ABSTRACT
This study aimed to explore the different pathogeneses of combined pulmonary fibrosis and emphysema (CPFE) from emphysema and pulmonary fibrosis. The levels of transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), Krebs Von Den Lungen-6 (KL-6), matrix metalloproteinase-9 (MMP-9), tissue inhibitors of metalloproteinases-1 (TIMP-1), cytokeratin 19 fragment (CYFRA21-1), squamous cell carcinoma antigen (SCC), and the telomerase activity in peripheral blood were measured in 38 CPFE patients, 50 pulmonary emphysema patients, and 34 idiopathic pulmonary fibrosis (IPF) patients. The results demonstrated that the levels of VEGF and TGF-β1 in IPF patients were significantly higher than those in emphysema patients (p < 0.05), and no significant differences were detected between CPFE patients and other two groups (p > 0.05). The levels of KL-6 and CYFRA21-1 in IPF patients were significantly higher than those in emphysema and CPFE patients (p < 0.05), and the latter had the similar levels (p > 0.05). Among the three groups, the levels of SCC, MMP-9, TIMP-1, MMP-9/TIMP-1 ratio, and telomerase activity were not different (p > 0.05). Our study showed that VEGF, TGF-β1, KL-6, and CYFRA21-1 may play a role in the pathogenesis of pulmonary fibrosis. The lower levels of KL-6 and CYFRA21-1 in CPFE patients may be one of the reasons why these patients develop emphysema on the basis of fibrosis.
Introduction
Emphysema and idiopathic pulmonary fibrosis (IPF) are two distinctive diseases with different clinical features, physiology and pathology. Emphysema is characterized by the loss of extracellular matrix and enlargement of pulmonary alveoli in pathology; however, IPF is characterized by accumulation of mesenchymal cells and extracellular matrix components in the lungs.
Traditionally, emphysema and IPF have been considered two distinct diseases. However, the coexistence of emphysema and cryptogenic fibrosing alveolitis was first reported in 8 patients by Wiggins et al. Citation(1) in 1990. In 2005, Cottin et al. Citation(2) named this disease as combined pulmonary fibrosis and emphysema (CPFE) for the first time. Since then, reports about CPFE have been increasing, among which most studies were retrospective clinical studies, and the reason why two opposite phenotypes can coexist in the same subject is still unresolved.
It is generally accepted that both emphysema and pulmonary fibrosis result from dysregulation of the synthesis and degradation of extracellular matrix proteins. Matrix metalloproteinases (MMPs) and the specific tissue inhibitors of metalloproteinases (TIMPs) are involved in the parenchymal destruction and repair processes. The development of emphysema is resultant to the matrix destruction due to the imbalance between proteases and anti-proteases. MMPs are also implicated in the pathogenesis of pulmonary fibrosis. In the early stage of pulmonary fibrosis, MMPs could facilitate the degradation of the basement membrane, and the inflammatory cell migration thereafter. In the late stage of pulmonary fibrosis, MMPs may participate in the process of repair Citation(3). These studies raise the possibility that MMPs and TIMPs may play a role in the pathogenesis of CPFE.
Both emphysema and IPF are regarded as diseases of accelerated aging. One of the hallmarks of aging is the progressive shortening of telomeres with increasing age. Telomeres are DNA-protein structures that protect chromosome termini from degradation. Telomerase is the enzyme complex that generates and maintains telomeres Citation(4). It was reported that specific mutations in the telomerase components resulted in IPF. However, several contradictory results were also published regarding its role in pulmonary fibrosis Citation(5,6). By far, little work about the telomerase role in emphysema is reported.
Transforming growth factor-β1 (TGF-β1), as the most important fibrogenic cytokine, is essential for the progression of pulmonary fibrosis. In emphysema, decreased TGF-β expression signaling could lead to increased MMP level and subsequent extracellular matrix degradation Citation(7). Recently, Lee et al. Citation(8) reported that TGF-β1 overexpression could generate complex phenotype of coexisting fibrosis and emphysema in studied animals.
Vascular endothelial growth factor (VEGF) is a potent growth factor that stimulates endothelial cell and type II cell growth and survival. There is growing evidence that human emphysema is associated with decreased VEGF gene expression Citation(9,10). The role of VEGF in pulmonary fibrosis is not clear yet, as contradictory reports that suggest either a protective or a harmful role are published Citation(11,12).
Current evidence indicates that IPF is an epithelial-driven disease. Alveolar epithelial cells produce mediators that induce the formation of fibroblast and myofibroblast foci. Krebs Von Den Lungen-6 (KL-6) Citation(13), cytokeratin 19 fragment (CYFRA21-1) Citation(14), and squamous cell carcinoma antigen (SCC) Citation(15) can be produced by alveolar epithelial cells. Moreover, serum KL-6 is a marker for interstitial pneumonia and reflects disease activity and prognosis Citation(13,16,17). So far, few studies investigate the role of CYFRA21-1 and SCC in IPF. Preliminary works show that the levels of CYFRA21-1 in serum and bronchoalveolar lavage fluid (BALF) Citation(18,19) and SCC in lung tissues are increased in IPF Citation(15). It is unclear if these three markers participate in the pathogenesis of CPFE.
In this study, we selected MMP-9, TIMP-1, TGF-β1, VEGF, KL-6, CYFRA21-1, SCC, and telomerase as pathological markers in an effort to explore the potential pathogenesis of CPFE by comparing these markers among patients with emphysema, IPF, or CPFE.
Methods
Subjects
Thirty-eight CPFE subjects were enrolled consecutively in the Department of Respiratory Medicine in Shanghai Sixth People's Hospital. CPFE was diagnosed according to the radiology criteria specified by Cottin et al. Citation(2). The first control group of emphysema consisted of 50 subjects. Emphysema phenotype was identified by a low attenuation area% of more than 5% in at least one of the six lung fields in high resolution computerized tomography (HRCT) according to the method of Ito et al. Citation(20). The second control group of 34 IPF subjects was recruited according to the ATS/ERS/JRS/ALAT consensus statement Citation(21).
Patients with connective tissue disease, occupational history, environmental exposure, or a history of drugs that resulted in interstitial lung diseases (ILD) were excluded.
This study was approved by Ethics Committee of Shanghai Sixth People's Hospital, and all the subjects provided written informed consent.
Measurement of TGF-β1, VEGF, KL-6, MMP-9, and TIMP-1
2 ml of peripheral venous blood was drawn into an ethylenediaminetetraacetic acid bottle, centrifuged at 1000 g for 15 minutes, and then, the plasma was separated and stored at −80°C before test. Serum samples were obtained from clotted blood following centrifugation at 1000 g for 15 minutes. The levels of VEGF in plasma and TGF-β1, KL-6, MMP-9, and TIMP-1 in serum were measured using an enzyme-linked immunosorbent assay (ELISA) kits (CUSABIO, Wuhan, China) according to the manufacturer's protocols.
Measurement of SCC and CYFRA21-1
Serum SCC level was measured with commercially available chemiluminescent microparticle immunoassay kit (Abbott, Sligo, Ireland). Serum CYFRA21-1 level was measured with commercially available electrochemiluminescence kit (Roche, Mannheim, Germany).
Telomerase activity assay
Lymphocytes in peripheral blood were isolated by density gradient centrifugation using Ficoll-Paque PREMIUM (GE, Uppsala, Sweden). Telomerase activity was assayed using a telomerase PCR ELISA kit (Roche, Mannheim, Germany) according to the manufacturer's recommendations. Negative controls were prepared at a high temperature (95°C, 10 min) before the incubation.
Statistical analysis
The normality of the variables was analyzed by Kolmogorov-Smirnov test. Based on the results, the Kruskal-Wallis test was used to compare the values of the different groups. In the case of a significant difference between the groups, intergroup comparisons were assessed by a nonparametric method using the Mann-Whitney U test. Correlations were examines by calculating the Spearman's correlation coefficient r. Statistical analysis was performed using SPSS 17.0 software. Statistical significance was defined by a p value of <0.05.
Results
Patient characteristics
The characteristics of patients in the three groups are shown in . The gender, age, and smoking history were matched among the groups (p > 0.05). The pulmonary function parameters differed significantly among the three groups (p < 0.05).
Table 1. Characteristics of the CPFE, emphysema, and IPF groups.
Comparison of TGF-β1, VEGF, KL-6, MMP-9, and TIMP-1 levels among patients with emphysema, CPFE, and IPF
As shown in , the levels of VEGF and TGF-β1 in IPF patients were significantly higher than those in emphysema patients (VEGF: p = 0.003; TGF-β1: p = 0.012). There were no significant differences in the levels of VEGF and TGF-β1 between CPFE patients and the other two groups (p > 0.05). The serum KL-6 level in IPF patients was significantly higher than those in emphysema patients (p = 0.000) and CPFE patients (p = 0.000). The serum KL-6 level was similar in emphysema patients and CPFE patients (p = 0.374). Among the three groups, the levels of MMP-9, TIMP-1, and MMP-9/TIMP-1 ratio were not different (p > 0.05) ().
Figure 1. The concentrations of VEGF (A), TGF-β1 (B) and KL-6 (C) in different groups. Data were expressed as scatterplots with median lines and interquartile range. CPFE: combined pulmonary fibrosis and emphysema. IPF: idiopathic pulmonary fibrosis. VEGF: vascular endothelial growth factor. TGF-β1: transforming growth factor-β1. KL-6: Krebs Von Den Lungen-6.
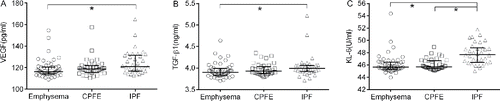
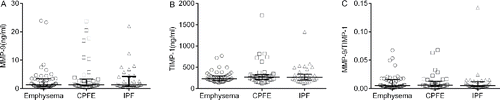
Figure 2. The concentrations of MMP-9 (A), TIMP-1 (B) and ratio of MMP-9/TIMP-1 (C) in different groups. Data were expressed as scatterplots with median lines and interquartile range. CPFE: combined pulmonary fibrosis and emphysema. IPF: idiopathic pulmonary fibrosis. MMP-9: matrix metalloproteinase-9. TIMP −1: tissue inhibitors of metalloproteinase-1. *: p < 0.05.
Comparison of SCC and CYFRA21-1 levels among patients with emphysema, CPFE, and IPF
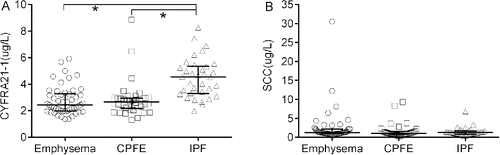
As shown in , the level of CYFRA21-1 in IPF patients was significantly higher when compared to those of emphysema patients (p = 0.000) and CPFE patients (p = 0.000), respectively. The CYFRA21-1 level in CPFE patients was not different from that in emphysema patients (p = 0.736). The SCC levels were similar in the three groups (p > 0.05).
Figure 3. The concentrations of CYFRA21-1 (A) and SCC (B) in different groups. Data were expressed as scatterplots with median lines and interquartile range. CPFE: combined pulmonary fibrosis and emphysema. IPF: idiopathic pulmonary fibrosis. CYFRA21-1: cytokeratin 19 fragment. SCC: squamous cell carcinoma antigen. *: p < 0.05.
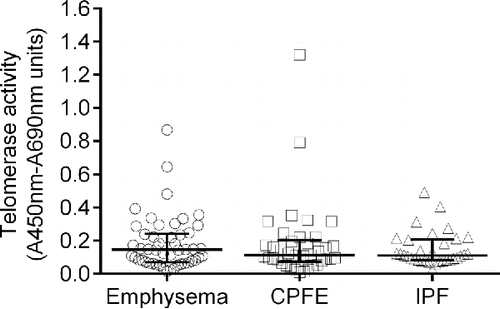
Comparison of telomerase activity among patients with emphysema, CPFE, and IPF
The telomerase activities in the study populations are shown in . There were no significant differences among the three groups (p > 0.05).
Correlations of different cytokines and tumor markers
MMP-9 was positively correlated with TIMP-1 (r = 0.367, p = 0.000) and KL-6 (r = 0.181, p = 0.046), and inversely correlated with TGF-β1 (r = −0.197, p = 0.030). CYFRA21-1 was positively correlated with TGF-β1 (r = 0.213, p = 0.019) and KL-6 (r = 0.324, p = 0.000).
Discussion
VEGF is crucial for maintaining structural homeostasis in the adult lung. The changes in its expression play a significant role in several acute and chronic lung diseases. Reduced VEGF has been reported in induced sputum Citation(9) and lungs Citation(10,22) of emphysema patients and animal models. VEGF receptor blockade Citation(23), VEGF inactivation Citation(24), or genetic deletion Citation(25) leads to emphysema phenotypes. Vascular endothelial growth factor A (VEGFA) induction by resveratrol alleviated elastase-induced emphysema in a mouse model Citation(26). Besides its stimulating activity on endothelial cells, VEGF could also act as a growth and anti-apoptotic factor for alveolar epithelial cells Citation(27). Therefore, deficient VEGF level may lead to the apoptosis of alveolar septal cell (endothelial and epithelial), which results in the development of air space enlargement. In animal models of pulmonary fibrosis, the VEGF concentration in the lung tissue was increased after the bleomycin instillation Citation(11,28), and a positive correlation was found between the VEGF level and the HRCT interstitial score of IPF patients Citation(29). Furthermore, delivery of VEGF aggravated pulmonary fibrosis in rats Citation(30), and anti-VEGF therapy or VEGF receptor-2 antagonist attenuated bleomycin-induced pulmonary fibrosis in mice Citation(11,28). This evidence indicates that VEGF participates in the pathogenesis of pulmonary fibrosis and emphysema. In accordance to these findings, serum VEGF level in IPF patients was higher than that in emphysema patients in the current study. Although the VEGF level in CPFE patients did not differ from that in emphysema patients, it was similar to that in IPF patients. Whether this is caused by coexistence of emphysema and pulmonary fibrosis should be further studied in the future.
TGF-β1 plays a pivotal role in diverse biologic processes during the development of many chronic lung diseases. Evidence from human and animal studies indicates that TGF-β1 is upregulated in the progression of fibrosis Citation(31,32). Rats overexpressing active TGF-β1 gene developed marked lung fibrosis Citation(33). TGF-β pathway activity is also involved in emphysema pathogenesis. Kamio et al. Citation(34) reported serum TGF-β1 level decreased significantly as emphysema progressed. Disruption of TGF-β1 signaling, at the level of either synthesis/activation or receptor ligation or post-receptor signal transduction, is critical to the pathobiology of emphysema Citation(35). Increased presence of TGF-β1 in the parenchyma could protect against emphysema Citation(36). Consistent with these studies, our study indicated that the serum TGF-β1 level was significantly higher in IPF patients than that in emphysema patients. When comparing CPFE patients with IPF or emphysema patients, no difference was found. It was reported that TGF-β1 inhibited MMP-9 expression in alveolar macrophages and monocytes Citation(7). We also found that serum TGF-β1 level was inversely correlated with MMP-9 level. Overall, these findings suggest an important role of the TGF-β -MMP axis in development of emphysema and fibrosis. Nevertheless, no significant difference in MMP-9 levels among IPF, emphysema, and CPFE patients was detected in our study, and a small sample size in our study could contribute to this insignificance. A larger study, preferentially a multicenter one, may help disclose the role and correlation of TGF-β1 and TGF-β-MMP axis in CPFE development.
KL-6 is one of the human MUC1 antigens secreted mainly by alveolar type II cells especially when these cells are regenerating Citation(13). The KL-6 expression is elevated in the serum of adult patients with different types of ILD, including IPF Citation(16,37). KL-6 can also be used to predict the onset of acute exacerbation and survival in the patients with IPF Citation(17,38). In vitro studies showed that KL-6 could promote proliferation, survival Citation(39), and differentiation Citation(40) of lung fibroblasts. Taken together, these observations suggest that KL-6 is involved in the pathogenesis of fibrosis in ILD. To our knowledge, there is only one study evaluating KL-6 level in emphysema, in which, the serum KL-6 level, in a decreasing order, was in IPF patients, CPFE patients, emphysema patients, and control Citation(41). In the present study, we found that the serum KL-6 level was significantly higher in IPF patients when compared with those in emphysema patients and CPFE patients. The KL-6 level in CPFE patients was similar with that in emphysema patients. To our surprise, MMP-9 was positively correlated with KL-6, and such relationship has never been reported. Thus, a further study is warranted to characterize the role of KL-6 in lung disease pathogenesis.
CYFRA21-1 is a tumor marker for lung cancer; however, the elevated serum or BALF level of CYFRA21-1 was reported in patients with IPF Citation(18,19). Dobashi et al. Citation(18) reported that serum Cytokeratin 19 level had a significant negative correlation with percent vital capacity in patients with pulmonary fibrosis. Our previous study also demonstrated that serum CYFRA21-1 level in IPF patients was inversely correlated with diffusing capacity of the lung for carbon monoxide (DLCO) and arterial oxygen tension (PaO2), and positively correlated with the alveolar–arterial oxygen difference (AaDO2) Citation(19). Serum CYFRA21-1 was also reported as a predictor for the mortality in IPF patients Citation(19). Interestingly, the CYFRA21-1 level in emphysema or CPFE patients was never reported. In this study, we found that the serum CYFRA21-1 level in IPF patients was higher than those in both emphysema patients and CPFE patients. However, the CYFRA21-1 level in CPFE patients was not different from that in emphysema patients. Despite the different expression levels among the three groups, a positive correlation of CYFRA21-1 with TGF-β1 and KL-6 was noticed in our study. The role of CYFRA21-1 in the pathogenesis of IPF and CPFE needs to be further studied.
Although the proteases and anti-proteases theory in emphysema is widely accepted, the role of MMP-9 and TIMP-1 in emphysema was controversy Citation(42–45). As well, contradicting results existed in the studies of pulmonary fibrosis Citation(46,47). Not surprisingly, no difference in MMP-9, TIMP-1, and MMP-9/TIMP-1 among emphysema, CPFE, and IPF patients was discovered in our study. All these findings suggest the complicated role of MMP-9 and TIMP-1 in the pathogenesis of emphysema and pulmonary fibrosis.
Telomerase plays an important role in maintaining the integrity of telomeres. It was reported that telomerase null mice developed emphysematous air space enlargement after cigarette smoke exposure Citation(48). Telomerase mutations are also a risk factor for IPF Citation(4). In a recent study, Diaz de Leon et al. Citation(49) examined radiographs of telomerase mutation carriers with IPF and found superimposed emphysema in 20% of cases. In the present study, we found no significant difference in the telomerase activity among emphysema, IPF, and CPFE.
The present study has several limitations. First, a relatively small number of patients were included in the study, due to the rareness of CPFE and IPF. Second, healthy controls were not recruited. Though the aim of our study is to determine whether the levels of cytokines, tumor markers, and the telomerase activity in CPFE were different from IPF and pulmonary emphysema, the inclusion of healthy controls could help us reach a more convincing conclusion. Third, cytokines were examined only in peripheral blood, but not in BALF or lung tissues. Above all, our results need to be validated by multicenter prospective studies, preferentially with a large study population, healthy volunteers participation, and BALF or lung tissue biopsy.
Conclusions
VEGF, TGF-β1, KL-6, and CYFRA21-1 may play a role in the pathogenesis of pulmonary fibrosis. The lower levels of KL-6 and CYFRA21-1 in CPFE patients may be one of the reasons why these patients develop emphysema on the basis of fibrosis, although the precise roles of these markers in the pathogenesis of CPFE remain unclear.
Acknowledgments
The authors thank the assistance of Mr Bing Li, Mr Ming-hao Gu, Ms Yi-wen Liu, Ms Cui-xia Yang, Ms Lian Cui and Mr Yi-qing He at Shanghai Jiao Tong University Affiliated Sixth People's Hospital.
Funding
This study was supported by a technology Foundation (12XJ30070) from the medical college of Shanghai Jiao Tong University.
References
- Wiggins J, Strickland B, Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution computed tomography in assessment. Respir. Med. 1990; 84(5):365–9.
- Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur. Respir. J. 2005; 26(4):586–93.
- Kim JY, Choeng HC, Ahn C, Cho SH. Early and late changes of MMP-2 and MMP-9 in bleomycin-induced pulmonary fibrosis. Yonsei Med. J. 2009; 50(1):68–77.
- Gansner JM, Rosas IO. Telomeres in lung disease. Transl. Res. 2013; 162(6):343–52.
- Liu T, Chung MJ, Ullenbruch M, Yu H, Jin H, Hu B, et al. Telomerase activity is required for bleomycin induced pulmonary fibrosis in mice. J. Clin. Invest. 2007; 117(12):3800–9.
- Le Saux CJ, Davy P, Brampton C, Ahuja SS, Fauce S, Shivshankar P, et al. A novel telomerase activator suppresses lung damage in a murine model of idiopathic pulmonary fibrosis. PLoS One 2013; 8(3):e58423.
- Königshoff M, Kneidinger N, Eickelberg O. TGF-β signalling in COPD: deciphering genetic and cellular susceptibilities for future therapeutic regimens. Swiss Med. Wkly. 2009; 139(39–40):554–63.
- Lee CG, Cho S, Homer RJ, Elias JA. Genetic control of transforming growth factor-β1-induced emphysema and fibrosis in the murine lung. Proc. Am. Thorac. Soc. 2006; 3(6):476–7.
- Kanazawa H, Hirata K, Yoshikawa J. Imbalance between vascular endothelial growth factor and endostatin in emphysema. Eur. Respir. J. 2003; 22(4):609–12.
- Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am. J. Respir. Crit. Care Med. 2001; 163(3 Pt 1):737–44.
- Ou XM, Li WC, Liu DS, Li YP, Wen FQ, Feng YL, et al. VEGFR-2 antagonist SU5416 attenuates bleomycin-induced pulmonary fibrosis in mice. Int. Immunopharmacol. 2009; 9(1):70–9.
- Stockmann C, Kerdiles Y, Nomaksteinsky M, Weidemann A, Takeda N, Doedens A, et al. Loss of myeloid cell-derived vascular endothelial growth factor accelerates fibrosis. Proc. Natl. Acad. Sci. U S A. 2010; 107(9):4329–34.
- Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL-6. Chest 1989; 96(1):68–73.
- Iyonaga K, Miyajima M, Suga M, Saita N, Ando M. Alterations in cytokeratin expression by the alveolar lining epithelial cells in lung tissues from patients with idiopathic pulmonary fibrosis. J. Pathol. 1997; 182(2):217–24.
- Calabrese F, Lunardi F, Giacometti C, Marulli G, Gnoato M, Pontisso P, et al. Overexpression of squamous cell carcinoma antigen in idiopathic pulmonary fibrosis: clinicopathological correlations. Thorax 2008; 63(9):795–802.
- Kobayashi J, Kitamura S. KL-6: a serum marker for interstitial pneumonia. Chest 1995; 108(2):311–5.
- Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 2006; 11(2):164–8.
- Dobashi N, Fujita J, Ohtsuki Y, Yamadori I, Yoshinouchi T, Kamei T, et al. Elevated serum and BAL cytokeratin 19 fragment in pulmonary fibrosis and acute interstitial pneumonia. Eur. Respir. J. 1999; 14(3):574–8.
- Xu L, Bian W, Gu J, Yang DR, Rong ZH, Shen C. Clinical significance of tumour markers in idiopathic pulmonary fibrosis. J. Clin. Pulm. Med. 2013; 18(10):1865–7. [ Article in Chinese].
- Ito M, Hanaoka M, Droma Y, Hatayama O, Sato E, Katsuyama Y, et al. The association of transforming growth factor beta 1 gene polymorphisms with the emphysema phenotype of COPD in Japanese. Intern. Med. 2008; 47(15):1387–94.
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al.. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011; 183(6):788–824.
- Kratzer A, Salys J, Nold-Petry C, Cool C, Zamora M, Bowler R, et al. Role of IL-18 in second-hand smoke–induced emphysema. Am. J. Respir. Cell Mol. Biol. 2013; 48(6):725–32.
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J. Clin. Invest. 2000; 106(11):1311–9.
- Takahashi Y, Izumi Y, Kohno M, Ikeda E, Nomori H. Airway administration of vascular endothelial growth factor siRNAs induces transient airspace enlargement in mice. Int. J. Med. Sci. 2013; 10(12):1702–14.
- Mura M, Binnie M, Han B, Li C, Andrade CF, Shiozaki A, et al. Functions of type II pneumocyte-derived vascular endothelial growth factor in alveolar structure, acute inflammation, and vascular permeability. Am. J. Pathol. 2010; 176(4):1725–34.
- Chen YB, Lan YW, Chen LG, Huang TT, Choo KB, Cheng WT, et al. Mesenchymal stem cell-based HSP70 promoter-driven VEGFA induction by resveratrol alleviates elastase-induced emphysema in a mouse model. Cell Stress Chaperones. 2015; 20(6):979–89.
- Roberts JR, Perkins GD, Fujisawa T, Pettigrew KA, Gao F, Ahmed A, et al. Vascular endothelial growth factor promotes physical wound repair and is anti-apoptotic in primary distal lung epithelial and A549 cells. Crit. Care Med. 2007; 35(9):2164–70.
- Hamada N, Kuwano K, Yamada M, Hagimoto N, Hiasa K, Egashira K, et al. Anti-vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J. Immunol. 2005; 175(2):1224–31.
- Ando M, Miyazaki E, Ito T, Hiroshige S, Nureki SI, Ueno T, et al. Significance of serum vascular endothelial growth factor level in patients with idiopathic pulmonary fibrosis. Lung 2010; 188(3):247–52.
- Farkas L, Farkas D, Ask K, Möller A, Gauldie J, Margetts P, et al. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J. Clin. Invest. 2009; 119(5):1298–311.
- Bergeron A, Soler P, Kambouchner M, Loiseau P, Milleron B, Valeyre D, et al. Cytokine profiles in idiopathic pulmonary fibrosis suggest an important role for TGF-beta and IL-10. Eur. Respir. J. 2003; 22(1):69–76.
- Xu L, Yang D, Zhu S, Gu J, Ding F, Bian W, et al. Bleomycin-induced pulmonary fibrosis is attenuated by an antibody against KL-6. Exp. Lung Res. 2013; 39(6):241–8.
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 1997; 100(4):768–76.
- Kamio K, Ishii T, Motegi T, Hattori K, Kusunoki Y, Azuma A, et al. Decreased serum transforming growth factor-b1 concentration with aging is associated with the severity of emphysema in chronic obstructive pulmonary disease. Geriatr. Gerontol. Int. 2013; 13(4):1069–75.
- Horowitz JC, Martinez FJ, Thannickal VJ. Mesenchymal cell fate and phenotypes in the pathogenesis of emphysema. COPD 2009; 6(3):201–10.
- Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, et al. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes MMP12-dependent emphysema. Nature 2003; 422(6928):169–73.
- Samukawa T, Hamada T, Uto H, Yanagi M, Tsukuya G, Nosaki T, et al. The elevation of serum napsin A in idiopathic pulmonary fibrosis, compared with KL-6, surfactant protein-A and surfactant protein-D. BMC Pulm. Med. 2012; 12:55.
- Ohshimo S, Ishikawa N, Horimasu Y, Hattori N, Hirohashi N, Tanigawa K, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir. Med. 2014; 108(7):1031–9.
- Ohshimo S, Yokoyama A, Hattori N, Ishikawa N, Hirasawa Y, Kohno N. KL-6, a human MUC1 mucin, promotes proliferation and survival of lung fibroblasts. Biochem. Biophys. Res. Commun. 2005; 338(4):1845–52.
- Xu L, Yan DR, Zhu SL, Gu J, Bian W, Rong ZH, et al. KL-6 regulated the expression of HGF, collagen and myofibroblast differentiation. Eur. Rev. Med. Pharmacol. Sci. 2013; 17(22):3073–7.
- Kokuho N, Ishii T, Kamio K, Hayashi H, Kurahara M, Hattori K, et al. Diagnostic values for club cell secretory protein (CC16) in serum of patients of combined pulmonary fibrosis and emphysema. COPD 2015; 12(4):347–54.
- Boschetto P, Quintavalle S, Zeni E, Leprotti S, Potena A, Ballerin L, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax 2006; 61(12):1037–42.
- D'Armiento JM, Goldklang MP, Hardigan AA, Geraghty P, Roth MD, Connett JE, et al. Increased matrix metalloproteinase (MMPs) levels do not predict disease severity or progression in emphysema. PLoS One 2013; 8(2):e56352.
- Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, et al. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am. J. Physiol. Lung Cell Mol. Physiol. 2008; 294(6):L1149–57.
- Atkinson JJ, Lutey BA, Suzuki Y, Toennies HM, Kelley DG, Kobayashi DK, et al. The role of matrix metalloproteinase-9 in cigarette smoke–induced emphysema. Am. J. Respir. Crit. Care Med. 2011; 183(7):876–84.
- Cabrera S, Gaxiola M, Arreola JL, Ramírez R, Jara P, D'Armiento J, et al. Overexpression of MMP9 in macrophages attenuates pulmonary fibrosis induced by bleomycin. Int. J. Biochem. Cell Biol. 2007; 39(12):2324–38.
- Jin X, Dai H, Ding K, Xu X, Pang B, Wang C. Rapamycin attenuates bleomycin-induced pulmonary fibrosis in rats and the expression of metalloproteinase-9 and tissue inhibitors of metalloproteinase-1 in lung tissue. Chin. Med. J. 2014; 127(7):1304–9.
- Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, et al. Telomere length is a determinant of emphysema susceptibility. Am. J. Respir. Crit. Care Med. 2011; 184(8):904–12.
- Diaz de Leon A, Cronkhite JT, Katzenstein AL, Godwin JD, Raghu G, Glazer CS, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS One 2010; 5(5):e10680.