ABSTRACT
This retrospective cohort study aimed to assess treatment patterns over 24 months amongst patients with chronic obstructive pulmonary disease (COPD), initiating a new COPD maintenance treatment, and to understand clinical indicators of treatment change. Patients included in the study initiated a long-acting β2-agonist (LABA), a long-acting muscarinic antagonist (LAMA), or a combination of LABA and an inhaled corticosteroid (ICS/LABA) between January 1, 2009, and November 30, 2013, as recorded in the United Kingdom Clinical Practice Research Datalink (UK CPRD). Treatment modifications (switching or adding maintenance treatments) over 24 months were assessed, and patient characteristics, disease burden, medication and healthcare resource use during the 30 days before treatment modification were evaluated. The cohort comprised 17,258 patients [LABA (8%), LAMA (39%) and ICS/LABA (54%)] with similar age, body mass index and dyspnoea distribution. LABA users were more likely than LAMA users to add a maintenance therapy. Distinct patterns of treatment augmentations were noted, whereby LABA users typically received dual therapy before moving to triple therapy, while LAMA users moved to triple therapy by directly adding an ICS/LABA. Exacerbation events immediately prior to treatment change were not frequently recorded; however, the need for rescue short-acting medication and assessment of dyspnoea in the 30 days prior to the treatment change suggest that dyspnoea is a remaining unmet need driving therapy change.
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality and morbidity worldwide and is considered a treatable disease Citation(1). Guidelines consider inhaled long-acting bronchodilator therapy convenient and more effective in maintaining symptom relief than short-acting bronchodilators (SABDs) Citation(1,2). Maintenance treatment with either a long-acting muscarinic antagonist (LAMA) or a combination of inhaled corticosteroids (ICS) and long-acting β2-agonist (LABA) is recommended for patients with more symptoms and higher risk of exacerbations. For those with persistent symptoms, the stepwise escalation to inhaled triple therapy (ICS/LABA/LAMA) is recommended. This combination improves lung function and quality of life and may further reduce exacerbations Citation(1).
The goal of treating COPD is to reduce symptoms; increase exercise tolerance and health status; and to reduce future risk of exacerbations, disease progression and mortality. Any change in therapy (escalation/addition) is ideally driven by persistence or worsening of symptoms, loss of functionality or health status and the need to reduce the future risk of exacerbations or mortality. Although all inhaled monotherapy and combination therapies have shown improvements in lung function, quality of life and exacerbations Citation(3), results from a primary care study have indicated that patients with COPD treated with long-acting bronchodilators alone continue to experience significant dyspnoea on exertion, exacerbations and reduced quality of life indicative of suboptimal control Citation(4). This may be attributable to either a lack of appropriate maintenance treatment or the suboptimal implementation of COPD guidelines in primary care clinical practice Citation(5–9). In constrast, deviation from guidelines may also result in overtreatment, particularly with inhaled corticosteriods, which can carry increased risks of side effects and also incur considerable cost to the healthcare system Citation(10).
Therefore, it is crucial to understand the characteristics of patients when presenting in a prodrome phase prior to a modifying therapy event and after initiation of a first maintenance treatment. Considering the current scenario of scant literature around disease burden and the economic impact of nonadherence to guidelines, evaluation of the switching and escalation of an initial long-acting bronchodilator treatment are likely to provide important new insights on clinical events associated with such treatment change. The current study aimed to evaluate 1) treatment modifications over 24 months amongst patients with COPD, initiating long-acting bronchodilator maintenance treatment, either alone or in combination with ICS; and 2) the drivers of treatment modification, as measured by clinical indicators such as exacerbations, non-COPD hospitalisations, oral corticosteroid use, SABD prescriptions, dyspnoea assessments, or spirometry measures recorded in the 30 days before treatment modification.
Materials and Methods
Study design
This retrospective cohort study evaluated patient characteristics and treatment patterns over 24 months amongst patients with COPD who were newly prescribed a COPD maintenance treatment including a LABA, LAMA, or ICS/LABA from the United Kingdom Clinical Practice Research Datalink (UK CPRD). The database contains longitudinal data on patient characteristics; medical history, including records of referrals to consultants and hospitalisations; and treatment history Citation(11). The date of the first qualifying prescription from January 1, 2009, to November 30, 2013, was identified as the index date. The electronic records were anonymised, and the protocol was approved by the UK CPRD Independent Scientific Advisory Committee (ISAC; protocol 13_073a2).
Patients were required to be aged ≥40 years and to have at least one COPD “definite” diagnostic code within ±12 months of the index date, have no history of an ICS prescription in the 12 months before the index date (LABA and LAMA groups only), and have at least 12 months of history before and 24 months of follow-up after the index date (unless death occurred).
All statistical analyses were conducted using SAS version 9.2. Mantel-Haenszel chi-square tests for categorical variables and analysis of variance for testing differences in mean age and mean body mass index were used with two-tailed p-values <0.05 indicating statistical significance.
Baseline characteristics
Patient demographics and disease characteristics, including Medical Research Council (MRC) dyspnoea grade and COPD exacerbations in the 12 months before the index date, were collected and compared between initial maintenance treatment groups. Exacerbations were identified using an algorithm recently validated in the UK CPRD Citation(12). Briefly, a moderate exacerbation was defined using a combination of medical diagnosis of COPD exacerbation and/or treatment with COPD-specific antibiotics combined with oral corticosteroids (OCSs) (treated outside the hospital), while a severe exacerbation was one resulting in hospitalisation or emergency department visit for COPD.
Treatment modifications during follow-up
In the 24-month follow-up period, treatment modifications, including first switch in treatment and all augmentations with other maintenance therapy, were recorded for the LABA, LAMA and LABA/ICS subgroups.
A ‘switch’ was recorded when another maintenance therapy was started after the index therapy was discontinued, or if it was initiated before index therapy discontinuation, and persisted for at least 60 days after index therapy discontinuation. Additions to treatment included those that resulted in dual therapy (ICS/LABA, ICS/LAMA or LABA/LAMA) or triple therapy (ICS/LABA/LAMA) and were considered when the new maintenance therapy was started more than 30 days after the index date and overlapped for at least 30 continuous days with the index therapy. If there were less than 30 days of continuous overlap but the patient had a future prescription for the add-on therapy, this was also classified as an augmentation. Switches and augmentations were considered separately, such that a patient could be included in both analyses if they met the appropriate definitions.
Patient characteristics, MRC dyspnoea grade, Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages of airflow obstruction, other COPD medication use, non-COPD hospitalisations, all-cause general practitioner (GP) interactions and COPD exacerbations in the 30 days before treatment modification were evaluated. These data were collected for each treatment modification separately, with the date of the first switch or first addition acting as the anchor for each analysis.
Results
Patient disposition and demographics
The cohort comprised 17,258 patients [LABA (8%), LAMA (39%) and ICS/LABA (54%)]. Patients on LAMA were slightly more likely to be male, older and a current smoker (). The three groups did not differ significantly on dyspnoea score; however, patients initiating ICS/LABA were more likely to have experienced a COPD exacerbation in the 12 months prior to the index date. Further details of patient and disease characteristics, adherence to therapy and costs for the same cohort are presented in a previous publication Citation(13). A total of 141 (11%) LABA initiators, 524 (8%) LAMA initiators and 1,733 (19%) ICS/LABA initiators did not receive another prescription for COPD maintenance therapy of any kind after their index prescription throughout the 24-month follow-up period and thus were excluded from the treatment modification analysis.
Table 1. Patient demographics prior to long-acting bronchodilator initiation.
Treatment modifications
Treatment modifications amongst LABA initiators
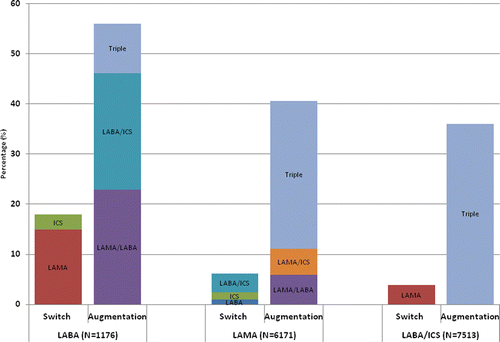
Amongst LABA users, 18% (N = 211) switched therapy during 24 months of follow-up (); the mean time to first switch was 271 days [standard deviation (SD), 187 days]. Switching to a LAMA was the most common option (83%), while the remaining switches were to ICS monotherapy. Further, 35% (N = 410) of LABA users experienced a total of 674 treatment augmentations (patients were allowed to contribute more than one). All 410 augmenters experienced one or more occasions of stepping up to a dual therapy (LABA/LAMA or ICS/LABA), while only 10% (N = 117) escalated to triple therapy through a dual pathway (). The mean time to first dual therapy was 262 (SD, 196) days, while escalation to triple therapy was, on average, a later event with a mean time of 347 (SD, 195) days.
Figure 1. Treatment augmentation and switches within 24 months of initiation of a first maintenance COPD therapy with LABA, LAMA or ICS/LABA. Note: This analysis excluded 141 LABA initiators, 323 LAMA initiators and 1733 ICS/LABA initiators who did not receive any COPD maintenance therapy prescription either replacing initiation therapy or adding a different kind of therapy after their index prescription throughout the 24-month follow-up period. In total, 18% (N = 211) of LABA users switched therapy and 35% (N = 410) augmented therapy (patients could contribute multiple augmentations, and thus, the height of the bar is greater than 35%). In total, 6% (N = 383) of LAMA users switched therapy, 37% (N = 2306) augmented therapy (patients could contribute multiple augmentations, and thus, the height of the bar is greater than 37%). In total, 4% (N = 287) of LABA/ICS users switched therapy (only switches to LAMA were considered as a change to LABA alone or ICS alone was considered a discontinuation of LABA/ICS combination therapy), 36% (N = 2708) augmented to triple therapy. COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
Treatment modifications amongst LAMA initiators
A small proportion of LAMA users (6%, N = 383) switched therapy within 24 months of follow-up, with a mean time to first switch of 274 (SD, 211) days (). Switching to ICS/LABA was the most common option (62%) with switches to LABA (16%) or ICS (22%) monotherapy occurring about equally. In addition, 37% of LAMA users (N = 2,306) experienced 2,522 augmentations, with the majority of augmentation events (72%) being direct escalations to triple therapy by adding ICS/LABA to their existing LAMA therapy (). Overall, the mean time to first dual therapy [271 (SD, 197) days] was similar to the mean time to first triple therapy [285 (SD, 202) days].
Treatment modifications amongst ICS/LABA initiators
Four percent (N = 287) of ICS/LABA users switched therapy to a LAMA within 24 months of follow-up, with a mean time to first switch of 259 (SD, 189) days (). Amongst ICS/LABA users, 36% (N = 2,708) escalated to triple therapy with a mean time of 282 (SD, 201) days.
Clinical events and healthcare resource utilisation in the 30 days prior to treatment modification
A majority of patients (88–94%) initiating any of the three maintenance therapies had a GP visit recorded in the 30 days immediately prior to switching or adding medications (). At least half of all patients (55–64%) also had an SABD prescription, with the exception of patients switching from ICS/LABA to LAMA (46% with a SABD prescription). Regarding the clinical assessment of patient symptoms, an MRC was recorded for about one-third of patients (32–41%), while 11–17% had a spirometry reading recorded. Amongst patients that had received these assessments, those stepping up to triple therapy, regardless of initial treatment group, were more likely to have a record of moderate to severe dyspnoea (MRC grade ≥ 3) or an FEV1 of <50% predicted. Exacerbations of COPD (moderate to severe) in the 30 days prior to treatment change occurred in fewer than one quarter of patients and were the only clinical event studied that appeared to possibly discriminate modification choice. Amongst LABA initiators, 24% who stepped up to triple therapy vs. 12-15% who switched or added to a dual had experienced an exacerbation in the 30 days before treatment change. Similarly, 18% of LAMA initiators who stepped up to triple vs. 11-15% of dual or switchers experienced an exacerbation. This pattern was also seen for ICS/LABA (15% progressing to triple therapy vs 8% with a treatment switch). Serious medical events, including non-COPD hospitalisations (3–7% across groups) or a hospitalised COPD exacerbation (2–5%), occurred rarely.
Table 2. Disease burden and healthcare resource utilisation in the 30 days prior to a treatment modification amongst the LABA, LAMA and ICS/LABA cohorts.
Discussion
This study evaluated treatment modifications over 24 months following initiation of maintenance treatment with LABA, LAMA or ICS/LABA in patients with COPD. Subsequently, we assessed clinical events and healthcare resource use during the 30 days prior to treatment modification to see if it was possible to identify particular events that may have guided treatment change.
With regard to treatment patterns, these results demonstrated that amongst the monobronchodilator-only users (LABA or LAMA), additions to therapy were more common occurrences than switches. LAMA users were much more likely to escalate directly to triple therapy, possibly due to the ease of adding an ICS/LABA combination to a LAMA in one step, whilst for LABA users, escalation favoured a dual therapy intermediate step up with only 10% further progressing to triple therapy. Our results are similar to those of other studies conducted in the United Kingdom. In a newly diagnosed COPD patient population, Wurst et al. Citation(14) also showed that switches were less common than treatment additions, and that LAMA (32%) and ICS/LABA users (24%) were more likely to progress to triple therapy over 2 years than those initiating LABA monotherapy (6%). A study in the Optimum Patient Care Research Database suggested that 32% of all COPD patients progressed to triple therapy during follow-up (up to 10 years after their initial COPD diagnosis), and that the most common pathway was the addition of LAMA to ICS/LABA Citation(15). It will be of interest to understand if the recent emergence of fixed combination LAMA/LABA dual bronchodilator therapies (vilanterol/umeclidinium and indacaterol/glycopyrronium, aclidinium/formoterol and tiotropium/olodaterol) Citation(16–19) may have a major influence on treatment patterns in the future.
The GOLD Global Strategy suggests that disease monitoring, including symptom evaluation and objective measures of airflow limitation, should determine when to modify therapy Citation(1). Amongst patients with a treatment modification during follow-up, we attempted to understand if symptom measurement, number of SABD prescriptions for rescue bronchodilation or disease worsening markers provided any indication of a preference for a particular treatment modification (switch versus escalation). Results showed that exacerbation events were only a minor influence on treatment augmentation. By contrast, the need to prescribe short-acting rescue medication in addition to bronchodilator maintenance therapy and the assessment of dyspnoea, using the MRC, suggest ongoing symptomatic disease burden for many patients in the 30 days prior to an augmentation or a switch event. The propensity for physicians to augment treatment based on elevated symptoms highlights the need for additional bronchodilation currently fulfilled most often by escalation to triple therapy amongst LAMA initiators and the addition of LAMA or ICS or both amongst LABA initiators. This suggests that a high proportion of patients in GOLD Stages A and B (low risk, few/significant symptoms) who remain symptomatic are prescribed triple therapy without having experienced an exacerbation event. Indeed, Price and colleagues showed that 49% of patients in GOLD Stage 2 with no exacerbations in the previous year were prescribed ICS Citation(20). Alternatively, for patients displaying continued breathlessness but who are not exacerbating, a phenotype-based pharmacotherapeutic approach suggests that new fixed combination LAMA/LABA dual bronchodilator therapies may be a reasonable management option Citation(21,22). Whilst no such analysis regarding events immediately preceding treatment modification has been conducted to date in the United Kingdom, Price and colleagues showed little variability in the pathway to triple therapy based on patient history of exacerbation, mMRC score, or lung function at baseline Citation(15,20). Our findings are similar to those of a study conducted in the United States using insurance claims data, which also found little difference in the frequency of exacerbation events and all-cause hospitalisation between patients adding or switching from LAMA therapy (LABA analysis not conducted) Citation(23). Compared to the US study, however, we found a considerably higher recording of dyspnoea (approximately one-third with an MRC recording compared to 10–12% with an International Classification of Disease ([ICD]-9) code for shortness of breath in the US study), suggesting that symptom monitoring may be more frequent in UK general practice. However, it cannot be ruled out that this difference is simply due to variability in coding practices between electronic medical record and reimbursement-based insurance records schemes.
These data only allow generation of hypotheses about treatment decision-making, as the electronic medical record alone lacks a recording of the physician rationale behind medication changes. Our data on treatment patterns must be interpreted in the context of the 2 year follow-up, as more patients may have had a treatment change if followed longer. It has previously been shown that maximum modification to treatment is likely in the first 6 months of diagnosis (with 15–49% of patients changing their initial therapy) Citation(14); however, studies with longer follow-up have shown that some patients do not progress to triple therapy until 3–7 years after diagnosis Citation(15). Furthermore, while this study includes a large sample size with reasonable follow-up and is one of the few studies to make inroads towards understanding clinical events that occur proximal to treatment modifications, there are limitations inherent in the CPRD database with regard to studying treatment patterns and possible reasons for treatment change. First, the CPRD database represents patients treated in primary care, and thus, prescriptions or clinical events that occur in hospital and may affect GP decision-making about treatment plans are not necessarily collected. As well, CPRD provides data on prescribed, rather than dispensed, medications. Second, the occurrence of events in the period outside of the 30-day window may have had some bearing on these treatment changes; moreover, mild self-reported exacerbations not related to increasing healthcare resource use, and thus not collected in our analysis, may have influenced physician prescribing behaviours. Third, the lack of sufficient clinical data on patient symptomatology and severity of airflow obstruction may simply reflect a lack of recording the results of MRC dyspnoea evaluation or spirometry, and does not necessarily mean that these were not performed or used for clinical decision-making. Finally, the database is unlikely to capture patient-driven reasons for treatment modification, such as patient preference or ability to use certain devices or adverse drug reactions Citation(24). These final two points are considerable limitations and additional longitudinal research with detailed capture of patient symptoms and outcomes, as well as the patient voice in treatment decision-making, is warranted.
Conclusions
Overall, we identified a trend for physicians to preferentially escalate therapy over switching therapy for both LABA and LAMA initiators. Amongst those with a treatment addition, direct step up to triple therapy was common for those initiating LAMA therapy, while those starting LABA had a slower transition and tended to step through a dual therapy first. The choice to switch versus escalate therapy occurred largely in the absence of a marked difference in exacerbation incidence and was primarily associated with the need for rescue short-acting medication and assessment of dyspnoea in the 30 days prior to the treatment change. This suggests that dyspnoea is a remaining unmet need that may be a driver of therapy change in UK primary care.
Declaration of interest
Sarah H. Landis, Keele Wurst, and Yogesh S. Punekar are employees of GSK and hold stock shares at GSK. Hoa V. Le was an employee of GSK at the time of the study and own stock shares at GSK. Kerina Bonar is a complementary worker on assignment to (and paid by) GSK.
Acknowledgments
The authors thank Dr. Chi T.L. Truong for her clinical consultations for medical coding lists used in the study.
Funding
This study was funded by GlaxoSmithKline (WEUSKOP6976). All listed authors met the criteria for authorship set forth by the International Committee for Medical Journal Editors. Editorial support, in the form of development, assembling tables and figures, collating author comments, copyediting, fact checking and referencing was provided by Dr. Annirudha Chillar of Cactus Communications Inc., and was funded by GlaxoSmithKline.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2015. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Feb18.pdf
- National Institute for Healthcare and Excellence (NICE) Guidelines. Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management 2010. Available from: http://www.nice.org.uk/guidance/cg101
- Rosenberg SR, Kalhan R. An integrated approach to the medical treatment of chronic obstructive pulmonary disease. Med ClinNorth Am 2012; 96(4):811–826.
- Dransfield MT, Bailey W, Crater G, Emmett A, O'Dell DM, Yawn B. Disease severity and symptoms among patients receiving monotherapy for COPD. Prim Care Respir J 2011; 20(1):46–53.
- Diette GB, Orr P, McCormack MC, Gandy W, Hamar B. Is pharmacologic care of chronic obstructive pulmonary disease consistent with the guidelines? Popul Health Manage 2010; 13(1):21–26.
- Fitch K, Iwasaki K, Pyenson B, Plauschinat C, Zhang J. Variation in adherence with Global Initiative for Chronic Obstructive Lung Disease (GOLD) drug therapy guidelines: a retrospective actuarial claims data analysis. Curr Med Res Opin 2011; 27(7):1425–1429.
- Salinas GD, Williamson JC, Kalhan R, Thomashow B, Scheckermann JL, Walsh J, et al. Barriers to adherence to chronic obstructive pulmonary disease guidelines by primary care physicians. Int J Chron Obstruct Pulmon Dis 2011; 6:171–179.
- Jochmann A, Neubauer F, Miedinger D, Schafroth S, Tamm M, Leuppi JD. General practitioner's adherence to the COPD GOLD guidelines: baseline data of the Swiss COPD Cohort Study. Swiss Med Wkly 2010; 140:1–8. [ Epub ahead of print]
- Perez X, Wisnivesky JP, Lurslurchachai L, Kleinman LC, Kronish IM. Barriers to adherence to COPD guidelines among primary care providers. Respir Med 2012; 106(3):374–381.
- White P, Thornton H, Pinnock H, Georgopoulou S, Booth HP. Overtreatment of COPD with inhaled corticosteroids–implications for safety and costs: cross-sectional observational study. PLoS One 2013; 8(10):e75221. doi: 10.1371/journal.pone.0075221.eCollection2013.
- Clinical Practice Research Datalink (CPRD). Accessed October 4, 2012. Available from: http://www.cprd.com/home/.
- Rothnie KJ, Müllerová H, Hurst JR, Smeeth L, Davis K, Thomas SL, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS ONE 2016; 11(3):e0151357.
- Punekar YS, Landis S, Wurst K, Le H. Characteristics, disease burden and costs of COPD patients up to two years after initiation of long-acting bronchodilators in UK primary care. Respir Res 2015; 16:141. doi: 10.1186/s12931-015-0295-2.
- Wurst KE, Punekar YS, Shukla A. Treatment evolution after COPD diagnosis in the UK primary care setting. PloS One 2014; 9(9):e105296.
- Brusselle G, Price D, Gruffydd-Jones K, Miravitlles M, Keininger DL, Stewart R, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis 2015; 10:2207–2217.
- Buhl R, Maltais F, Abrahams R, Bjermer L, Derom E, Ferguson G, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4). Eur Respir J 2015; 45(4):969–979.
- Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med 2014; 2(6):472–486.
- Maleki-Yazdi MR KT, Richard N, Zvarich M, Church A. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir Med 2014; 108(12):1752–1760.
- Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J 2013; 42(6):1484–1494.
- Price D, West D, Brusselle G, Gruffydd-Jones K, Jones R, Miravitlles M, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis 2014; 9:889–904.
- Montuschi P, Malerba M, Santini G, Miravitlles M. Pharmacological treatment of chronic obstructive pulmonary disease: from evidence-based medicine to phenotyping Drug Discovery Today 2014; 19(12):1928–1935.
- Montuschi P, Ciabattoni G. Bronchodilating drugs for chronic obstructive pulmonary disease: current status and future trends. J Med Chem 2015; 58:4131–4164.
- Wurst KE, St Laurent S, Mullerova H, Davis KJ. Characteristics of patients with COPD newly prescribed a long-acting bronchodilator: a retrospective cohort study. Int J Chron Obstruct Pulmon Dis 2014; 9:1021–1031.
- Osman LM, Hyland ME. Patient needs and medication styles in COPD. Eur Respir Rev 2005; 14(96):89–92.

