ABSTRACT
Mucolytics are potentially useful for the management of chronic obstructive pulmonary disease (COPD), although there is conflicting advice on their use in different guideline documents. Furthermore, there is paucity of data comparing the efficacy of the different mucolytic agents in reducing the odds of COPD exacerbations. We performed pair-wise and network meta-analyses to evaluate the impact of mucoly-tics in COPD. Randomized clinical trials lasting at least 3 months and investigating the effects of mucolytics on COPD exacerbations were identified from published studies and repository databases. Mucolytics significantly reduced the odds of exacerbation vs. placebo (11 studies analyzed: odds ratio (OR) 0.51, 95% confidence interval (CI) 0.39–0.67; p < 0.001). The most effective drugs were carbocysteine, erdosteine, and N-acetylcysteine 1,200 mg/day (SUCRA 68.0–79.0%), whereas the OR was similar to placebo for ambroxol and N-acetylcysteine 600 mg/day. Only N-acetylcysteine 1,200 mg/day significantly protected against exacerbations vs. placebo (2 studies analyzed: OR 0.56, 95% CI 0.35–0.92; p < 0.05; high quality of evidence). A signal of effectiveness was detected for carbocysteine (2 studies analyzed: OR 0.45, 95% CI 0.20–1.01; p ≥ 0.05; moderate quality of evidence). Specific differences in study designs and patient-related characteristics, such as history of exacerbations and ethnicity, were potential effect modifiers for our statistical models, whereas neither respiratory function nor the use of corticosteroids influenced the analysis. This meta-analysis demonstrates that mucolytics are useful in preventing COPD exacerbations as maintenance add-on therapy to patients with frequent exacerbations. The effectiveness of mucolytics is independent of the severity of airway obstruction and the use of inhaled corticosteroids.
KEYWORDS:
Introduction
The 2017 version of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy states that in “in COPD patients not receiving inhaled corticosteroids, regular treatment with mucolytics such as carbocysteine and N-acetylcysteine (NAC) may reduce exacerbations and modestly improve health status” Citation(1). However, currently available data do not permit to assess the real target population for mucolytic agents because of the heterogeneity of chronic obstructive pulmonary disease (COPD) patients enrolled in the studies, doses of treatments, and concomitant medications Citation(1,2).
A number of national and international bodies have recognized the potential utility of mucolytics in the management of stable COPD, although wide variability in their use exists, as evidenced by examining the national guidelines for management of COPD in Europe Citation(3–6).
In order to shed light on a very controversial topic, a recent Cochrane meta-analysis aimed to establish whether treatment with mucolytics reduces frequency of exacerbations and/or days of disability in patients with chronic bronchitis or COPD demonstrated that mucolytics reduce the number of exacerbations by a small amount, and do not appear to cause any harm Citation(2). The reduction was approximately one fewer exacerbation every three years. Remarkably, one person out of eight may avoid having an exacerbation, provided all take treatment every day for an average of 10 months.
These findings are definitely interesting, but they have been obtained by comparing active agents vs. placebo. Consequently, we have evidence that mucolytics as a class are potentially useful, but we still do not know if there is a mucolytic agent that is more effective than others in reducing frequency of exacerbations, also because head-to-head comparisons are lacking. This lack of information may explain why the choice of the mucolytic agent to be used is difficult and remains empiric, and likely why there are so many conflicting opinions on the use of these agents.
Pooling of direct and indirect evidence from randomized clinical trials (RCTs), known as mixed treatment comparisons (also called network meta-analysis), is becoming increasingly common in the clinical literature. A network meta-analysis allows coherent judgments on which of the several treatments is the most effective and produces estimates of the relative effects of each treatment compared with every other treatment in a network Citation(7). Therefore, considering the confounding divergences on the use of mucolytics in the treatment of stable COPD in different national and international guidelines and strategies Citation(3,8), the positive outcome of the Cochrane meta-analysis Citation(2), and the lack of head-to-head RCTs of treatment interventions, we carried out a treatment comparison by systematic review and synthesis on the currently available clinical evidence by using an initial pair-wise meta-analysis to evaluate the overall impact of mucolytic agents on COPD exacerbations, and then a network meta-analysis to compare different mucolytic agents.
Materials and methods
Searching strategy
This pair-wise and network meta-analysis has been registered in PROSPERO (registration number: CRD42016053762 available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016053762), and performed in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement () Citation(9). Furthermore, this synthesis satisfied all the recommended items reported by the PRISMA-P 2015 checklist Citation(10).
Figure 1. PRISMA flow diagram for the identification of studies included in the meta-analysis concerning the impact of mucolytic agents on COPD exacerbations (A). Diagram displaying the network of the five arms involved in the Bayesian analysis. The links between nodes indicate the direct comparisons between pairs of treatments. The numbers shown along the link lines indicate the number of COPD patients comparing pairs of treatments head-to-head (B).
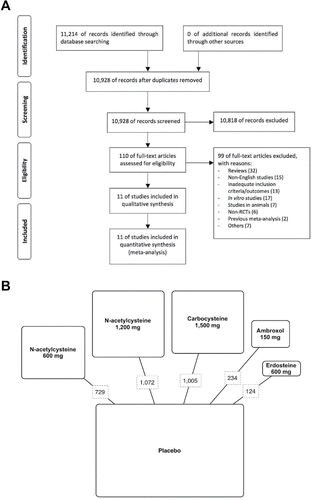
We undertook a comprehensive literature search for RCTs evaluating the influence of mucolytic agents on the odds of exacerbations in patients suffering from COPD (not chronic bronchitis) confirmed by pulmonary function testing. Due to seasonal variation, it has been suggested that the evaluation of COPD exacerbation should require a period of ≥ 1 year Citation(11,12). However, since the rate of exacerbation was successfully investigated in studies with 3 months of follow-up Citation(13), in this meta-analysis, we included RCTs lasting at least 3 months.
The identification of mucolytic agents was performed in agreement with GOLD 2014 and the American College of Chest Physicians and Canadian Thoracic Society Guideline for prevention of COPD exacerbations Citation(14,15). In particular, the terms “ambroxol,” “carbocysteine,” “erdosteine,” “iodinated glycerol,” and “N-acetylcysteine” were searched for the mucolytic agents, and the terms “chronic obstructive pulmonary disease” and “COPD” were searched for the disease. The search was performed in PubMed, Scopus, Embase, Google Scholar, and the repository database clinicaltrials.gov Citation(16) through March 2017, to identify relevant studies published in the English language and published up to March 31, 2017. RCTs reporting the impact of oral mucolytic agents vs. placebo on COPD exacerbations have been included in the analysis.
Two reviewers independently checked the relevant RCTs identified from literature searches and databases. RCTs were selected in agreement with the previously mentioned criteria, and any difference in opinion about eligibility was resolved by consensus.
Quality score, risk of bias and evidence profile
The Jadad score, with a scale of 1 to 5 (score of 5 being the best quality), was used to assess the quality of the RCTs concerning the likelihood of biases related to randomization, double blinding, withdrawals, and dropouts Citation(17). The risk of bias for each included study was also assessed via the Cochrane Collaboration's tool Citation(18). Two reviewers independently assessed the quality of individual studies, and any difference in opinion about the quality score was resolved by consensus.
The risk of publication bias was assessed by applying the funnel plot and Egger's test through the following regression equation: SND = a + b × precision, where SND represents the standard normal deviation (treatment effect divided by its standard error (SE)), and precision represents the reciprocal of the standard error. Evidence of asymmetry from Egger's test was considered to be significant at p < 0.1, and the graphical representation of 90% confidence bands is presented Citation(17).
Meta-regression analysis was performed to examine the source of heterogeneity between studies, leading to asymmetry of funnel plot Citation(19–21). Further analysis was carried out by using the meta-regression model to adjust for statistically significant (p < 0.05) confounders Citation(22,23).
The quality of the evidence has been assessed in agreement with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system Citation(24) overall for all the studies included in the synthesis, and for the subset meta-analyses.
Data extraction
Data from included studies were extracted from published papers, and/or online supplementary files, and/or the public database clinicaltrials.gov. Data extraction was carried out in agreement with the recommendations provided by the Cochrane Handbook for Systematic Reviews of Interventions Citation(25). Data were extracted and checked for study characteristics and duration, doses of medications, patient characteristics, age, gender, smoking habits, FEV1, Jadad score, and number of exacerbations.
End points
The end point of this meta-analysis was to assess the protective effect of mucolytic drugs against COPD exacerbation, vs. placebo. In particular, we have measured the association between the treatment with mucolytic agent and COPD exacerbation. This allowed to evaluate the odds that a COPD exacerbation occurred in patients treated with mucolytic drugs, compared to the odds that a COPD exacerbation occurred in untreated patients Citation(26).
Data analysis
We performed both pair-wise and network meta-analyses to evaluate the impact of mucolytic agents on COPD exacerbations. Since the follow-up duration was not consistent among the RCTs included in this meta-analysis, the data have been normalized as a function of person-season, where one season lasts 3 months Citation(27–29). This method involved the conversion of the measures into a common metric (events per person-time) before meta-analyzing the data, leading to increased estimates of effect, precision, and clinical interpretability of results Citation(25,30).
Results are expressed as odds ratio (OR) and 95% confidence interval (95%CI) in pair-wise meta-analysis. Since data were selected from a series of studies performed by researchers operating independently, and a common effect size cannot be assumed, we used the random-effects model to perform the pair-wise meta-analysis in order to balance the study weights and adequately estimate the 95%CI of the mean distribution of drugs effect on the investigated variable Citation(27). In fact, although the mathematics behind the fixed-effects model are much simpler than those of the random-effects model, results of this quantitative synthesis cannot be generalized via fixed-effects model since the included studies were quite dissimilar Citation(31), as reported in . Therefore, the greater the degree of difference among the studies incorporated in the analysis, the more important it becomes to employ the random-effects model Citation(32).
Table 1. Patient demographics, baseline and study characteristics.
A subset analysis was performed with regard to the effect of specific mucolytic drugs, doses, and quality of studies. High-quality studies were identified by considering the outcomes resulting from the summary of the Cochrane Collaboration's tool and having Jadad score ≥ 3. Furthermore, a sensitivity analysis was carried out for studies that lasted ≥ 1 year compared with those that lasted < 1 year.
The network meta-analysis was performed to indirectly compare the effect of specific mucolytic drugs and doses. A full Bayesian evidence network was used (chains: 4; initial values scaling: 2.5; tuning iterations: 20.000; simulation iterations: 50.000; tuning interval: 10), and the convergence diagnostics for consistency and inconsistency was assessed via the Brooks-Gelman-Rubin method, as previously reported Citation(33). Due to the characteristics of parameters besides the available data, the proper non-informative distributions specified the prior densities, in agreement with the Bayesian Approaches to Clinical Trials and Health-Care Evaluation Citation(34,35). Since the distributions were sufficiently vague, the reference treatment, study baseline effects, and heterogeneity variance were unlikely to have a noticeable impact on model results. In this condition, GeMTC software automatically generates and runs the required Bayesian hierarchical model and selects the prior distributions and starting values as well, via heuristically determining a value for the outcome scale parameter (i.e. outcome scale S) Citation(36,37). The posterior mean deviance of data points in the unrelated mean effects model were plotted against their posterior mean deviance in the consistency model in order to provide information for identifying the loops in the treatment network where evidence was inconsistent Citation(38). Results of the network meta-analysis are expressed as relative effect and 95% credible level (95%Crl). The probability that each intervention arm was the most effective was calculated by counting the proportion of iterations of the chain in which each intervention arm had the highest mean difference, and the surface under the cumulative ranking curve (SUCRA), representing the summary of these probabilities, was also calculated. The SUCRA is 100% when a treatment is certain to be the best, and 0% when a treatment is certain to be the worst Citation(33,39).
The optimal information size (OIS) was calculated as previously described Citation(40), and the statistical significance was assessed for p < 0.05.
OpenMetaAnalyst Citation(41) and GeMTC Citation(36) software were used for performing the meta-analysis, SPSS Statistics (NY, US) for meta-regression, GraphPad Prism (CA, US) software for graphing the data, and GRADEpro for assessing the quality of evidence Citation(24). The statistical significance was assessed for p < 0.05, and moderate to high levels of heterogeneity were considered for I2 > 50%.
Results
Studies characteristics
Results obtained from 3,164 COPD patients (1,587 treated with a mucolytic agent, 1,577 treated with placebo) were selected from 11 published studies, including 6 RCTs on NAC Citation(42–47), 3 on carbocysteine Citation(48–50), one on erdosteine Citation(51), and one on ambroxol Citation(52) (). Eight RCTs, including 7 full papers Citation(42–45, 48, 51, 52) and a correspondence Citation(50), had a Jadad score ≥ 3. The risk of bias for each included study calculated via Jadad score was consistent with that assessed via the Cochrane Collaboration's tool (). The period of treatment ranged from 16 to 156 weeks. shows the definition of exacerbation as reported by the studies included in the synthesis.
Table 2. Summary of the risk of bias for each included study assessed via the Cochrane Collaboration's tool Citation(18).
Table 3. Definition of COPD exacerbations as reported by studies included in the meta-analysis.
Pair-wise meta-analysis
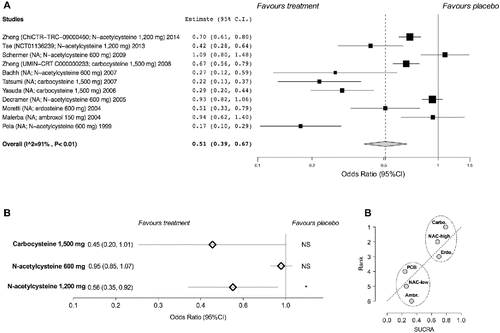
Treatment with mucolytic agents significantly reduced the odds of COPD exacerbations vs. placebo (11 studies analyzed: OR 0.51, 95% CI 0.39–0.67; p < 0.001) (). The sensitivity analysis identified greater effectiveness of mucolytics agents in studies lasting ≥ 1 year vs. shorter RCTs (OR 0.61, 95% CI 0.47–0.79 vs. OR 0.29, 95% CI 0.14–0.60, respectively). The subset analysis performed by including in the synthesis exclusively high-quality studies indicated that only the treatment with NAC administered at high dose (1,200 mg/day) significantly protected COPD patients against exacerbations (2 high-quality studies analyzed: OR 0.56, 05% CI 0.35–0.92), whereas NAC at lower dose (600 mg/day) did not significantly improve the odds of COPD exacerbations vs. placebo (2 high-quality studies analyzed: OR 0.95, 95% CI 0.85–1.07; p ≥ 0.05) (). Carbocysteine did not significantly reduce the exacerbation frequency vs. placebo (2 high-quality studies analyzed: OR 0.45, 95% CI 0.20–1.01; p ≥ 0.05); however, a signal of effectiveness was detected for this medication since the higher CI of the effect estimate only slightly overlapped the OR of 1 (no effect) (). The subset pair-wise meta-analysis for erdosteine and ambroxol was not carried out since only one high-quality RCT was identified for each drug Citation(51,52).
Figure 2. Forest plot of pair-wise meta-analysis of the impact of the mucolytic drugs on the odds of COPD exacerbations vs. placebo (A), subset analysis performed on specific mucolytic drugs and doses investigated in high-quality studies (B). Ranking plot for the network of mucolytic agents on the odds of COPD exacerbations (C) in which the treatments have been ranked according to SUCRA (rank of 6 being the worst treatment). Ambr, ambroxol; carbo, carbocysteine; erdo, erdosteine; PCB, placebo; NAC-high, N-acetylcysteine high-dose; NAC-low, N-acetylcysteine low-dose; SUCRA, surface under the cumulative ranking curve. NS, not significant (p ≥ 0.05); * p < 0.05.
Network meta-analysis
The ranking plot resulting from the network meta-analysis carried out on high-quality studies identified two distinct clusters of effectiveness. Specifically, carbocysteine, erdosteine, and NAC high-dose were effective drugs in reducing COPD exacerbations, whereas ambroxol and NAC at low dose were less-effective medications (). The SUCRA values of the effective medications ranged from 68.0% to 79.0%, whereas similar values to placebo were detected for both ambroxol and NAC at low dose ().
Table 4. SUCRA values for the impact of mucolytic agents in reducing the odds of COPD exacerbations.
Bias and quality of evidence
A substantial level of heterogeneity was detected in the pair-wise meta-analysis (I2 91%, p < 0.001), whereas the assessment of statistical consistency of the network meta-analysis indicated that all the points fit adequately with the line of equality (R2 0.99; slope 0.96, 95%CI 0.95–0.97).
Overall, the cumulative number of enrolled COPD patients in the studies reached the OIS for a binary outcome meta-analysis on mucolytic agents (OIS: 414; delta: +2,750). Specifically, OIS was reached for carbocysteine (OIS: 210; delta: +795), NAC high-dose (OIS: 502; delta: +570), and NAC low-dose (OIS: 380; delta: +349), whereas the number of patients in the studies on ambroxol (OIS: >5,000; delta: <−5,000) and erdosteine (OIS: 310: delta: −186) did not meet the OIS criteria.
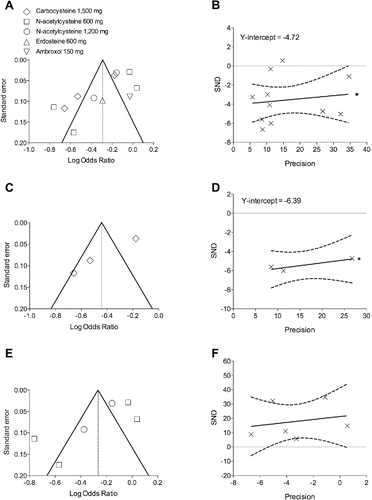
The analysis performed via funnel plot and Egger's tests indicated that smaller studies may have significantly (p < 0.1) distorted the results of this meta-analysis because of inflated estimates of effect size for both the overall pair-wise meta-analysis on mucolytic agents ( and ) and the subset analysis on carbocysteine ( and ). On the contrary, no significant (p ≥ 0.1) publication bias was detected for NAC ( and ). Results of Egger's test should be interpreted with caution in case of insufficient number of studies, due to the low power of 10 or fewer RCTs for meta-analyses Citation(53). The analysis of publication bias for erdosteine and ambroxol was not carried out since only one RCT was identified for each drug Citation(51,52).
Figure 3. Publication bias assessment via funnel plots (left panels) and Egger's test (right panels) for the impact of mucolytic agents (A and B) on the rate of COPD exacerbations vs. placebo, and subset analysis of carbocysteine (C and D) and N-acetylcysteine (E and F). SND, standard normal deviate. *p < 0.1.
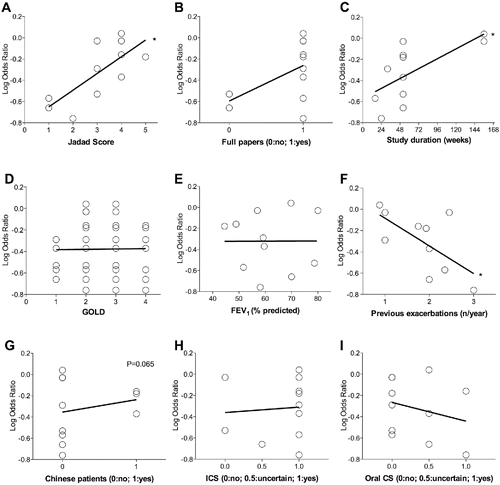
The meta-regression model indicated that several explanatory variables might have influenced the size of intervention effect. The characteristics of the studies, such as duration and quality, and the history of exacerbation rate before patients were enrolled into the RCTs, are potential effect modifiers that may have significantly (p < 0.05) altered the impact of mucolytic drugs on the frequency of COPD exacerbations. A signal (p = 0.065) of effect modifier was detected also for ethnicity, with Chinese COPD patients being less responsive to the protective effect of mucolytic agents compared with non-Chinese populations. However, neither respiratory function nor the use of corticosteroids represented significant modifier covariates (p ≥ 0.05). Detailed results on meta-regression analysis are reported in . After adjusting for the significant confounders detected in the meta-regression model, therapy with mucolytic agents remained as a statistically significant treatment against COPD exacerbations (nonadjusted p < 0.001; adjusted for Jadad score p < 0.01; adjusted for studies duration p < 0.05; adjusted for history of exacerbation rate p < 0.001).
Figure 4. Meta-regression analysis for study and publication characteristics (A–C), disease characteristics (D–F), ethnicity (G) and corticosteroid therapy (H and I). The lower the log odds ratio the greater the treatment effect on reduction of COPD exacerbations. FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroids; CS, corticosteroids. *p < 0.05: significant interaction between the treatment with mucolytic drugs vs. exacerbation rate and the potential modifier covariates.
The GRADE approach indicated moderate quality of evidence for the overall impact of mucolytic agents on the odds of COPD exacerbation. The GRADE subset analysis of specific mucolytic agents is reported in .
Table 5. GRADE evidence profile: mucolytic agents and COPD exacerbations.
Discussion
The results of this meta-analysis demonstrate that mucolytic drugs are effective in protecting patients against COPD exacerbations, and that this beneficial effect was greater in patients treated for one year or longer. Carbocysteine, erdosteine, and NAC administered at high dose (600 mg twice daily, corresponding to 1,200 mg/day) were the most effective agents, whereas neither ambroxol nor NAC administered at low dose (600 mg/day) reduced the odds of COPD exacerbations, compared with placebo.
NAC high-dose elicited a significant protective effect against COPD exacerbations among the most effective compounds, as supported by GRADE analysis, and a signal of effectiveness was detected for carbocysteine. Small studies on carbocysteine, such as those conducted by Tatsumi et al. Citation[49] and Yasuda et al. Citation[50], were characterized by low-quality according to the Jadad score, and elicited the so called “small study effect”, in which the treatment effect estimate was greater than that observed in larger and well-designed studies. Although only one RCT on erdosteine was eligible to be included in this synthesis Citation(51), the network approach permitted a comparison of the efficacy of this medication with the other mucolytic agents. Erdosteine was within the cluster of the most effective drugs, regardless of the level of evidence.
Indeed, this meta-analysis reports substantial level of heterogeneity. Nevertheless, distribution of I2 values of 509 meta-analyses in the Cochrane Database of Systematic Reviews showed that about a quarter of meta-analyses had I2 values over 50%, and that that the distribution of heterogeneity was roughly flat even for I2≈90% Citation(54). Moreover, the quantification of heterogeneity is only one component of a wider investigation of variability across studies, and the importance of I2 values depends also on direction of effects Citation(54). Thus, heterogeneity of this quantitative synthesis should be interpreted considering that most of the estimates showed the same direction of effect toward protective impact of mucolytic agents against COPD exacerbations.
Actually, NAC did not reach the suggested minimal clinical important difference (MCID) of 22% reduction in exacerbation rate Citation(12,55). However, the GRADE analysis indicated a 95% probability of up to 17.8% risk reduction of COPD exacerbations in patients treated with high-dose NAC compared with placebo. This finding is intriguing, but it must be pointed out that mucolytic drugs were administered as add-on to standard COPD therapy in all the high-quality RCTs that have been included in this meta-analysis, suggesting that their beneficial protective role may be additive to that already elicited by standard therapy for COPD.
The definition of COPD exacerbation was assessed by a clinical point of view in all studies included in this meta-analysis, whereas little importance was given to the duration of symptoms. Nevertheless, it must also be pointed out that the identification of a correct MCID for COPD exacerbations is difficult because of the lack of a uniform clinical definition and the commonly underreported frequency and severity of exacerbations, which are potential targets for intervention Citation(55). Interventions reducing exacerbations by as little as 11% appear to be regarded widely as clinically important Citation[56]. Thus, NAC high-dose may even reach the MCID for COPD exacerbations if the studies that have influenced the 2017 GOLD recommendations are considered. Citation(56).
Referring to the recent findings of a previous meta-analysis by our group Citation(27), the 2017 GOLD recommendations indicated that regular treatment with mucolytics may reduce COPD exacerbations and improve health status Citation(1). The GOLD 2017 document cited also a Cochrane review Citation(2) to recommend the use of mucolytic agents only in COPD patients not receiving inhaled corticosteroids, and to report that due to the heterogeneity of study populations, treatment dosing and concomitant treatments, currently available data do not allow one to identify precisely the potential target population for this class of drugs Citation(1). Probably the conclusions of the Cochrane review Citation(2) have been misunderstood, since the authors stated that mucolytics may be considered as Citation(1) a treatment option for patients with frequent exacerbations who cannot take other therapies such as inhaled corticosteroids or long-acting bronchodilators, which have a stronger evidence base for their effectiveness; or as Citation(2) add-on treatment once all other therapies to reduce exacerbations have been utilized Citation(2).
The present meta-analysis updates the data analyzed by Poole and colleagues Citation(2). Our network approach allowed the efficacy of the specific mucolytic agents to be ranked, and we also considered the quality of evidence, focusing on high-quality RCTs. Moreover, we used for the first time a meta-regression to identify the explanatory variables that influence the effect size of the interventions. The concomitant use of corticosteroids, administered either systemically or via inhalation, did not modify the effectiveness of mucolytic agents with regard to COPD exacerbations. Furthermore, mucolytics were more effective in frequent exacerbators, and the extent of bronchial obstruction was not a confounder. Furthermore, the meta-regression analysis further confirms that the quality of RCTs is a relevant effect modifier. In any case, the identified confounders did not alter the findings reported in this quantitative synthesis, since adjusting data for the significant mediators confirmed the protective effect of mucolytic agents against COPD exacerbations.
The current international guidelines for COPD do not differentiate treatment options for any drug class based on potential ethnical differences Citation(57). However, since the only well-designed RCTs that have documented an effect for NAC or carbocysteine on COPD exacerbations (HIACE, PANTHEON and PEACE) were performed in China Citation(42,43,48), it is difficult to say whether this is a true ethnicity effect, or a local study effect. The findings of this study indicate that a specific population effect may exist but, unexpectedly, it seems that Chinese patients are less responsive to the protective effect of mucolytic agents compared with non-Chinese populations, suggesting that the impact of mucolytic drugs may be greater in the Caucasian populations.
A meta-analysis provides only the effect estimates that, by definition, reflect an estimate of the possible impact of the intervention on the investigated outcome(s) and, in any case, result by indirect comparisons. Therefore, the real impact of the most effective mucolytic drugs should be confirmed by performing well-designed head-to-head and placebo-controlled RCTs.
It is unlikely that comparative pragmatic RCTs in the field of respiratory research will be performed, due to their cost and lack of interest of pharmaceutical companies Citation(17). However, a well-powered RCT that will directly compare the efficacy of carbocysteine, erdosteine, and NAC is highly desirable. Furthermore, pre-clinical studies may help to understand if the mechanism of action leading to the protective effect of mucolytics is specific for each compound, as demonstrated for NAC in a validated ex vivo model of COPD exacerbation Citation(58), or if it is an effect of class.
Concluding, this quantitative synthesis supports the use of mucolytic drugs as add-on therapy to prevent COPD exacerbations, especially when administered to patients with frequent exacerbations. The effectiveness of mucolytics seems to be independent of the level of bronchial obstruction and the use of corticosteroids. Consequently, mucolytic agents should be offered to GOLD group C and group D patients. However, results of further RCTs are needed to improve the quality of evidence of most of the analyzed mucolytic agents, in order to correctly rank their effectiveness and suggest their use in these COPD patients.
Conflict of interest
MC has participated as a lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Bio Futura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Lallemand, Mundipharma, Novartis, Pfizer, Verona Pharma, and Zambon, and has been a consultant to Chiesi Farmaceutici, Lallemand, Novartis, Verona Pharma, and Zambon. His department was funded by Almirall, Boehringer Ingelheim, Novartis, and Zambon.
PR participated as a lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis. His department was funded by Almirall, Boehringer Ingelheim, Novartis, and Zambon.
LC has participated as advisor in scientific meetings under the sponsorship of Boehringer Ingelheim, received non-financial support by AstraZeneca, received a research grant partially funded by Boehringer Ingelheim, Novartis and Almirall, and has been a consultant to Zambon and Verona Pharma. His department was funded by Almirall, Boehringer Ingelheim, Novartis, and Zambon.
NAH served as a consultant to Boehringer Ingelheim GmbH, Sunovion Pharmaceuticals Inc, Novartis AG, Mylan Inc, Pearl Therapeutics Inc, and Pfizer Inc. His institution received grant support on his behalf from GlaxoSmithKline plc, Boehringer Ingelheim GmbH, Pfizer Inc, Pearl Therapeutics Inc, and Sunovion Pharmaceuticals Inc.
MGM has participated as a lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline and Novartis, and has been a consultant to Chiesi Farmaceutici.
Study registration
PROSPERO 2016:CRD42016053762
Author contributions
MC contributed to study conception and design; contributed to interpretation of data; drafted the submitted article, revised it critically for important intellectual content, and provided final approval of the version to be published; and has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
PR contributed to study conception and design; contributed to acquisition and interpretation of data; revised the submitted article critically for important intellectual content, and provided final approval of the version to be published; and has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
LC has the responsibility for the content of the manuscript, including the data and analysis; contributed to study conception and design; contributed to acquisition, analysis, and interpretation of data; drafted the submitted article, revised it critically for important intellectual content, and provided final approval of the version to be published; and has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
MGM and NAH contributed to study conception and design; contributed to interpretation of data; revised it critically for important intellectual content and provided final approval of the version to be published; and has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was supported by institutional funds (University of Rome “Tor Vergata”, Rome, Italy).
References
- GOLD. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of COPD – 2017. Available at: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/ (accessed April 6, 2017).
- Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD001287.
- Miravitlles M, Vogelmeier C, Roche N, Halpin D, Cardoso J, Chuchalin AG, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J. 2016; 47:625–637.
- Cazzola M, Floriani I, Page CP. The therapeutic efficacy of erdosteine in the treatment of chronic obstructive bronchitis: a meta-analysis of individual patient data. Pulm Pharmacol Ther. 2010; 23:135–144.
- DARE. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews (Internet) - Centre for Reviews and Dissemination (UK). The therapeutic efficacy of erdosteine in the treatment of chronic obstructive bronchitis: a meta-analysis of individual patient data. Available at: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0029749/ (accessed January 22, 2017).
- Koblizek V, Chlumsky J, Zindr V, Neumannova K, Zatloukal J, Zak J, et al. Chronic Obstructive Pulmonary Disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013; 157:189–201.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010; 29:932–944.
- NICE. National Clinical Guideline Centre. Chronic obstructive pulmonary disease in over 16 s: diagnosis and management. Available at: https://www.nice.org.uk/guidance/CG101 (accessed January 22, 2017).
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009; 3:e123–e130.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4:1.
- Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala JL, Masa F, et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004; 59:387–395.
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008; 31:416–469.
- Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010; 65:423–428.
- GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Available from: http://goldcopd.org (accessed June 12, 2017).
- Criner GJ, Bourbeau J, Diekemper RL, Ouellette DR, Goodridge D, Hernandez P, et al. Prevention of acute exacerbations of COPD: American college of chest physicians and Canadian thoracic society guideline. Chest J 2015; 147:894–942.
- Rogliani P, Calzetta L, Cavalli F, Matera MG, Cazzola M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm Pharmacol Ther. 2016; 40:95–103.
- Calzetta L, Rogliani P, Matera MG, Cazzola M. A systematic review with meta-Analysis of Dual Bronchodilation With LAMA/LABA for the Treatment of Stable COPD. Chest. 2016; 149:1181–1196.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration‘s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000; 53:1119–1129.
- Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009; 9:1.
- Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol. 1999; 28:1–9.
- Jardim DL, Schwaederle M, Wei C, Lee JJ, Hong DS, Eggermont AM, et al. Impact of a biomarker-based strategy on oncology drug development: a meta-analysis of clinical trials leading to FDA approval. J Natl Cancer Inst. 2015; 107: djv253.
- Kelley GA, Kelley KS. Statistical models for meta-analysis: A brief tutorial. World J Methodol. 2012; 2:27–32.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011; 64:383–394.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. ( updated March 2011). Available from www.cochrane-handbook.org. The Cochrane Collaboration. 2011.
- Szumilas M. Explaining odds ratios. J Can Acad Child Adolesc Psychiatry. 2010; 19:227–229.
- Cazzola M, Calzetta L, Page C, Jardim J, Chuchalin AG, Rogliani P, et al. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015; 24:451–461.
- Shen Y, Cai W, Lei S, Zhang Z. Effect of high/low dose N-acetylcysteine on chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD. 2014; 11:351–358.
- Calzetta L, Matera MG, Braido F, Contoli M, Corsico A, Di Marco F, et al. Withdrawal of inhaled corticosteroids in COPD: a meta-analysis. Pulm Pharmacol Ther. 2017; 45:148–156
- Guevara JP, Berlin JA, Wolf FM. Meta-analytic methods for pooling rates when follow-up duration varies: a case study. BMC Med Res Methodol. 2004; 4:17.
- DeCoster J. Meta-analysis notes. http://www.stat-help.com/meta.pdf (accessed June 14, 2017. 2004).
- Turner JR, Durham TA. Meta-methodology: conducting and reporting meta-analyses. J Clin Hypertens (Greenwich). 2014; 16:91–93.
- Calzetta L, Rogliani P, Ora J, Puxeddu E, Cazzola M, Matera MG. LABA/LAMA combination in COPD: a meta-analysis on the duration of treatment. Eur Respir Rev. 2017; 26: 160043.
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Statist Assoc. 2012.
- Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian approaches to clinical trials and health-care evaluation: John Wiley & Sons. 2004.
- van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synthesis Method. 2012; 3:285–299.
- Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synthesis Methods. 2016; 7:80–93.
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013; 33:641–656.
- Cazzola M, Calzetta L, Rogliani P, Matera MG. Tiotropium formulations and safety: a network meta-analysis. Ther Adv Drug Saf. 2017; 8:17–30.
- Rogliani P, Calzetta L, Cazzola M, Matera MG. Drug safety evaluation of roflumilast for the treatment of COPD: a meta-analysis. Expert Opin Drug Saf. 2016:1–14.
- Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012; 49:1–15.
- Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014; 2:187–194.
- Tse HN, Raiteri L, Wong KY, Yee KS, Ng LY, Wai KY, et al. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest. 2013; 144:106–118.
- Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir Med. 2009; 103:542–551.
- Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005; 365:1552–1560.
- Bachh AA, Shah NN, Bhargava R, Ahmed Z, Pandey DK, Dar KA, et al. Effect of oral N-acetylcysteine in COPD-A randomised controlled trial. JK Pract. 2007; 14:12–16.
- Pela R, Calcagni AM, Subiaco S, Isidori P, Tubaldi A, Sanguinetti CM. N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respiration. 1999; 66:495–500.
- Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet. 2008; 371:2013–2018.
- Tatsumi K, Fukuchi Y. Carbocisteine improves quality of life in patients with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2007; 55:1884–1886.
- Yasuda H, Yamaya M, Sasaki T, Inoue D, Nakayama K, Tomita N, et al. Carbocisteine reduces frequency of common colds and exacerbations in patients with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2006; 54:378–380.
- Moretti M, Bottrighi P, Dallari R, Da Porto R, Dolcetti A, Grandi P, et al. The effect of long-term treatment with erdosteine on chronic obstructive pulmonary disease: the EQUALIFE study. Drugs Exp Clin Res. 2004; 30:143–152.
- Malerba M, Ponticiello A, Radaeli A, Bensi G, Grassi V. Effect of twelve-months therapy with oral ambroxol in preventing exacerbations in patients with COPD. Double-blind, randomized, multicenter, placebo-controlled study (the AMETHIST Trial). Pulm Pharmacol Ther. 2004; 17:27–34.
- Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007; 176:1091–6.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.
- Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189:250–255.
- Chapman KR, Bergeron C, Bhutani M, Bourbeau J, Grossman RF, Hernandez P, et al. Do we know the minimal clinically important difference (MCID) for COPD exacerbations? COPD. 2013; 10:243–249.
- Tse HN, Raiteri L, Wong KY, Ng LY, Yee KS, Tseng CZ. Benefits of high-dose N-acetylcysteine to exacerbation-prone patients with COPD. Chest. 2014; 146:611–623.
- Cazzola M, Calzetta L, Facciolo F, Rogliani P, Matera MG. Pharmacological investigation on the anti-oxidant and anti-inflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation. Respir Res. 2017; 18:26.

