after bronchodilator inhalation differing by less than 5% or 100 ml from each other had to be produced. The largest volume was used in this analysis. Patients were also asked to produce a sputum sample which was analysed by microscopy and culture. A satisfactory sample for sputum analysis was defined by the presence of at least 3+ polymorphs and < 3+ squamous epithelial cells as previously described (Citation[11]). Bronchiectasis was diagnosed by a consultant radiologist using standard criteria (Citation[12], Citation[13]) and scans were scored by a previously described method (Citation[6], Citation[10], Citation[14]).
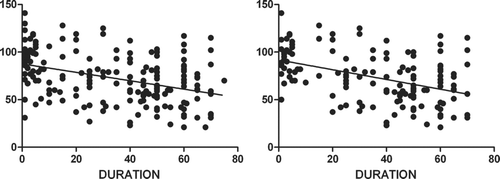
The FEV1 has been shown to be the most important measure of outcome in COPD (Citation[16]). FEV1 has not been reported in detail in the context of bronchiectasis but it is an important measurement of lung function in this condition. Linear regression was used to correlate the relationship between FEV1 and duration of chronic productive cough.
Statistical analysis
Comparisons between categorical variables used the Pearson's chi-squared statistic (or the Fisher's exact test for small samples). When comparing continuous outcome variables by categorical predictors, the Student's t-test was used (or its non-parametrical equivalent, the Wilcoxon rank-sum test). Linear regression was used to explore the relationship between FEV1 and duration of chronic productive cough with the Wald statistic. Two-tailed p-values of 0.05 or less were considered statistically significant.
RESULTS
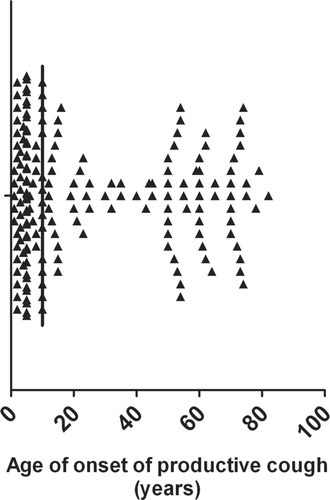
The cohort of 182 subjects had a mean age of 58 years and was predominantly female (64%). The median age of onset of productive cough was 10 years with an inter-quartile range of 5–52 years. There was also a bi-modal distribution of age onset with the onset of productive cough most common in the first 15 years of life followed by onset of productive cough in subjects over the age of 50. There were relatively few subjects who developed the onset of productive cough from the ages of 16 to 50 years ().
Figure 1 This figure demonstrates the age of onse of a productive cough in the bronchiectasis cohort. The median value is 10 years of age.
The cohort was separated into patients who had developed a chronic productive cough in childhood (i.e. before 16 years of age) and those who had developed a cough in adulthood (i.e., 16 years of age or older). One hundred and seven subjects (59%) had childhood onset and 75 subjects (41%) had adult onset. There were 13 subjects who were diagnosed as having bronchiectasis in childhood (10 with bronchography and 3 by pathology after lobectomy). The adult onset group was slightly older and had a higher proportion of males. Similar to previous studies most subjects had idiopathic disease (Citation[6], Citation[8], Citation[10], Citation[16]). In the childhood onset group 24% of subjects had a potentially causative factor identified including 13 post-infectious, 7 with IgG subclass deficiency, 4 with allergic bronchopulmonary aspergillosis, one with Kartagener's syndrome and one with Young's syndrome.
In the adult onset group 27% of subjects had an identifiable cause; there were 11 post-infectious, 4 with hypogammaglobulinaemia, 2 with IgG subclass deficiency, one with reflux, one with IgA deficiency and one with chronic lymphocytic leukaemia. No subject had cystic fibrosis (although there were 6 subjects with single CF mutations; all were sweat test negative). Several other potential etiologic factors were identified in the adult group. Four patients developed a cough whilst pregnant. Almost half of the adult group developed a chronic productive cough whilst smoking. The majority of patients in the adult onset group (62 patients or 83%) described the insidious onset of productive cough which persisted. Features of the patient group are listed in .
Table 1 Demographics of patient group
Clinical features of childhood onset and adult onset groups
The clinical features of both groups differed significantly for a number of parameters. The childhood onset group had a markedly longer duration of productive cough; median duration of 50 years compared with adult onset group with a median duration of 5 years (p < 0.001). The childhood onset group had a higher prevalence of rhinosinusitis, exacerbations per year and presence of crepitations. The prevalence of rhinosinusitis was almost 3 times higher in the childhood onset group (73% versus 27% in adult onset) and most of these subjects in the childhood onset group (62/78 patients or 79%) described awareness of this symptom before the age of 16 years (rhinosinusitis was diagnosed by combination of symptoms and changes on plain X-ray or CT of sinuses). Clinical features are listed in .
Table 2 Features of childhood onset and adult onset groups
Spirometry and CT findings in childhood onset and adult onset groups
The spirometry and CT findings of both groups also demonstrated significant differences. The FEV1 and FVC both in terms of absolute numbers and % predicted were significantly lower in the childhood onset group (p < 0.001). The numbers of lobes with evidence of bronchiectasis on HRCT scanning was slightly higher in the childhood onset group 2.50 ± 1.06 compared to 2.15 ± 1.09 in the adult group (p = 0.017). The HRCT score was higher in the childhood group 29 ± 17 compared to 20 ± 13 in the adult group (p < 0.001). This difference reflected a higher number of involved segments per lobe and the presence of cystic changes. Features are listed in .
Microbiologic findings in groups
Adequate sputum specimens for microbiologic analysis were obtained in 98 (92%) of the childhood onset group and 48 (65%) of the adult onset group. The adult group had a lower volume of sputum and this appeared to contribute to the difficulty in obtaining samples. Overall results were fairly similar in both groups. The most common pathogens isolated were Haemophilus influenzae and Pseudomonas aeruginosa (other pathogens isolated included; Moxarella catarrhalis, Streptococcus pneumoniae and non-tuberculus mycobacterium). There was a higher incidence of P. aeruginosa in the childhood group (11% versus 6%) and a higher incidence of no growth in the adult group (43% versus 30%) (p = 0.048). Results are shown in .
Table 3 Microbiologic findings in groups
Features of non-smokers and smokers in adult onset group
The adult onset group could be divided into subjects who developed a productive cough whilst smoking and those who had the onset of productive cough with no clear association with smoking. Forty-five subjects (60%) were classified in the non-smoking group and 30 subjects (40%) in the smoking group. The smoking group had a mean pack-year number of 34 ± 14. The onset of productive cough occurred 36 ± 8 years after the onset of smoking. There were features that were similar in both groups e.g. age, volume of sputum, frequency of exacerbations and lobar involvement but there were also some significant differences. Smoking subjects had a higher MRC dyspnoea scale; 2.5 ± 1.2 compared to 1.6 ± 1.0 (P < 0.001). This was also reflected in a lower % predicted FEV1 in smokers; 75 ± 26 compared to 91 ± 20 (P = 0.004). There was a higher incidence of rhinosinusitis in the non-smoking group (36% versus 13%, P = 0.037). Four of the subjects in the non-smoking group had a smoking history but all had stopped at least 15 years before the onset of productive cough. Results are listed in .
Table 4 Features of non-smokers and smokers in adult onset group
Relationship between duration of productive cough and FEV1
The differences in the childhood onset and adult onset group may be explained by the duration of productive cough. Lung function was inversely related to length of chronic cough (−0.44; 95% CI −0.59, −0.29; p < 0.001) %predicted FEV1 per year of chronic cough (). This relationship was strengthened when participants with a history of smoking were removed (, −0.51 % predicted FEV1 per year of chronic cough, 95% CI −0.69, −0.33; p < 0.001). However, the data demonstrated that the relationship was only partial with the results showing a broad range between the duration of productive cough and the decline in FEV1 (rather than demonstrating a tight clustering).
DISCUSSION
In this study we described the features of 182 adult subjects who had either developed a chronic productive cough in childhood or adulthood. There were significant differences in a number of parameters between the childhood onset and adult onset group. The adult onset group could be subdivided into those who were non-smokers and smokers. The duration of productive cough was significantly associated with decline in FEV1. The clinical, spirometry and micobiologic findings of the whole cohort in this study are similar to recent studies (Citation[7], Citation[10], Citation[17]).
The clinical features of the childhood onset and adult onset groups differed for several factors. The median duration of productive cough was 10-fold longer in the childhood onset group. The volume of daily sputum production was also higher in the child hood onset group but this did not achieve statistical significance. The prevalence of haemoptysis showed a trend to be higher in the childhood onset group which may reflect increased airway inflammation. The incidence of rhinosinusitis was almost 3-fold higher in the childhood group. Rhinosinusitis has not been commonly recognized as a symptom in bronchiectasis but several previous studies have reported rates of between 50 and 70% (Citation[8], Citation[9]). In most cases patients were aware of rhinosinusitis symptoms commencing in childhood generally about the same time as a productive cough; suggesting a generalised abnormality of the respiratory tract. The childhood onset group had more than three times the prevalence of crepitations which may reflect the increased sputum production that was noted.
The adult onset cough group could be divided into 2 subgroups: those who had developed a cough in association with chronic smoking and non-smokers. Previous literature has highlighted many smokers with COPD have associated bronchiectasis (Citation[2], Citation[3]) although the phenotypic features have not been clearly described. The 2 groups were fairly similar in a number of characteristics. The smoking group had worse lung function and this was reflected in a higher MRC dyspnoea score. In subjects with bronchiectasis it is often difficult to clearly establish the role of potential etiologic factors. Of the 30 smokers only 2 had another identifiable cause of bronchiectasis (hypogammaglobulinaemia and low IgA) and subjects developed the onset of productive cough after smoking for a number of years. None of the other 152 subjects had developed a productive cough whilst smoking (3 of the subjects who had child-onset productive cough smoked as adults (duration of smoking from 5–15 years) but this had no clear effect on symptoms). There is also considerable overlap between COPD and bronchiectasis.
An important issue in this cohort was the relationship between the duration of productive cough and the features of bronchiectasis. Recent studies have emphasized that most subjects appear to gradually deteriorate both in respect to clinical features and lung function (Citation[6], Citation[8], Citation[10], Citation[16], Citation[18], Citation[19]). In this cohort there was a significant relationship between duration of cough and severity of bronchiectasis. However this only partly explained the difference between the 2 groups. The relationship between the onset of productive cough and the actual development of radiologically detectable bronchiectasis could not be determined in this group, nor has it to the authors' knowledge been done in any other studies. The great majority of the adult subjects in this group developed the insidious onset of productive cough rather than having a clearly identifiable acute event (e.g., severe pneumonia). This suggests that subjects develop low-grade respiratory tract infection that results in persistent airway colonization and inflammation eventually resulting in destructive pulmonary disease as has been described in the “vicious cycle hypothesis” (Citation[20]).
Recently Chang et al. have described the condition of persistent bacterial bronchitis (PBB) in children who develop an isolated productive cough of more than 4 weeks in duration that resolves with antibiotic treatment (Citation[21], Citation[22]). Whether this entity develops into bronchiectasis is not known but it certainly may be a precursor condition.
A major problem in the study of bronchiectasis in adulthood is the heterogeneity of this condition. The pathogenesis is poorly understood and there are a large number of etiologic factors. It is often hard to definitively establish the actual importance of a potential etiologic factor in the development of bronchiectasis. The high prevalence of idiopathic disease in recent studies (Citation[6], Citation[8], Citation[10], Citation[16]) including the current one reflects the current lack of knowledge. Arguably the most definitive study of the aetiology of bronchiectasis was performed by Pasteur et al. who described 53% of a population having idiopathic disease compared to the current study with an incidence of 75% of idiopathic disease (Citation[10]). The main difference between the 2 studies was the significantly higher report of post-infective disease in the study of Pasteur (29% versus 13%). In our investigation we did not study production of specific antibody to bacterial pathogens as done by Pasteur but we did measure neutrophil response to bacterial pathogens, lymphocyte subsets and complement which has not been done by most other investigators. The current study suggests that smoking should also be considered as an important etiologic factor.
The childhood group may have an intrinsic susceptibility of the respiratory tract to develop infection which could also be reflected by the high incidence of rhinosinusitis. The immature immune system in childhood particularly in the first 5 years of life may explain the susceptibility of the subjects to develop persistent respiratory tract sepsis. A further modifying factor may be chronic inflammation occurring in the developing lung.
The onset of chronic productive cough was relatively rare between the ages of 16–50 years. This finding suggests that immune function is optimal over that time and can compensate for any deficiency that may have caused problems in childhood. Field in her large prospective study of bronchiectasis patients (Citation[23]) described that as children became adults their symptoms improved regardless of treatment. Immune function has been well recognized to decline with age starting from about 50 (Citation[24]) and this phenomenon may explain the development of productive cough and bronchiectasis in older adults.
The division of subjects into childhood and adult onset cohorts is arbitrary. The age of 16 to divide the groups was chosen as this cohort was predominantly female and only one subject had the onset of a productive cough between 16 and 18 year of age. Subjects in this study were very well known to the investigators and they were asked multiple times about the onset of a productive cough. Subjects who could not give a clear history were excluded from the study. Recall bias is a limitation of this study.
A problem with studying bronchiectasis is that subjects have nearly always have had disease for a long time before they are studied and as such virtually every study of this condition has used retrospective recall of symptoms. Another issue is that bronchiectasis is heterogeneous and populations are likely to vary between individual studies. However this cohort represents a large study (by bronchiectasis standards) and results are likely to be applicable to other groups of subjects with bronchiectasis. There were generally many years between the onset of productive cough and the diagnosis of bronchiectasis in the childhood group; this probably reflects the lack of availability of CT (and difficulty in performing bronchography). The study may also be affected by survivor bias in which children with severe disease had premature mortality.
This study has demonstrated that there were significant differences in this cohort of bronchiectasis patient between subjects who developed a productive cough in childhood phenotypes which reflect different underlying pathological processes.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Health and Medical Research Council of Australia (NHMRC) to PK. The authors would like to thank the Respiratory Function Laboratory and the Department of Radiology at Monash Medical Centre/Southern Health.
REFERENCES
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med 2005; 4: 205–209
- O'Brien C, Guest P J, Hill S L, Stockley R A. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax 2000; 55: 635–642
- Patel I S, Vlahos I, Wilkinson T M, Lloyd-Owen S J, Donaldson G C, Wilks M, Reznek R H, Wedzicha J A. Bronchiectasis, exacerbation indices and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170: 400–407
- Barker A F. Bronchiectasis. N Engl J Med 2002; 346: 1383–1393
- King P, Holdsworth S, Freezer N, Holmes P. Bronchiectasis. Intern Med J 2006; 36: 729–737
- King P T, Holdsworth S R, Freezer N J, Villanueva E, Gallagher M, Holmes P W. Outcome in adult bronchiectasis. COPD: J Chron Obstruct Pulmona Dis 2005; 2: 27–34
- Martinez-Garcia M A, Soler-Cataluna J J, Perpina-Tordera M, Roman-Sanchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007; 132: 1565–1572
- Tsang K W, Tipoe G L. Bronchiectasis: Not an orphan disease in the east. Int J Tuberc Lung Dis 2004; 8: 691–702
- King P T, Holdsworth S R, Freezer N J, Villanueva E, Holmes P W. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med 2006; 100: 2183–2189
- Pasteur M C, Helliwell S M, Houghton S J, Webb S C, Foweraker J E, Coulden R A, Flower C D, Bilton D, Keogan M T. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000; 162: 1277–1284
- King P T, Holdsworth S R, Freezer N J, Villanueva E, Holmes P W. Microbiologic follow-up study in adult bronchiectasis. Respir Med 2007; 101: 1633–1638
- McGuinness G, Naidich D P, Leitman B S, McCauley D I. Bronchiectasis: Ct evaluation. AJR Am J Roentgenol 1993; 160: 253–259
- McGuinness G, Naidich D P. Ct of airways disease and bronchiectasis. Radiol Clin North Am 2002; 40: 1–19
- Reiff D B, Wells A U, Carr D H, Cole P J, Hansell D M. Ct findings in bronchiectasis: Limited value in distinguishing between idiopathic and specific types. A J R 1995; 165: 261–267
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977; 1: 1645–1648
- Twiss J, Stewart A W, Byrnes C A. Longitudinal pulmonary function of childhood bronchiectasis and comparison with cystic fibrosis. Thorax 2006; 61: 414–418
- Nicotra M B, Rivera M, Dale A M, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest 1995; 108: 955–961
- Sheehan R E, Wells A U, Copley S J, Desai S R, Howling S J, Cole P J, Wilson R, Hansell D M. A comparison of serial computed tomography and functional change in bronchiectasis. Eur Respir J 2002; 20: 581–587
- Evans S A, Turner S M, Bosch B J, Hardy C C, Woodhead M A. Lung function in bronchiectasis: The influence of pseudomonas aeruginosa. Eur Respir J 1996; 9: 1601–1604
- Cole P J. Inflammation: A two-edged sword–the model of bronchiectasis. Eur J Respir Dis Suppl 1986; 147: 6–15
- Marchant J M, Masters I B, Taylor S M, Chang A B. Utility of signs and symptoms of chronic cough in predicting specific cause in children. Thorax 2006; 61: 694–698
- Chang A B, Landau L I, Van Asperen P P, Glasgow N J, Robertson C F, Marchant J M, Mellis C M. Cough in children: Definitions and clinical evaluation. Med J Aust 2006; 184: 398–403
- Field E. Bronchiectasis: A long-term follow-up of medicial and surgical cases from childhood. Arch Dis Child 1961; 36: 587–603
- Weksler M E, Szabo P. Aging and the immune system. Clinical Immunology; Principles and Practice, R R Rich, T A Fleicher, W T Shearer, B L Kotzin, H W Schroeder. Mosby, London 2001; 41.41–48