ABSTRACT
The concept of asthma and COPD as separate conditions has been questioned, and the term asthma–COPD overlap syndrome has been introduced. We assessed the prevalence, symptoms, and lifestyle factors of asthma–COPD overlap (ACO) in a large Norwegian population-based study.
From 2006 to 2008, a total of 50,777 residents of Nord-Trøndelag participated in the Nord-Trøndelag Health Study, Norway. They completed questionnaires regarding respiratory symptoms, disease status, and medication use. We estimated the prevalence and 95% confidence intervals of ACO. Additionally, spirometry was used to estimate the prevalence of ACO in a subgroup.
The prevalence of self-reported ACO was 1.9%, and in age groups <40, 40–60 and ≥60 years it was 0.7%, 1.4%, and 3.2%, respectively. Among those reporting COPD, the proportion of ACO was 0.56. In the spirometry subgroup when ACO was defined as doctor diagnosed asthma ever and FEV1/FVC < 0.70, the prevalence of ACO was 2.0%. All respiratory symptoms, separately or in combination, as well as medication use were reported most frequently in those with ACO compared to the other groups. Strikingly, we observed a two-fold higher proportion of allergic rhinitis in ACO compared to COPD only.
In this Norwegian population, the prevalence of self-reported ACO was 1.9%, and the corresponding proportion of ACO among those with COPD was 0.56. Participants with ACO generally had the highest proportions of respiratory symptoms compared to asthma or COPD.
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are both obstructive lung diseases (OLD) characterized by chronic airway inflammation and airflow limitation. Current clinical practice is based upon distinguishing between asthma and COPD, and clinicians use separate guidelines for each condition. However, it is not always possible to clearly differentiate between adult asthma and COPD because of common risk factors, such as tobacco smoking and air pollution, and the convergence of symptoms and typical clinical features (Citation1).
While asthma is characterized by widespread but variable and usually reversible airflow obstruction, COPD is characterized by persistent airflow limitation (Citation2). However, patients with severe asthma may have persistent airway obstruction (Citation2), and about 14% of patients with COPD have significant reversibility (Citation3). Hence, there can be an overlap between the two obstructive pulmonary diseases when persistent airway obstruction is present (Citation4,Citation5). During the past decade, the need to re-evaluate the concept of asthma and COPD as separate conditions has been addressed by several authors (Citation6,Citation7), and in 2013, the term asthma–COPD overlap syndrome (ACOS) was introduced (Citation8). In 2015, the GOLD guidelines presented a consensus-based description of ACOS (Citation2). Also a COPD phenotype with variable airflow limitation (COPD-VAL) has been described (Citation9). Medical treatment for asthma in general is more efficient than treatment for COPD, and it is important to identify the unique set of patients among those with COPD who also have asthma, i.e., the asthma–COPD overlap (ACO) group. Because it is still debated whether asthma and COPD overlap should be defined as a syndrome, the term ACO is used for the overlap group in the present paper (Citation10,Citation11).
To our knowledge, there have not been any studies on the prevalence of ACO in the general population in Norway, and only a few have been conducted worldwide. In studies from Italy, Latin America, and South-Korea, the prevalence of ACOS was 1.6–4.5%, 1.8%, and 2.3%, respectively (Citation12–14). Additionally, several studies have estimated the prevalence of ACOS in patients with COPD, and they range from 5.8% in Spain to 55% in the UK (Citation13,Citation4–22).
We aimed to investigate the prevalence of ACO in the general population from a large population-based study; the Nord-Trøndelag Health Study (HUNT), Norway. Additionally, we described the distribution of demographic, lifestyle factors, atopy, and symptom characteristics of these subjects in comparison with subjects with only asthma or only COPD.
Methods
Study population
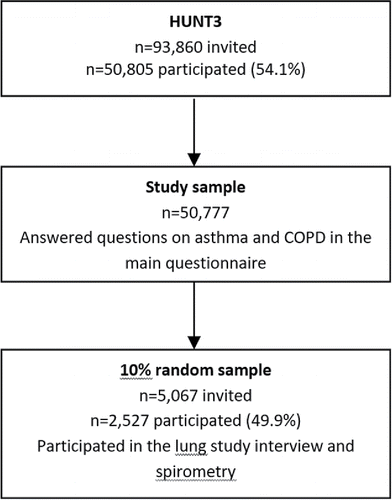
The Nord-Trøndelag Health Study was conducted in the county of Nord-Trøndelag, Norway. The study consisted of three health surveys, HUNT1 (1984–86), HUNT2 (1995–97), and HUNT3 (2006–08), where all adults 20 years or older were invited to attend clinical interviews and complete questionnaires (Citation23). This study utilized data from the HUNT3 as questions on COPD were limited in the previous surveys. The population of Nord-Trøndelag was approximately 128,000 in 2005. Of the 93,860 adults invited to attend HUNT3, 54.1% participated.
Study cohort
The study cohort consisted of 50,777 adults with self-reported information on asthma and COPD. Asthma only was defined as those answering “yes” to “Do you have or have you had asthma?” but “no” to “Have you had or do you have any of the following: chronic bronchitis, emphysema or chronic obstructive pulmonary disease (COPD)”. COPD only was defined as “no” and “yes” to these questions, respectively, and asthma–COPD overlap (ACO) was defined as “yes” to both questions.
Spirometry subgroup
Within the study cohort, a 10% sample (spirometry subgroup) (n = 5,067) was selected for further examinations, including spirometry and structured interview (). Of these subjects, a total of 2,527 participants had complete information on asthma and COPD. In this spirometry subgroup, asthma only was defined as “yes” to “Has a doctor diagnosed you as having asthma?” and pre-bronchodilator (pre-BD) FEV1/FVC ≥ 0.70. COPD only was defined as answering “no” to the asthma question above and a FEV1/ FVC < 0.70. Finally, ACO was defined as having doctor diagnosis of asthma and pre-BD FEV1/FVC < 0.70.
General questionnaire
Participants attending HUNT3 were asked to complete two comprehensive questionnaires, which included questions on respiratory symptoms and lifestyle factors that are common features of obstructive lung diseases (OLD). These included questions on coughing daily in the last year, coughing daily for the last three months, phlegm while wheezing, attacks of wheezing in the last year, allergic rhinitis ever, smoking status, family history of asthma and physical activity. Information on participants' age, sex, and body mass index was also available. More detail on the HUNT questionnaires can be found elsewhere (Citation23).
Lung interview and spirometry
Participants in the spirometry subgroup were also asked to attend an interview on respiratory health and perform spirometry testing. Spirometry was instructed by trained health professionals as per recommendations and criteria from the American Thoracic Society/ European Respiratory Society (Citation24).
Statistical analysis
Percentages and 95% confidence intervals (95%CI), and means and standard deviations (SD) were calculated. Comparison of proportions and means across two or more groups was done using Pearson's chi-square test or analysis of variance, respectively. Risk ratios (RR) and 95% CIs calculated from log-binomial regression models were used to compare the proportion of symptoms between groups adjusted for age, sex, and smoking status (never, former, and current). Additionally, we present prevalence using the lower limit of normal, defined as GLI-2012 z –score (z) for FEV1/FVC < –1.64, to define COPD, and in subgroups stratified by smoking status (Citation25,Citation26). Complete cases analysis was preformed when investigating the prevalence by characteristic. Statistical analyses were performed using R 3.2.2 (Citation27).
Ethics
This study was approved by the Regional Committee for Medical Research Ethics (2015/837/REK midt), Norway and all participants gave informed written consent.
Results
There were no large variations in characteristics between the study cohort (n = 50,777) and the spirometry subgroup (n = 2,527), except for a slightly higher percentage of never smokers in the total sample (48.6% versus 46.4%), ().
Table 1. Characteristics of the Nord–Trøndelag Health Study 3 (2006–2008), Norway.
In the study cohort, the lifetime prevalence of self-reported ACO was 1.9%, asthma only 9.8%, and COPD only 1.5% (p value < 0.01), (). The mean age of each group was 61.6, 51.0, and 62.5 years, respectively (p value < 0.01). Among those with COPD, the prevalence of ACO was 56%. The prevalence of ACO and COPD only increased with age while asthma only showed the opposite trend. In the spirometry subgroup, the prevalence of self-reported ACO was 1.9%, COPD only 1.6%, and asthma only 11.3% ().
Table 2. Prevalence and 95% CI of asthma, COPD, and ACO by self-report in the study cohort (n = 50,777) and the spirometry subgroup (n = 2,527) of Nord–Trøndelag Health Study 3 (2006–2008), Norway.
In the study cohort, when comparing the characteristics of the four groups (ACO, asthma only, COPD only, and no asthma or COPD), all respiratory symptoms, separately or in combination, as well as medication use are reported most frequently in those with ACO compared to the other groups (). In all the three OLD groups, attacks of wheezing last year was the most frequent symptom, 87.3% in ACO, 57.9% in asthma only, and 72.7% in COPD only. The percentage with allergic rhinitis ever was higher in the groups with asthma only (65.1%) and ACO (59.9%) compared to those with COPD only (29.9%) (ACO verses COPD, adjusted RR 2.01, 95% CI 1.75–2.31, p value < 0.001). Combined cough, wheeze, and allergy was reported most frequently by those with ACO 40.4%. Further, the percentages of both former and current smokers were highest in the groups with ACO (45.9% and 29.7%, respectively) and COPD only (44.1% and 33.3%, respectively), whereas the percentage of those with very high physical activity was lowest in the ACO group (14.0%).
Table 3. Percentage and 95% CI of usual features in asthma, COPD, and ACO in the study sample (n = 50,777) of Nord–Trøndelag Health Study 3 (2006–2008), Norway.
presents additional respiratory questions and measures included in an interview at the spirometry station and not included in the questionnaire given to the study sample. The percentage of all symptoms was highest among those with ACO and lowest among those with no asthma or COPD (). The most apparent difference in percentages between ACO and asthma only or COPD only was found regarding the question dyspnea at rest (p value < 0.01).
Table 4. Percentage and 95% CI of usual features in self-reported asthma, COPD, and ACO in the spirometry subgroup (n = 2,527) of Nord–Trøndelag Health Study 3 (2006–2008), Norway.
presents the prevalence of ACO, COPD only, and asthma only in the spirometry subgroup defined by doctor diagnoses and pre-BD FEV1/FVC; the prevalence of ACO was 2.0%, COPD only 9.0%, and asthma only 8.9%. Twenty percent of those reporting COPD had ACO. The prevalence of ACO was similar in females compared to males (2.0% versus 3.0%, p value 0.57). Among age categories, the highest prevalence of ACO was among those aged ≥60 years (p value 0.003).
Table 5. The prevalence of asthma only defined as the combination of a reported doctor diagnosis of asthma and FEV1/FVC ratio ≥0.70, prevalence of COPD only defined as no asthma and a FEV1/FVC ratio < 0.70, and the prevalence of ACO defined as a doctor diagnosis of asthma and FEV1/FVC ratio < 0.70 in the spirometry subgroup (n = 2,527) of the Nord–Trøndelag Health Study 3 (2006–2008), Norway.
In supplementary , the prevalence of ACO, COPD only, and asthma only were defined using FEV1/FVC below the LLN, as opposed to FEV1/FVC ratio < 0.70. The prevalence of ACO (2.0%) was similar to both the self-reported prevalence of ACO in the study cohort (1.9%) and the prevalence of ACO in the spirometry subgroup defined by doctor diagnosed asthma and FEV1/FVC < 0.70 (2.0%).
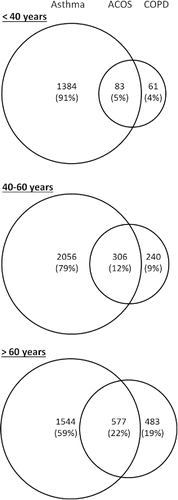
Among participants with OLD, in the age group < 40 years the percentage of those with asthma only was by far the largest, 91% compared to 5% with ACO and 4% with COPD only. In the age group 40–60 years, the corresponding percentages were 79%, 12%, and 9%, and among those >60 years, the percentages were 59%, 22%, and 19% respectively ().
Figure 2. Venn diagram of asthma, COPD, and ACO in subjects with OLD (n = 6734) in the study sample stratified by age in the Nord–Trøndelag Health Study 3 (2006–2008), Norway.
In the age group older than 60 years, the prevalence of asthma only, COPD only, and ACO among never smokers was 8.3%, 1.2%, and 1.8% respectively (supplementary ). In ever smokers (former and current smokers), the corresponding numbers were 8.9%, 3.9%, and 4.4% respectively (supplementary ). This corresponded to a prevalence of any OLD of 11.3% in never smokers versus 17.2% in ever smokers, and the percentage of subjects with ACO among those with OLD was substantially lower in never smokers (16%) compared to ever smokers (26%). Similar results were observed in the spirometry subgroup (supplementary and ).
Discussion
To our knowledge, this is the first Norwegian population study and the largest European prevalence study on ACO. In this Norwegian population, the prevalence of self-reported ACO was 1.9%, and COPD only 2.0%. Among all participants reporting COPD, the percentage of subjects with ACO was 56%. In the spirometry subgroup with spirometry defined COPD and asthma, the prevalence of ACO was 2.0% and COPD only 9%, corresponding to a percentage of subjects with ACO among all subjects with COPD of 18%. The main driver of this variation seemed to be undiagnosed COPD in the study cohort.
Participants with ACO generally had the highest frequency of respiratory symptoms compared to asthma only or COPD only. Intriguingly, the percentage with allergic rhinitis was two-fold higher among those with ACO compared to COPD only.
Comparisons with previous literature
In recent studies, several authors have defined the group with ACO as a separate syndrome (Citation19,Citation28). In the review article by Wurst et al. from 2016, the authors aimed to characterize the prevalence of ACOS and examine how different definitions of ACOS affect prevalence estimates (Citation29). They found that at least three substantially different classification schemes for defining ACOS were used: (1) Reported physician diagnosis of both asthma and COPD ever, (2) reported physician diagnosis of asthma and spirometry-defined COPD, and (3) self-reported symptoms and FEV1/FVC ratio >0.70 (asthma) and ≤0.70 (COPD). When we compared our findings in the study sample to other studies using similar definitions, we found the prevalence of ACO to be similar. For example, in a US population-based study by Diaz-Guzman et al., the prevalence of ACOS among those older than 25 years was 2.7% (Citation30). A study by de Marco et al. found a prevalence of 1.6% among Italians aged 20–44 years and 2.1% among those aged 45–64 years (Citation12), and in a population-based study from the United States by Kumbhare et al. from 2016, they found a prevalence of ACOS of 3.2% (Citation31). Finally, in the North Carolina Behavioral Risk Factor Surveillance System study from 2014, the age-adjusted prevalence of any self-reported COPD and current asthma (ACOS) was 2.4% (Citation32). Hence the ACO group in the present study is comparable to the ACOS groups in the above studies.
The prevalence of ACO defined as physician diagnosed asthma and FEV1/FVC < 0.70 of 2% in those aged between 40 and 60 years and 4% in those over 60 years in the spirometry subgroup is rather similar to the prevalence of ACOS of 2.9% found in the population-based study of five Latin American cities using similar definitions and including those aged over 40 years (Citation13).
In a review and meta-analysis by Alshabanat et al. from 2015, all studies included FEV1/FVC ratio as part of their definition of COPD (Citation33). They found that 27% of those with COPD had ACOS in population studies. In comparison, in the spirometry subgroup when using FEV1/FVC ratio to define COPD, we found an overlap between ACO and COPD of only 18%. However, when using only self-reported diagnoses, the overlap between ACO and COPD was as high as 56%. Based on our observations, the reason for this substantially reduced overlap between ACO and COPD when using objective diagnostic criteria is the fourfold increase in COPD with age compared to only a twofold increase when using self-reported diagnoses.
Regarding demographic and lifestyle factors for ACO, several previous studies have reported an increasing prevalence with age, which is in-line with our findings (Citation29,Citation31,Citation34). The high percentage of current and former cigarette smokers and the low percentage of participants with high levels of physical activity were very similar between the ACO and COPD only groups. The most striking finding regarding characteristics of those with ACO compared to those with COPD only was the two-fold increase in the occurrence of allergic rhinitis and an increased occurrence in all the typical respiratory symptoms, such as cough, wheeze, and use of medication ().
Clinical significance
From a patient point of view, it is important to be labeled with the most correct diagnosis in order to achieve the best treatment. Over time, some individuals with severe asthma develop persistent airflow obstruction and approximately 30% of asthmatics are smokers and thus have an increased risk of developing COPD (Citation35), which makes differentiation from COPD difficult (Citation36). Additionally, more than 10% of patients with COPD demonstrate significant airflow reversibility (post bronchodilator increase in FEV1 ≥ 12% and 200 ml), which traditionally has been regarded as a diagnostic criterion for asthma (Citation3). There will be patients in the borderline between asthma and ACO and between COPD and ACO who will be defined as patients with ACO in some studies and as asthmatics or COPD patients in other studies due to differences in the perception of the diagnostic criteria. The diagnosis will to some extent influence the treatment and follow-up they are offered.
It can be debated whether participants with ACO in the present study have a specific syndrome, ACOS, or is merely a subgroup of COPD patients. The finding that nearly 60% of the subjects with ACO also had allergic rhinitis in addition to more respiratory symptoms and more medication use, emphasizes the perception by several authors that subjects with ACO are more severely ill (Citation12,Citation13,Citation18). In their recent paper, Agusti et al. propose a strategy based on the presence of treatable traits, such as eosinophilic airway inflammation instead of “Oslerian diagnostic labels” such as asthma and COPD (Citation10). An increased focus on treatable traits may contribute to move the field of airway diseases toward precision medicine. However, introduction of the ACOS concept by several researchers in the past decade has contributed to an increased understanding of differences in the burden of symptoms among patients with COPD. The finding that more than half of those with self-reported COPD have ACO is important because it indicates that a COPD diagnosis is insufficient in explaining the symptoms and clinical findings presented by these patients. One may hypothesize that those in the present study who report all three diagnoses asthma, COPD, and allergic rhinitis represent a specific endotype or a treatable trait (Citation10) with a better response to anti-inflammatory medication compared to those with ACO and not allergic rhinitis.
Strengths and limitations
As the first Norwegian population study and by far the largest European prevalence study on ACO, including comprehensive interviews, questionnaires, and clinical examination, we were able to calculate fairly precise estimates of the prevalence of ACO, and present detailed demographic, lifestyle, and symptom information of this sample. Furthermore, because of this detailed information, we were able to present three alternative definitions of ACO for comparison.
There were several limitations of our study. Those participating in the HUNT, particularly the HUNT Lung Study may have had a more severe disease than the general population, as those with disease symptoms were more likely to attend clinical interviews than those without symptoms. This may have increased the proportions of symptoms compared to the general population. However, with respect to prevalence, this might have been underestimated as non-participants were more likely to have COPD than participants (Citation37). Additionally, we did not have access to post-bronchodilator measurements for this sample and did not use the formal definition of ACOS as specified in the GINA guidelines (Citation2). Therefore, our estimates from the spirometry subgroup may not be directly comparable to other studies. Additionally, the use of pre-bronchodilator measurement will overestimate the prevalence of COPD in the population. The spirometry criteria for the diagnosis of ACO in our study may have also included participants with persistent asthma who otherwise are never-smokers (27% of those identified as ACO were never smokers in the spirometry subgroup) and, thus, are less likely to have COPD. Finally, we did not have complete information on all demographic and lifestyle characteristics and for variables such as physical activity, it is unlikely that missing was random. This may have directly influenced the prevalence of disease within these subgroups.
Conclusion
We found that approximately one out of seven adults in the general population had OLD, and about 14% of those have ACO. Participants with ACO had the most severe disease as they generally had the highest frequency of respiratory symptoms compared to those with only asthma or COPD, and the percentage with allergic rhinitis was two-fold higher among those with ACO compared to those with COPD only. Doctors need meaningful labels for various disease subgroups in order to choose the best treatment regimen for their patients, and recognizing subjects with ACO may represent a step toward a more specific diagnosing practice for patients with OLD. Future research is needed to better characterize the airway inflammation and disease characteristics in patients with ACO.
Declaration of interest statement
The authors declare that they have no competing interests.
Acknowledgments
The Nord-Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology, NTNU), Nord-Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health.Funding
Additional information
Funding
References
- Abramson MJ, Perret JL, Dharmage SC, McDonald VM, McDonald CF. Distinguishing adult-onset asthma from COPD: a review and a new approach. Int J Chronic Obstruct Pulm Dis. 2014;9:945–962. doi:10.2147/COPD.S46761.
- Global initiative for Chronic Obstructive Lung Disease. Asthma, COPD and Asthma-COPD Overlap Syndrome. GOLD [Internet]. 2015. Available from: http://goldcopd.org/asthma-copd-asthma-copd-overlap-syndrome/
- Global initiative for Chronic Obstructive Lung Disease. Global strategy for the Diagnosis, Management, and prevention of chronic obstructive pulmonary disease. GOLD [Internet]. 2016. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
- Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124(2):474–481. doi:10.1378/chest.124.2.474.
- Viegi G, Matteelli G, Angino A, Scognamiglio A, Baldacci S, Soriano JB, et al. The proportional Venn diagram of obstructive lung disease in the Italian general population. Chest. 2004;126(4):1093–1101. doi:10.1378/chest.126.4.1093.
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728–735. doi:10.1136/thx.2008.108027.
- Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Resp Crit Care. 2003;167(3):418–424. doi:10.1164/rccm.200203-183OC.
- Louie S, Zeki AA, Schivo M, Chan AL, Yoneda KY, Avdalovic M, et al. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol. 2013; 6(2):197–219. doi:10.1586/ecp.13.2.
- Matsumoto K, Seki N, Fukuyama S, Moriwaki A, Kan-o K, Matsunaga Y, et al. Prevalence of asthma with airflow limitation, COPD, and COPD with variable airflow limitation in older subjects in a general Japanese population: the Hisayama Study. Respir Investig. 2015;53(1):22–29. doi:10.1016/j.resinv.2014.08.002.
- Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi:10.1183/13993003.01359-2015.
- Rodrigo GJ, Neffen H, Plaza V. Asthma–chronic obstructive pulmonary disease overlap syndrome: a controversial concept. Curr Opin Allergy Clin Immunol. 2017;17(1):36–41. doi:10.1097/ACI.0000000000000326.
- De Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8(5):e62985. doi:10.1371/journal.pone.0062985.
- Menezes AMB, de Oca MM, Rogelio P, Nadeau G, Fernando C, Lopez-Varela MV, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297–304. doi:10.1378/chest.13-0622.
- Chung JW, Kong KA, Lee JH, Lee SJ, Ryu YJ, Chang JH. Characteristics and self-rated health of overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2014;9:795–804.
- Rhee CK, Yoon HK, Yoo KH, Kim YS, Lee SW, Park YB, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2014;11(2):163–170. doi:10.3109/15412555.2013.831061.
- Marsh S, Travers J, Weatherall M, Williams M, Aldington S, Shirtcliffe P, et al. Proportional classifications of COPD phenotypes. Thorax. 2008;63(9):761–767. doi:10.1136/thx.2007.089193.
- Miravitlles M, Soriano JB, Ancochea J, Muñoz L, Duran-Tauleria E, Sánchez G, et al. Characterisation of the overlap COPD–asthma phenotype. Focus on physical activity and health status. Resp Med. 2013;107(7):1053–1060. doi:10.1016/j.rmed.2013.03.007.
- Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12(1):127. doi:10.1186/1465-9921-12-127.
- Louie S, Zeki AA, Schivo M, Chan AL, Yoneda KY, Avdalovic M, et al. The asthma–chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol. 2013;6(2):197–219. doi:10.1586/ecp.13.2.
- Izquierdo-Alonso JL, Rodriguez-GonzálezMoro JM, de Lucas-Ramos P, Unzueta I, Ribera X, Antón E, et al. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD). Resp Med. 2013;107(5):724–731. doi:10.1016/j.rmed.2013.01.001.
- Miravitlles M, Huerta A, Fernández-Villar JA, Alcázar B, Villa G, Forné C, et al. Generic utilities in chronic obstructive pulmonary disease patients stratified according to different staging systems. Health Qual Life Out. 2014;12(1):1. doi:10.1186/s12955-014-0120-5.
- Golpe R, López PS, Jiménez EC, Anón OC, de Llano LAP. Distribution of clinical phenotypes in patients with chronic obstructive pulmonary disease caused by biomass and tobacco smoke. Archivos de Bronconeumología (English Edition). 2014;50(8):318–324. doi:10.1016/j.arbr.2014.06.003.
- Krokstad S, Langhammer A, Hveem K, Holmen T, Midthjell K, Stene T, et al. Cohort profile: The HUNT study, Norway. Int J Epidemiol. 2012;42(4):968–977. doi:10.1093/ije/dys095.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805.
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312.
- Langhammer A, Johannessen A, Holmen TL, Melbye H, Stanojevic S, Lund MB, et al. Global Lung Function Initiative 2012 reference equations for spirometry in the Norwegian population. Eur Respir J. 2016;48(6):1602–1611.
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ISBN 3-900051-07-0; 2014.
- Postma DS, Rabe KF. The asthma–COPD overlap syndrome. New Engl J Med. 2015;373(13):1241–1249. doi:10.1056/NEJMra1411863.
- Wurst KE, Kelly-Reif K, Bushnell GA, Pascoe S, Barnes N. Understanding asthma-chronic obstructive pulmonary disease overlap syndrome. Resp Med. 2016;110:1–11. doi:10.1016/j.rmed.2015.10.004.
- Diaz-Guzman E, Khosravi M, Mannino DM. Asthma, chronic obstructive pulmonary disease, and mortality in the US population. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2011;8(6):400–7. doi:10.3109/15412555.2011.611200.
- Kumbhare S, Pleasants R, Ohar JA, Strange C. Characteristics and prevalence of asthma/chronic obstructive pulmonary disease overlap in the United States. Ann Am Thorac Soc. 2016;13(6):803–810. doi:10.1513/AnnalsATS.201508-554OC.
- Pleasants RA, Ohar JA, Croft JB, Liu Y, Kraft M, Mannino DM, et al. Chronic obstructive pulmonary disease and asthma–patient characteristics and health impairment. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2014;11(3): 256–266.
- Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald J. Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis. PLoS One. 2015;10(9):e0136065. doi:10.1371/journal.pone.0136065.
- De Marco R, Marcon A, Rossi A, Antó JM, Cerveri I, Gislason T, et al. Asthma, COPD and overlap syndrome: a longitudinal study in young European adults. Eur Respir J. 2015; 46(3): 671–679.
- Kim HJ, Baek S, Kim HJ, Lee JS, Oh YM, Lee SD, et al. The impact of smoking on airflow limitation in subjects with history of Asthma and inactive tuberculosis. PLoS One. 2015;10(4):e0125020. doi:10.1371/journal.pone.0125020.
- Broekema M, Timens W, Vonk JM, Volbeda F, Lodewijk ME, Hylkema MN, et al. Persisting remodeling and less airway wall eosinophil activation in complete remission of asthma. Am J Respir Crit Care Med. 2011;183(3):310–316. doi:10.1164/rccm.201003-0494OC.
- Langhammer A, Krokstad S, Romundstad P, Heggland J, Holmen J. The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol. 2012;12:143. doi:10.1186/1471-2288-12-143.
Appendix
Supplementary Table 1. The prevalence of asthma only defined as the combination of a reported doctor diagnosis of asthma and lung function above LLN (Z-score ≥ -1.64), prevalence of COPD only defined as no asthma and lung function below LLN (Z-score < -1.64), and the prevalence of ACO defined as a doctor diagnosis of asthma and lung function below LLN (Z-score < -1.64) in the spirometry subgroup (n=2527) of the Nord–Trøndelag Health Study 3 (2006-2008), Norway.
Supplementary Table 2. Prevalence and 95% CI of asthma, COPD and ACO in the study sample, restricted to never smokers (n=24,669) of Nord–Trøndelag Health Study 3 (2006-2008), Norway.
Supplementary Table 3. Prevalence and 95% CI of asthma, COPD and ACO in the study sample, restricted to former and current smokers (n=24,682) of Nord–Trøndelag Health Study 3 (2006-2008), Norway.
Supplementary Table 4. Prevalence and 95% CI of self-reported asthma only, COPD only and ACO in the spirometry subgroup, restricted to never smokers (n=1,172) of Nord–Trøndelag Health Study 3 (2006-2008), Norway.
Supplementary Table 5. Prevalence and 95% CI of self-reported asthma only, COPD only and ACO in the spirometry subgroup, restricted to former and current smokers (n=1,289) of Nord–Trøndelag Health Study 3 (2006-2008), Norway.